Is Continuous Wound Infiltration a Better Option for Postoperative Pain Management after Open Nephrectomy Compared to Thoracic Epidural Analgesia?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Data Selection
2.2. ERAS® Protocol
2.3. Anesthesiology Protocol
2.3.1. TEA Placement
2.3.2. CWI Placement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Robson, C.J. Radical Nephrectomy for Renal Cell Carcinoma. J. Urol. 1963, 89, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Dillenburg, W.; Poulakis, V.; Skriapas, K.; de Vries, R.; Ferakis, N.; Witzsch, U.; Melekos, M.; Becht, E. Retroperitoneoscopic Versus Open Surgical Radical Nephrectomy for Large Renal Cell Carcinoma in Clinical Stage cT2 or cT3a: Quality of Life, Pain and Reconvalescence. Eur. Urol. 2006, 49, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Hemal, A.K.; Kumar, A.; Kumar, R.; Wadhwa, P.; Seth, A.; Gupta, N. Laparoscopic Versus Open Radical Nephrectomy for Large Renal Tumors: A Long-Term Prospective Comparison. J. Urol. 2007, 177, 862–866. [Google Scholar] [CrossRef]
- Junejo, N.; Alkhateeb, S.; Alrumayyan, M.; Alkhatib, K.; Alzahrani, H.; Alotaibi, M.; Alothman, K.; Al-Hussain, T.; Altaweel, W. Trends in the surgical management of renal cell carcinoma in a contemporary tertiary care setting. Urol. Ann. 2021, 13, 111. [Google Scholar] [CrossRef]
- Kehlet, H.; Holte, K. Effect of postoperative analgesia on surgical outcome. Br. J. Anaesth. 2001, 87, 62–72. [Google Scholar] [CrossRef]
- Wu, C.; Rowlingson, A.; Partin, A.; Kalish, M.A.; Courpas, G.E.; Walsh, P.C.; Fleisher, L.A. Correlation of Postoperative Pain to Quality of Recovery in the Immediate Postoperative Period. Reg. Anesth. Pain Med. 2005, 30, 516–522. [Google Scholar] [CrossRef]
- Pöpping, D.M. Protective Effects of Epidural Analgesia on Pulmonary Complications after Abdominal and Thoracic Surgery: A Meta-Analysis. Arch. Surg. 2008, 143, 990. [Google Scholar] [CrossRef]
- Kooij, F.O.; Schlack, W.S.; Preckel, B.; Hollmann, M.W. Does Regional Analgesia for Major Surgery Improve Outcome? Focus on Epidural Analgesia. Anesth. Analg. 2014, 119, 740–744. [Google Scholar] [CrossRef]
