Abstract
The aim of this systematic review was to evaluate the efficacy of oral medication or intrauterine device-delivered progestins in patients with endometrial hyperplasia (EH) with or without atypia. We systematically examined PubMed, EMBASE, the Cochrane Library, and clinicaltrials.gov to identify studies reporting the regression rate of patients with EH who received progestins or non-progestins. The regression rates after different treatments were compared using a network meta-analysis in terms of the relative ratios (RRs) and 95% confidence intervals (CIs). Begg–Mazumdar rank correlation and funnel plots were performed to evaluate the publication bias. Five non-randomized studies and 21 randomized controlled trials involving 2268 patients were included in the network meta-analysis. The levonorgestrel-releasing intrauterine system (LNG-IUS) was associated with a higher regression rate than medroxyprogesterone acetate (MPA) (RR 1.30, 95% CI 1.16–1.46) in patients with EH. Among those without atypia, the LNG-IUS was associated with a higher regression rate than any of the three types of oral medications (MPA, norethisterone, or dydrogesterone (DGT)) (RR 1.35, 95% CI 1.18–1.55). According to the network meta-analysis, combining the LNG-IUS with MPA or metformin increased regression rate, while DGT was associated with the highest regression rate among all oral medications. The LNG-IUS may be the best choice for patients with EH, and combining it with MPA or metformin may further improve its efficacy. DGT may be the preferred choice for patients who are unwilling to use the LNG-IUS or who cannot tolerate its side effects.
1. Introduction
Endometrial hyperplasia (EH) is a non-invasive, abnormal proliferation of endometrial glands or stroma of the uterus, and it increases the risk of endometrial cancer [1]. The World Health Organization defines two types of EH, with or without atypia [2]. The type with atypia is thought to be highly precancerous, with the condition becoming malignant in almost 60% of cases within five years of diagnosis [3]. Both types rarely occur in women younger than 30 years; the type with atypia is diagnosed most often in women 60–64 years old, and the type without atypia in women 50–54 years old [4]. Among premenopausal women, the incidence rate of EH with atypia is 7 per 100,000 woman-years, while that of EH without atypia is 30 per 100,000 woman-years [5].
The most common symptom of EH is abnormal uterine bleeding, which means more frequent or severe bleeding in the case of premenopausal women or any uterine bleeding in the case of postmenopausal women [6]. A systematic review of studies on premenopausal women concluded that the risk of endometrial carcinoma is higher among women who experience inter-menstrual bleeding than among women who experience heavy menstrual bleeding [7]. The most important risk factor of EH is chronic exposure to endogenous or exogenous estrogen, which can occur in women who have yet to give birth or who are infertile; who experience earlier menarche or later menopause; who receive tamoxifen; or who experience anovulation, menopausal transition, or polycystic ovarian syndrome [8]. Obesity, diabetes, hypertension, and Lynch syndrome also increase the risk of EH [9].
EH with atypia can be treated using a total hysterectomy with or without a bilateral salpingo-oophorectomy (BSO) to eliminate the cancer risk if the woman does not wish to bear children. If she does wish to preserve fertility or she cannot tolerate a hysterectomy, she can be given oral or local progestins, aromatase inhibitors, or gonadotropin-releasing hormone agonists [10,11].
EH without atypia can be managed through watchful waiting [12], or it can be treated using oral progestins, aromatase inhibitors, and letrozole (LET) [1], or a levonorgestrel-releasing intrauterine system (LNG-IUS). The LNG-IUS may be the better choice for many patients, because of its efficacy and generally tolerable side effects [13], which include pelvic pain, breast tenderness, ovarian cysts, weight gain, and acne [14,15]. On the other hand, nearly 30% of patients experience irregular bleeding or spotting during the first three months after the involvement of the LNG-IUS. In addition, the LNG-IUS has the risk of displacement, embedment, and perforation. Patients with LNG-IUS require regular follow-up in hospital to examine the locations of intrauterine devices (IUD) by using ultrasound or other imaging examination methods, and for some patients with a large uterus cavity or irregular shape of uterine cavity, the LNG-IUS may easily migrate down to the cervical canal or even fall out of the body [16,17]. For women who are unwilling to use the LNG-IUS or who cannot tolerate its side effects, the best alternative oral medication is unclear.
Here, we performed a systematic review and network meta-analysis of the available clinical evidence to compare the efficacy of oral progestins, other oral medications, or the LNG-IUS for EH with or without atypia.