- Pöpping, D.M.; Zahn, P.K.; Van Aken, H.K.; Dasch, B.; Boche, R.; Pogatzki-Zahn, E. Effectiveness and safety of postoperative pain management: A survey of 18925 consecutive patients between 1998 and 2006 (2nd revision): A database analysis of prospectively raised data. Br. J. Anaesth. 2008, 101, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Crettenand, F.; Martel, P.; Lucca, I.; Daneshmand, S.; Cerantola, Y. ERAS for Major Urological Procedures: Evidence Synthesis and Recommendations. In Enhanced Recovery after Surgery; Ljungqvist, O., Francis, N.K., Urman, R.D., Eds.; Springer International Publishing: Cham, Switzerland, 2000; pp. 421–431. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 9. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.C. Measurement of Pain. Lancet 1974, 304, 1127–1131. [Google Scholar] [CrossRef]
- Cerantola, Y.; Valerio, M.; Persson, B.; Jichlinski, P.; Ljungqvist, O.; Hubner, M.; Kassouf, W.; Muller, S.; Baldini, G.; Carli, F.; et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery after Surgery (ERAS®) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef]
- Isbister, J.P. The three-pillar matrix of patient blood management—An overview. Best Pract Res. Clin. Anaesthesiol. 2013, 27, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Feldheiser, A.; Aziz, O.; Baldini, G.; Cox, B.P.B.W.; Fearon, K.C.H.; Feldman, L.S.; Gan, T.J.; Kennedy, R.H.; Ljungqvist, O.; Lobo, D.N.; et al. Enhanced Recovery after Surgery (ERAS) for gastrointestinal surgery, part 2: Consensus statement for anaesthesia practice. Acta Anaesthesiol. Scand. 2016, 60, 289–334. [Google Scholar] [CrossRef] [PubMed]
- Lovich-Sapola, J.; Smith, C.E.; Brandt, C.P. Postoperative Pain Control. Surg. Clin. N. Am. 2015, 95, 301–318. [Google Scholar] [CrossRef]
- Gramigni, E.; Bracco, D.; Carli, F. Epidural analgesia and postoperative orthostatic haemodynamic changes: Observational study. Eur. J. Anaesthesiol. 2013, 30, 398–404. [Google Scholar] [CrossRef]
- Ukai, T.; Ebihara, G.; Watanabe, M. Opioid administration via epidural catheter is a risk factor for postoperative nausea and vomiting in total hip arthroplasty: A retrospective study. J. Orthop. Sci. 2018, 23, 973–976. [Google Scholar] [CrossRef]
- Forastiere, E.; Sofra, M.; Giannarelli, D.; Fabrizi, L.; Simone, G. Effectiveness of continuous wound infusion of 0.5% ropivacaine by On-Q pain relief system for postoperative pain management after open nephrectomy. Br. J. Anaesth. 2008, 101, 841–847. [Google Scholar] [CrossRef]