2. Materials and Methods
This network meta-analysis was performed in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The study protocol was registered in PROSPERO (CRD42022345837).
2.1. Search Strategy
The following electronic databases were searched: PubMed, EMBASE, the Cochrane Library, and clinicaltrials.gov. We searched all databases from their respective inception to 17 May 2022. The search algorithm was based on the key terms: (endometrial hyperplasia) AND ((levonorgestrel) OR (medroxyprogesterone acetate) OR (megestrol acetate) OR (progesterone) OR (norethisterone) OR (dydrogesterone)). Reference lists of included studies and previous systematic reviews were manually reviewed in order to identify additional studies. In cases of multiple studies reporting on the same patient population, only the largest study was included.
2.2. Study Eligibility
Studies were included in the present meta-analysis if they fulfilled the following criteria: (1) patients were diagnosed with endometrial hyperplasia with or without atypia; (2) patients were treated with the LNG-IUS, oral progestins (medroxyprogesterone acetate (MPA), megestrol acetate (MA), progesterone, norethisterone (NET), dydrogesterone (DGT), micronized progesterone (MP), or lynestrenol (LYN)), or oral non-progestins (metformin (MET), LET); (3) data sufficient to calculate regression rates were reported; (4) the study design was randomized controlled trial (RCT) or observational cohort study, whether prospective or retrospective.
We excluded studies if: (1) the participants were diagnosed with endometrial carcinoma; (2) the original data were not reported, such as in the case of reviews, study protocols, comments, or letters; (3) necessary data could not be obtained; (4) the studies had a single-arm cohort design; or (5) the studies were published in a language other than English.
2.3. Study Selection
All literature searches were screened independently by two reviewers, and any discrepancies were resolved by discussion between them or together with the corresponding author. The studies were screened for eligibility initially based on their titles and abstracts, then based on a review of the full text.
2.4. Quality Assessment
The quality of the RCTs was evaluated using the Cochrane Risk of Bias (RoB) assessment tool 2.0 (RoB 2.0) [18], and the risk of bias in the following domains was classified as low, high, or unclear: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The quality of non-randomized studies was assessed using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool, and the risk of bias in the following domains was classified as low, moderate, high, or critical: confounding, selection of participants, classification of interventions, deviation from intended intervention, missing data, measurement and reporting of outcomes [19]. The publication bias was assessed using Begg–Mazumdar rank correlation and funnel plots [20]. Any discrepancies during the quality assessment were resolved through discussion with the corresponding author.
2.5. Data Extraction and Calculations of Outcome
Two reviewers independently extracted the following data from each study: name of authors, publication year, study design, EH subtypes (with or without atypia), numbers of total patients and patients who experienced regression, and follow-up. Regression was defined as when the endometrial biopsy during follow-up was described as appearing “proliferative”, “secretory”, “inactive”, or “atrophic”, or as indicating a “progesterone effect” [9]. The regression rate was calculated as the number of patients with regression, divided by the total number of patients who received medication [13].
2.6. Statistical Analysis
The meta-analysis was performed using Stata 14.0 (StataCorp, College Station, TX, USA). Results associated with p values < 0.05 were considered significant. The rates of regression were compared in terms of the relative ratios (RRs) and 95% confidence intervals (CIs) using the random-effect and DerSimonian–Laird methods [21]. Heterogeneity was assessed based on I2 values and a visual analysis of forest plots. We considered I2 > 50% as high heterogeneity, in which case we conducted subgroup and sensitivity analyses, and drew Galbraith plot to obtain more detailed insights and to assess potential sources of heterogeneity [22]. The subgroup analyses were based on the EH subtype, country, and study design. The sensitivity analyses were performed by removing one study at a time and repeating the meta-analysis.
A network meta-analysis was performed using Aggregate Data Drug Information System (ADDIS) 1.16.8, which uses a Bayesian approach and allows comparisons among all treatment arms of studies, including direct and indirect comparisons simultaneously [23].
3. Results
3.1. Study Selection
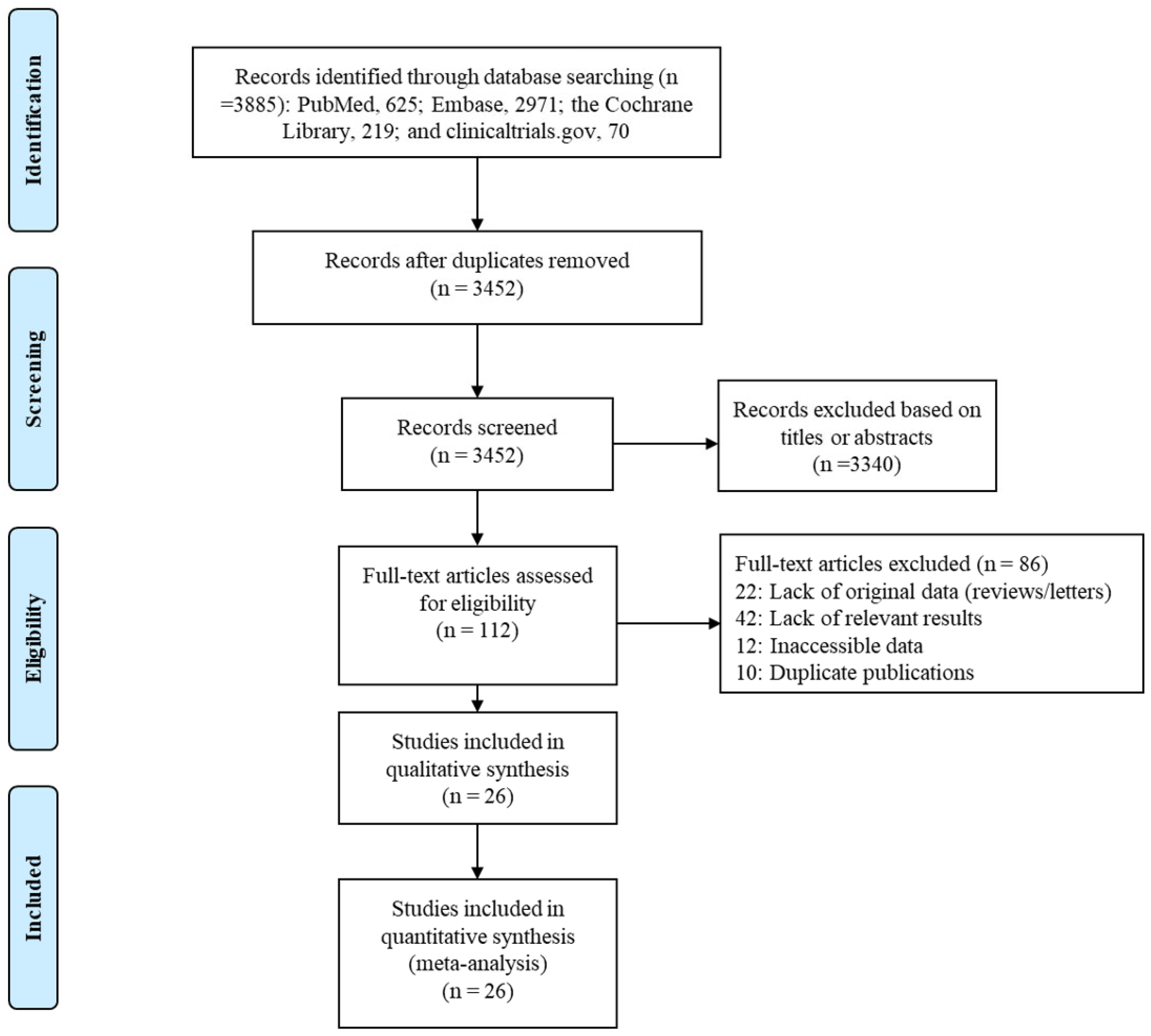
Our search found a total of 3885 published articles—625 in PubMed, 2971 in Embase, 219 in the Cochrane Library, and 70 on clinicaltrials.gov. We removed 433 duplicate articles and excluded another 3340 based on the titles or abstracts. A full-text review of the remaining 112 articles led to the inclusion of 26 in the systematic review and network meta-analysis (Figure 1).
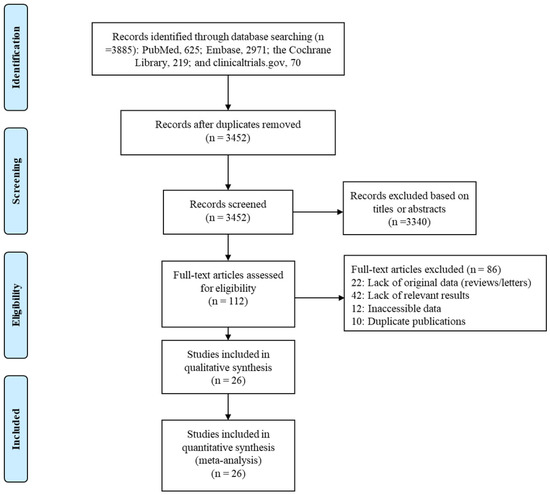
Figure 1.