- Gebhardt, R.; John Mehran, R.; Soliz, J.; Cata, J.P.; Smallwood, A.K.; Feeley, T.W. Epidural Versus ON-Q Local Anesthetic-Infiltrating Catheter for Post-Thoracotomy Pain Control. J. Cardiothorac. Vasc. Anesth. 2013, 27, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Richman, J.M.; Thirlby, R.C.; Wu, C.L. Efficacy of Continuous Wound Catheters Delivering Local Anesthetic for Postoperative Analgesia: A Quantitative and Qualitative Systematic Review of Randomized Controlled Trials. J. Am. Coll. Surg. 2006, 203, 914–932. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.J.; Harrison, E.M.; Peel, N.J.; Stutchfield, B.; McNally, S.; Beattie, C.; Wigmore, S.J. Randomized clinical trial of perioperative nerve block and continuous local anaesthetic infiltration via wound catheter versus epidural analgesia in open liver resection (LIVER 2 trial). Br. J. Surg. 2015, 102, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.; Ward, D.; Jeffery, J.; Toogood, G.J.; Lodge, J.A.; Rao, K.; Lotia, S.; Hidalgo, E. A Randomized Controlled Trial Comparing Epidural Analgesia Versus Continuous Local Anesthetic Infiltration via Abdominal Wound Catheter in Open Liver Resection. Ann. Surg. 2019, 269, 413–419. [Google Scholar] [CrossRef]
- Mungroop, T.H.; Veelo, D.P.; Busch, O.R.; van Dieren, S.; van Gulik, T.M.; Karsten, T.M.; de Castro, S.M.; Godfried, M.B.; Thiel, B.; Hollmann, M.W.; et al. Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): A randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 105–113. [Google Scholar] [CrossRef]
- Othman, A.H.; Ahmed, D.G.; Abd El-Rahman, A.M.; El Sherif, F.A.; Mansour, S.; Aboeleuon, E. Effect of Preperitoneal Versus Epidural Analgesia on Postoperative Inflammatory Response and Pain Following Radical Cystectomy: A Prospective, Randomized Trial. Clin. J. Pain 2019, 35, 328–334. [Google Scholar] [CrossRef]
- An, G.; Wang, G.; Zhao, B.; Zhang, X.; Li, Z.; Fu, J.; Zhao, X. Opioid-free anesthesia compared to opioid anesthesia for laparoscopic radical colectomy with pain threshold index monitoring: A randomized controlled study. BMC Anesthesiol. 2022, 22, 241. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Wilson, M.; Stewart, G.D.; Adeyoju, A.; Cartledge, J.; Kimuli, M.; Datta, S.; Hanbury, D.; Hrouda, D.; Oades, G.; et al. Challenges of early renal cancer detection: Symptom patterns and incidental diagnosis rate in a multicentre prospective UK cohort of patients presenting with suspected renal cancer. BMJ Open 2020, 10, e035938. [Google Scholar] [CrossRef]
- MacLennan, S.; Imamura, M.; Lapitan, M.C.; Omar, M.I.; Lam, T.B.; Hilvano-Cabungcal, A.M.; Royle, P.; Stewart, F.; MacLennan, G.; MacLennan, S.J.; et al. Systematic Review of Oncological Outcomes Following Surgical Management of Localised Renal Cancer. Eur. Urol. 2012, 61, 972–993. [Google Scholar] [CrossRef]
- Capitanio, U.; Terrone, C.; Antonelli, A.; Minervini, A.; Volpe, A.; Furlan, M.; Matloob, R.; Regis, F.; Fiori, C.; Porpiglia, F.; et al. Nephron-sparing Techniques Independently Decrease the Risk of Cardiovascular Events Relative to Radical Nephrectomy in Patients with a T1a–T1b Renal Mass and Normal Preoperative Renal Function. Eur. Urol. 2015, 67, 683–689. [Google Scholar] [CrossRef]
- Anastasiadis, E.; O’Brien, T.; Fernando, A. Open partial nephrectomy in renal cell cancer—Essential or obsolete? Int. J. Surg. 2016, 36, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Fernando, A.; Fowler, S.; O’Brien, T.; the British Association of Urological Surgeons (BAUS). Nephron-sparing surgery across a nation—Outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int. 2016, 117, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Lee, Y.J.; Jo, M.H.; Park, H.J.; Lim, G.W.; Cho, H.; Nam, E.J.; Kim, S.W.; Kim, J.H.; Kim, Y.T.; et al. The ON-Q pain management system in elective gynecology oncologic surgery: Management of postoperative surgical site pain compared to intravenous patient-controlled analgesia. Obstet. Gynecol. Sci. 2013, 56, 93. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef] [PubMed]
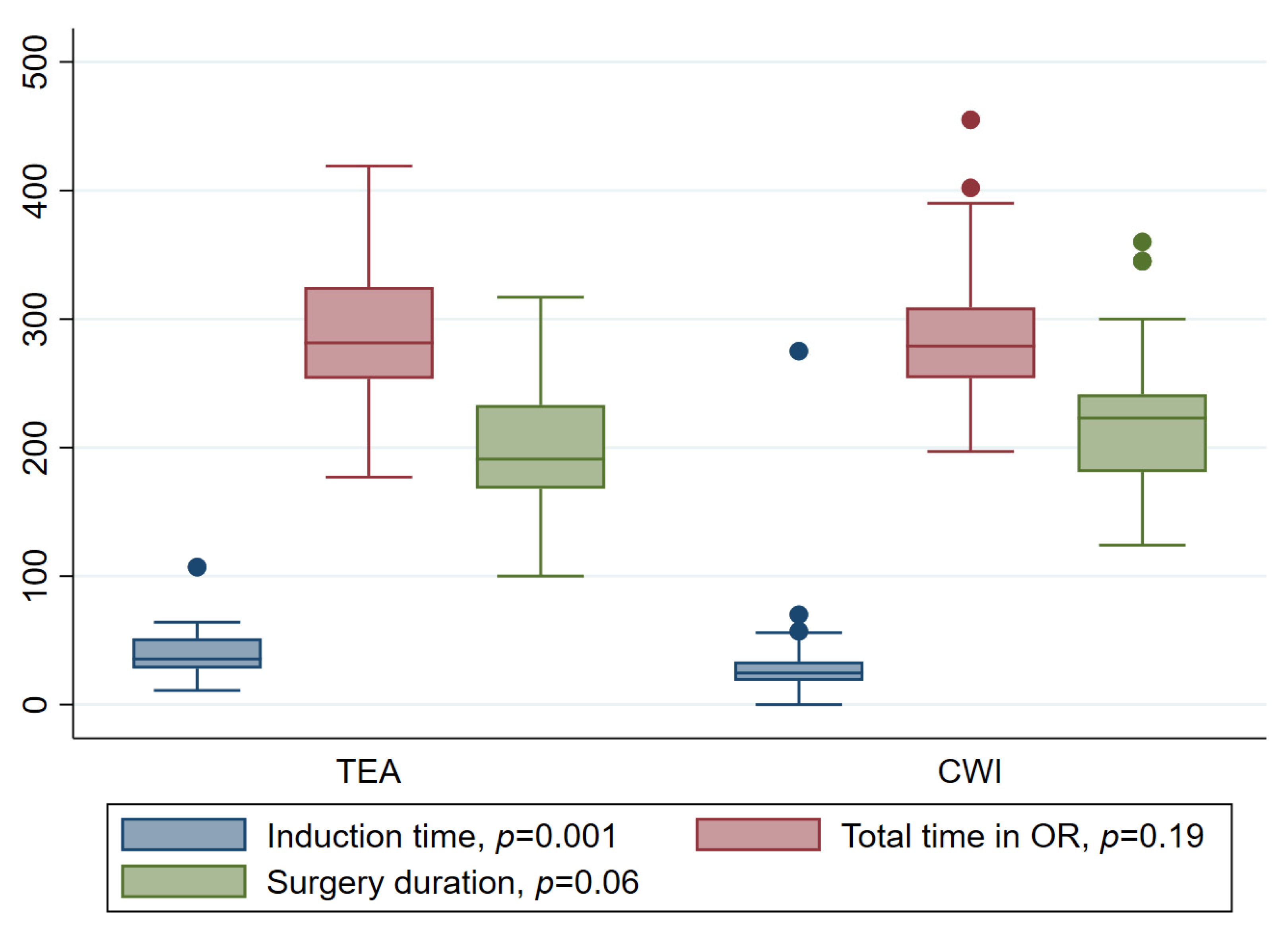
| CWI | TEA | ||
|---|---|---|---|
| N | 64 | 28 | p |
| Age—median (IQR) | 62 (51.5–67.5) | 63.5 (54.5–75) | 0.35 |
| Gender—n (%) | 0.65 | ||
| Female | 22 (34.4) | 11 (39.3) | |
| Male | 42 (65.6) | 17(60.7) | |
| Smoking—n (%) | 33 (55) | 19 (67) | 0.25 |
| BMI—median (IQR) | 26.4 (22.9–31.1) | 25.5 (22.3–30.2) | 0.11 |
| Diabetes—n (%) | 11 (17.2) | 6 (21.4) | 0.72 |
| Hypertension—n (%) | 11 (17.7) | 5 (18.2) | 0.49 |
| Procedure—n (%) | 0.39 | ||
| radical nephrectomy | 29 (45.3) | 10 (35.7) | |
| partial nephrectomy | 35 (54.7) | 18 (64.3) | |
| pT stages—n (%) | 0.28 | ||
| pT1 | 32 (50) | 12 (42.9) | |
| pT2 | 6 (9.4) | 0 | |
| pT3 | 18 (28.1) | 7 (25) | |
| pT4 | 1 (1.6) | 0 | |
| non-oncological | 7 (10.9) | 9 (32.1) | |
| pN stage—n (%) | 0.08 | ||
| pN0 | 17 (26.5) | 8 (28.6) | |
| pN1 | 0 | 2 (7.2) | |
| pNx | 40 (62.5) | 9 (32.1) | |
| non-oncological | 7 (11) | 9 (32.1) | |