Flow diagram of study selection process.
3.2. Characteristics of Included Studies
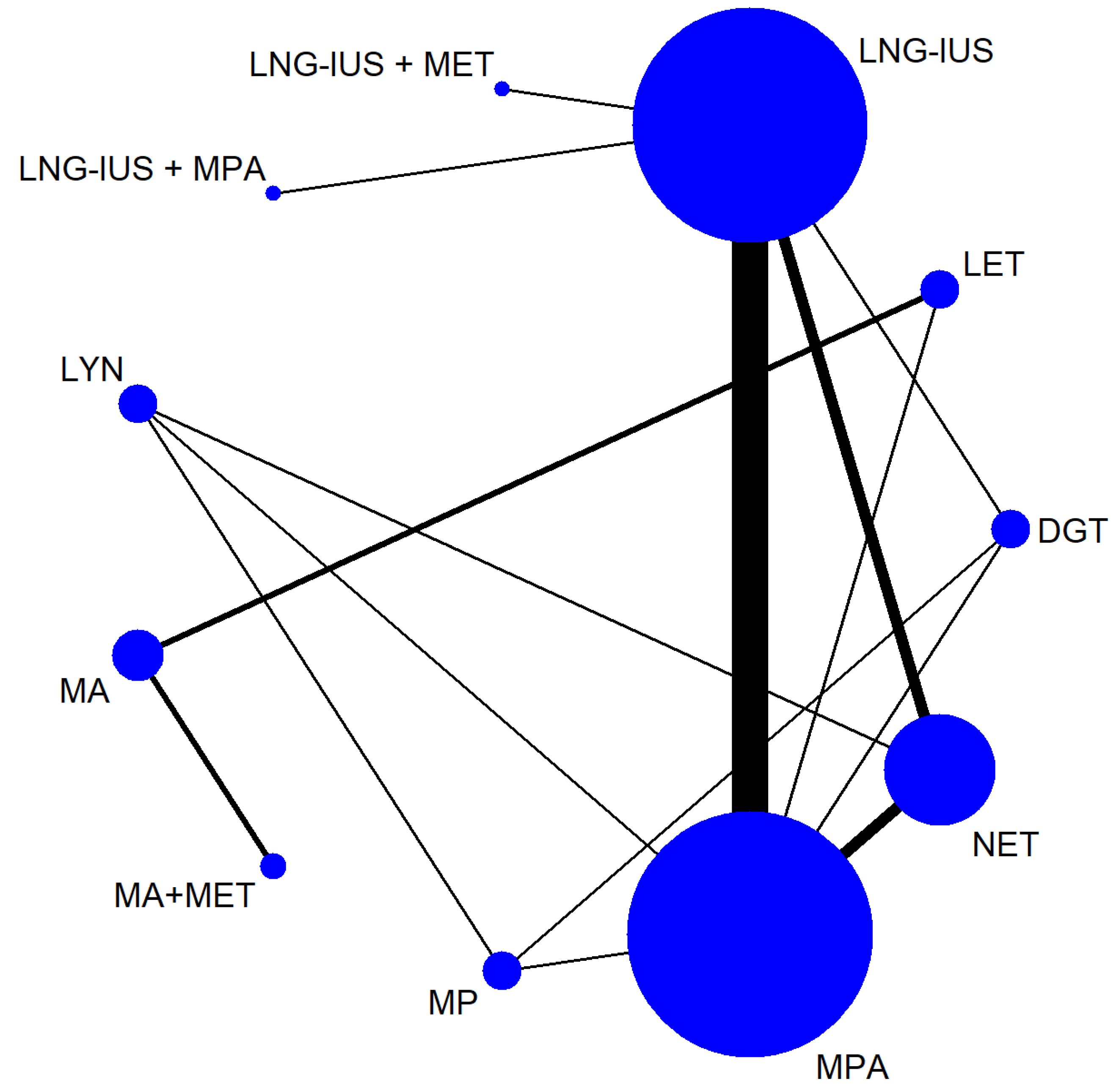
Table 1 shows the characteristics of the 26 studies, of which 5 were non-randomized and 21 were RCTs [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Altogether, the trials involved 2268 patients with EH with or without atypia. The samples in the studies ranged from 40 to 215 patients, and the studies were carried out in the following countries: Egypt (n = 6), Iran (n = 7), Turkey (n = 4), Norway (n = 4), Italy (n = 1), China (n = 1), Russia (n = 1), India (n = 1), and Pakistan (n = 1). The baseline patient characteristics were similar among the studies, allowing a network of comparisons involving 11 treatments to be analyzed (Figure 2).

Table 1.
Characteristics of included studies of patients with endometrial hyperplasia.

Figure 2.
Network of comparisons among treatments for endometrial hyperplasia.
In the figure, the line width is proportional to the number of trials comparing the treatments. The node size is proportional to the number of participants randomly assigned to that treatment.
Abbreviations: DGT, dydrogestrone; LET, letrozole; LNG-IUS, levonorgestrel-releasing intrauterine system; LYN, lynestrenol; MA, megestrol acetate; MET, metformin; MP, micronized progesterone; MPA, medroxyprogesterone acetate; NET, norethisterone.
3.3. Quality Assessment of Included Studies
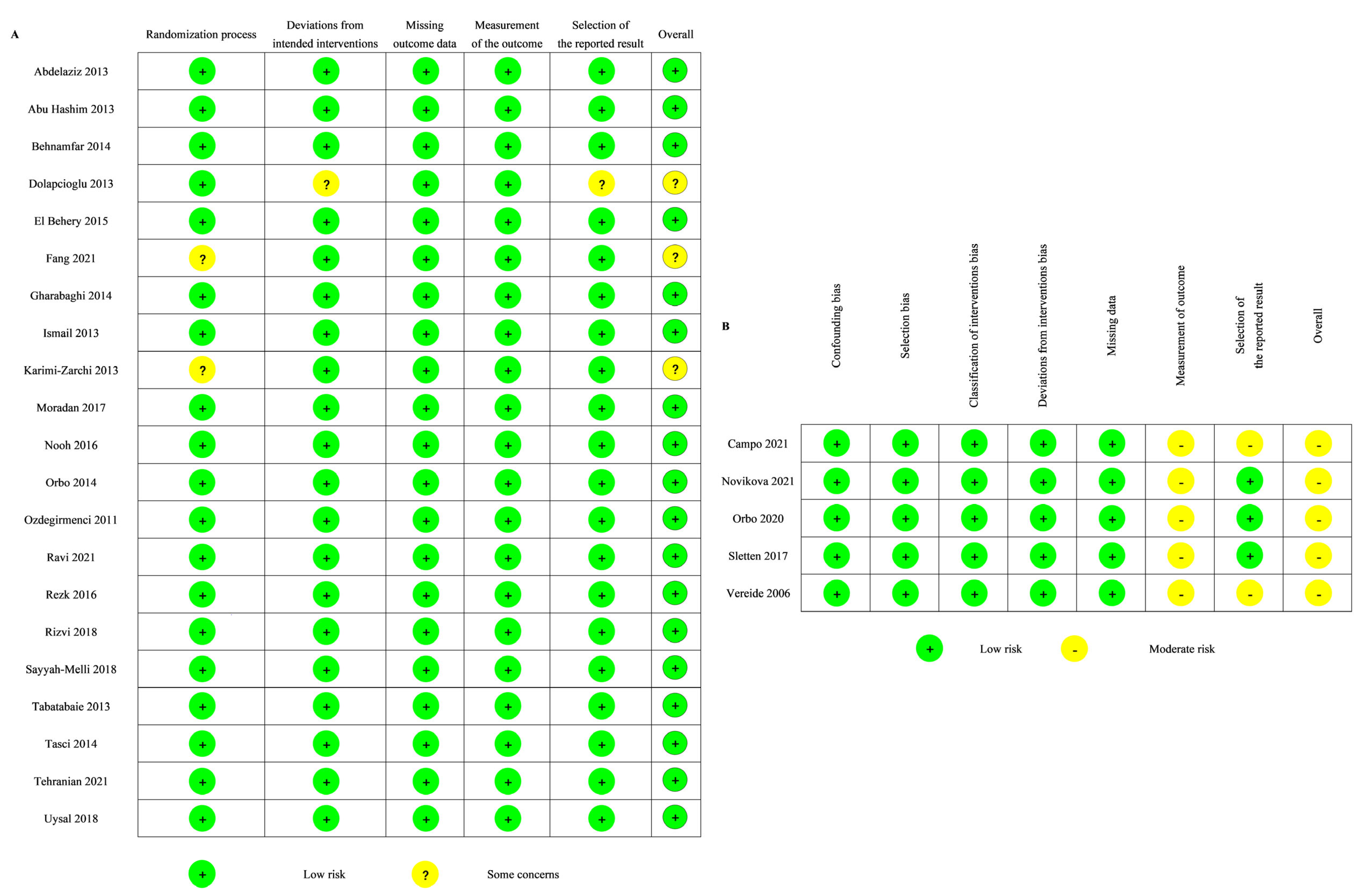
The quality of the RCTs was evaluated using the RoB 2.0 tool. Nearly all RCTs (18 of 21) were classified as being at low risk of bias (Figure 3A). Two of the 21 RCTs had some concerns regarding the randomization process. One of the 21 RCTs had some concerns regarding the deviations from intended intervention and the selection of the reported results.