| Complications—n (%) | 0.17 | ||
| Clavien I | 8 (12.5) | 6 (21.4) | |
| Clavien II | 13 (20.3) | 7 (25) | |
| Clavien IIIa | 7 (10.9) | 0 | |
| Clavien IIIb | 1 (1.6) | 2 (7.2) | |
| No complication | 35 (54.7) | 13 (46.4) |
| VAS Score at | Analysis of Variance | Mean VAS—(SD) | ||
|---|---|---|---|---|
| F | p | CWI | TEA | |
| POD 0 | (1, 87) = [9.95] | 0.002 | 5.1 (2.9) | 2.8 (3.4) |
| POD 1 | (1, 88) = [5.02] | 0.03 | 4.9 (2.6) | 3.5 (2.7) |
| POD 2 | (1, 89) = [1.48] | 0.96 | 3.5 (2.6) | 3.6 (2.8) |
| POD 3 | (1, 87) = [4.51] | 0.025 | 3.8 (2.5) | 2.6 (2.3) |
| CWI | TEA | ||
|---|---|---|---|
| N | 64 | 28 | p |
| Opioids at—n (%) | |||
| POD 0 | 41 (64) | 0 | <0.001 |
| POD 1 | 41 (64) | 2 (7.1) | 0.004 |
| POD 2 | 33 (51.5) | 2 (7.1) | 0.04 |
| POD 3 | 27 (42.2) | 5 (17.9) | 0.68 |
| Nausea Score at | Mean Nausea Score—(SD) | |||
|---|---|---|---|---|
| z | p | CWI | TEA | |
| POD 0 | 0.795 | 0.42 | 0.6 (2.1) | 0.9 (2.4) |
| POD 1 | 2.730 | 0.02 | 0.8 (2.4) | 2.7 (4.1) |
| POD 2 | 3.081 | 0.002 | 0.9 (2.5) | 3.3 (4.4) |
| POD 3 | 3.000 | 0.003 | 0.6 (1.8) | 2.1 (3.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crettenand, F.; Assayed-Leonardi, N.; Rohrer, F.; Martinez Carrique, S.; Roth, B. Is Continuous Wound Infiltration a Better Option for Postoperative Pain Management after Open Nephrectomy Compared to Thoracic Epidural Analgesia? J. Clin. Med. 2023, 12, 2974. https://doi.org/10.3390/jcm12082974
Crettenand F, Assayed-Leonardi N, Rohrer F, Martinez Carrique S, Roth B. Is Continuous Wound Infiltration a Better Option for Postoperative Pain Management after Open Nephrectomy Compared to Thoracic Epidural Analgesia? Journal of Clinical Medicine. 2023; 12(8):2974. https://doi.org/10.3390/jcm12082974
Chicago/Turabian StyleCrettenand, François, Nady Assayed-Leonardi, Felix Rohrer, Silvia Martinez Carrique, and Beat Roth. 2023. "Is Continuous Wound Infiltration a Better Option for Postoperative Pain Management after Open Nephrectomy Compared to Thoracic Epidural Analgesia?" Journal of Clinical Medicine 12, no. 8: 2974. https://doi.org/10.3390/jcm12082974
APA StyleCrettenand, F., Assayed-Leonardi, N., Rohrer, F., Martinez Carrique, S., & Roth, B. (2023). Is Continuous Wound Infiltration a Better Option for Postoperative Pain Management after Open Nephrectomy Compared to Thoracic Epidural Analgesia? Journal of Clinical Medicine, 12(8), 2974. https://doi.org/10.3390/jcm12082974