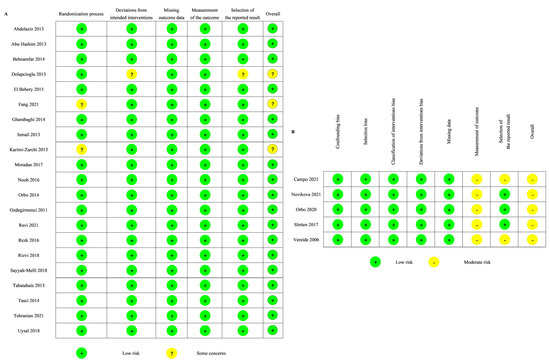
Figure 3.
Risk of bias of the included studies: (A) RoB2 [24,25,26,28,29,30,31,32,33,34,35,38,39,40,41,42,43,45,46,47,48]; (B) ROBIN-I [27,36,37,44,49].
The quality of the non-randomized studies was assessed using the ROBINS-I tool. Five non-randomized studies were classified as being at moderate risk of bias (Figure 3B). All of them were evaluated as having a moderate risk of bias in the measurement of the outcome, and two of them were also evaluated as having a moderate risk of bias in the selection of the reported result.
3.4. Comparisons of Regression Rates after Different Treatments
3.4.1. LNG-IUS vs. MPA
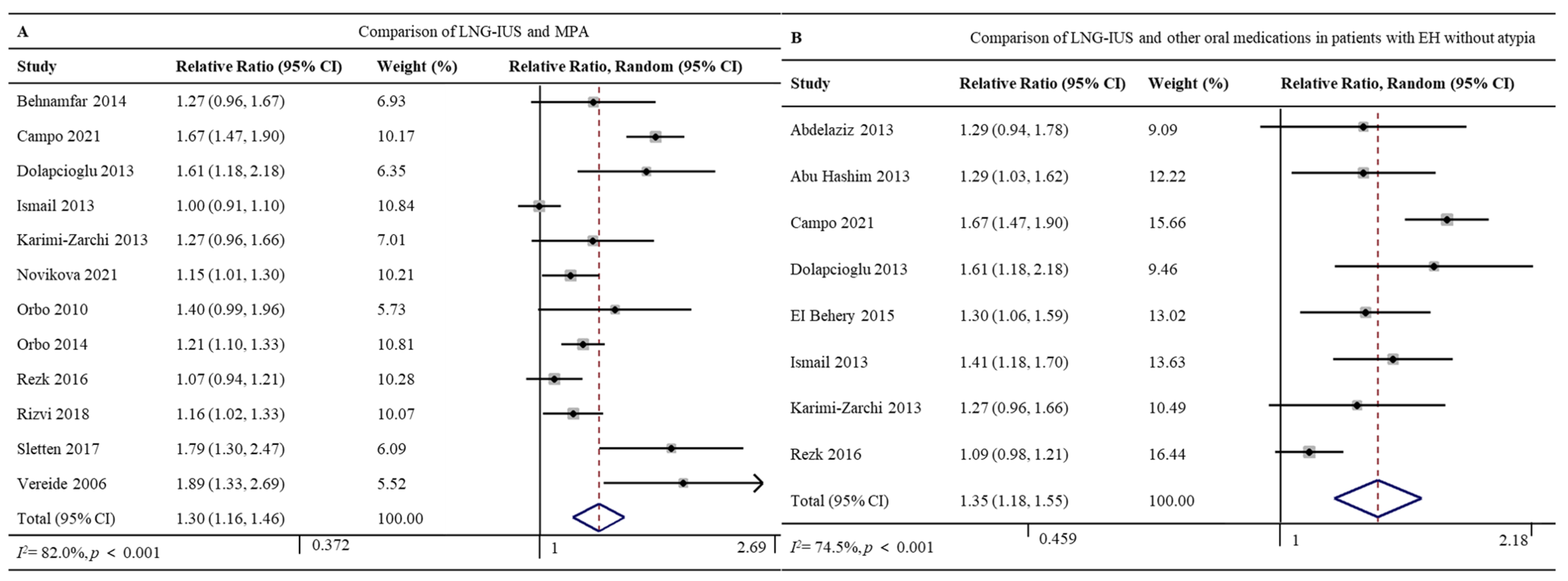
Twelve studies involving 1047 patients reported the regression rates for the LNG-IUS (96.7%, 408/422) and MPA (71.7%, 448/625) [26,27,28,32,33,36,37,38,41,42,44,49]. The LNG-IUS was associated with a significantly higher regression rate (RR 1.30, 95% CI 1.16–1.46, p < 0.001; I2 = 82.0%; Figure 4A). Given the high heterogeneity of the pooled data, we did subgroup analyses and sensitivity analyses but failed to uncover clear differences among subgroups. The Galbraith plot showed four studies might be the potential sources of heterogeneity [27,32,44,49] (Supplementary Figure S1).

Figure 4.
Forest plot of the meta-analysis of regression rates after treatment with (A) a comparison of the LNG-IUS and the MPA [26,27,28,32,33,36,37,38,41,42,44,49] or (B) a comparison of the LNG-IUS and other oral medications in patients with EH without atypia [24,25,27,28,29,32,33,36,41,44]. Abbreviations: RR, relative ratio; CI, confidence interval.
3.4.2. LNG-IUS vs. Oral Medications in Patients with EH without Atypia
Eight studies involving 882 patients with EH without atypia reported regression rates for the LNG-IUS (88.8%, 277/312) and oral medications (MPA, NET, or DGT) (66.5%, 379/570) [24,25,27,28,29,32,33,41]. The LNG-IUS was associated with a significantly higher regression rate (RR 1.35, 95% CI 1.18–1.55, p < 0.001; I2 = 74.5%; Figure 4B). Given the high heterogeneity of the pooled data, we did subgroup analyses and sensitivity analyses but failed to uncover clear differences among subgroups. The Galbraith plot showed two studies might be the potential sources of heterogeneity [27,41] (Supplementary Figure S2).
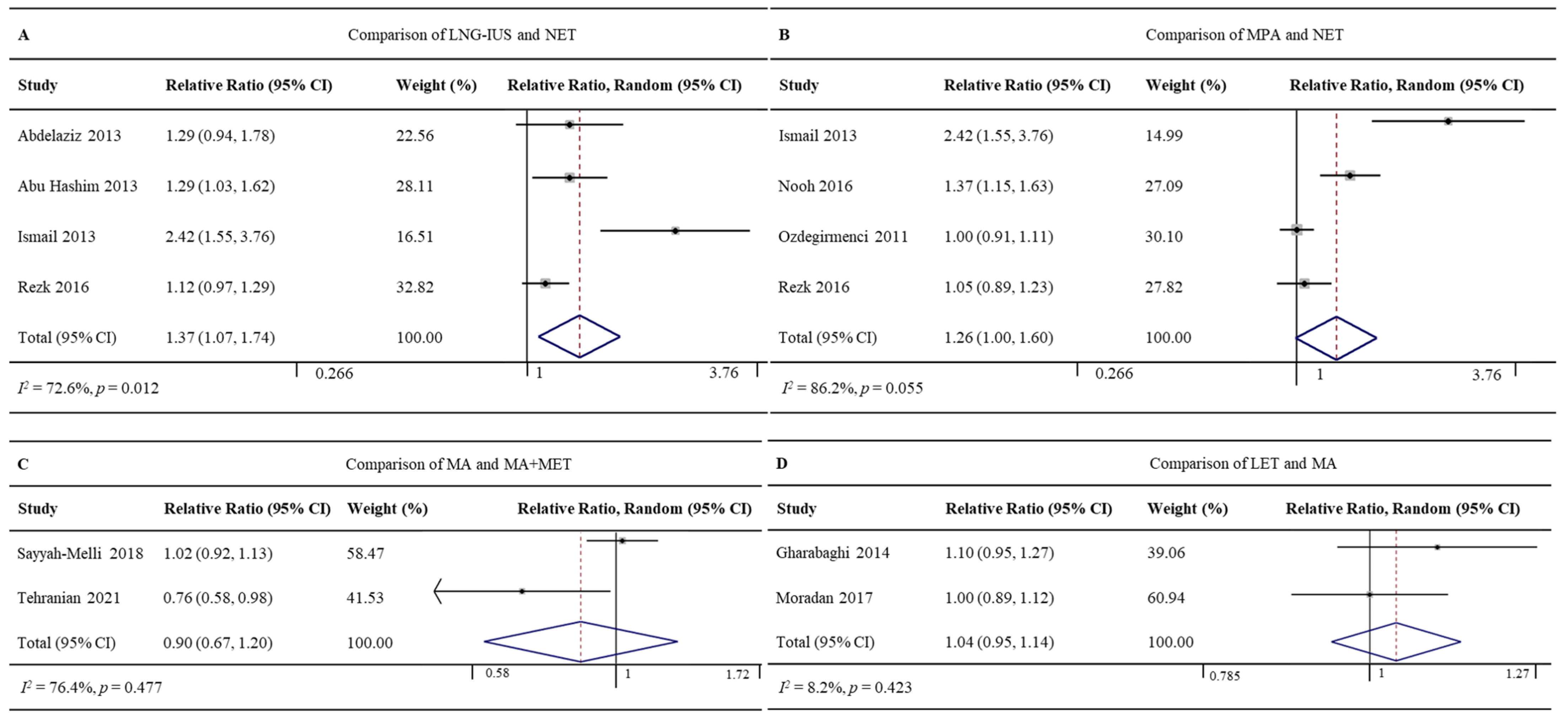
3.4.3. LNG-IUS vs. NET
Four RCTs involving 357 patients reported regression rates for the LNG-IUS (86.5%, 154/178) and NET (64.2%, 115/179) [24,25,32,41]. The LNG-IUS was associated with a significantly higher regression rate (RR 1.37, 95% CI 1.07–1.74, p = 0.012; I2 = 72.6%; Figure 5A). Given the high heterogeneity of the pooled data, we did subgroup analyses but failed to uncover clear differences among subgroups. Sensitivity analyses showed one study might be the potential source of heterogeneity [32]. Excluding this study led to the same result as the full meta-analysis, but with lower heterogeneity (RR 1.18, 95%CI 1.06–1.32, p = 0.003; I2 = 0.0%; Supplementary Figure S3).
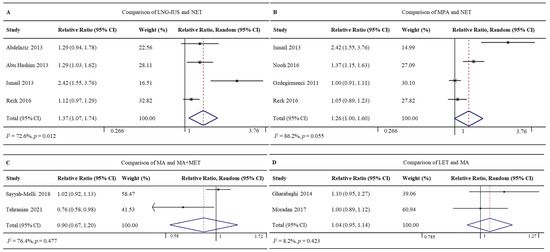
Figure 5.
Forest plot of the meta-analysis of regression rates in (A) a comparison of LNG-IUS and the NET [24,25,32,41], (B) a comparison of MPA and NET [32,35,39,41], (C) a comparison of MA and MA + MET [43,47], or (D) a comparison of LET and MA [31,34].
3.4.4. MPA vs. NET
Four RCTs involving 363 patients reported regression rates for MPA (92.3%, 169/183) and NET (71.7%, 129/180) [32,35,39,41]. The rates did not differ significantly between the two groups (RR 1.26, 95% CI 1.00–1.60, p = 0.055; I2 = 86.2%; Figure 5B). Given the high heterogeneity of the pooled data, we did subgroup analyses and sensitivity analyses but failed to uncover clear differences among subgroups. The Galbraith plot showed four studies might be the potential sources of heterogeneity [32,35] (Supplementary Figure S4).
3.4.5. MA vs. MA+MET
Two RCTs involving 140 patients reported regression rates for MA (85.1%, 57/67) and MA+MET (93.2%, 68/73) [43,47]. The rates did not differ significantly between the two groups (RR 0.90, 95% CI 0.67–1.20, p = 0.477; I2 = 76.4%; Figure 5C).
3.4.6. LET vs. MA
Two RCTs involving 142 patients reported regression rates for LET (94.4%, 67/71) and MA (88.7%, 63/71) [31,34]. The rates did not differ significantly between the two groups (RR 1.04, 95% CI 0.95–1.14, p = 0.423; I2 = 8.2%; Figure 5D).
3.5. Network Meta-Analysis of Regression Rates
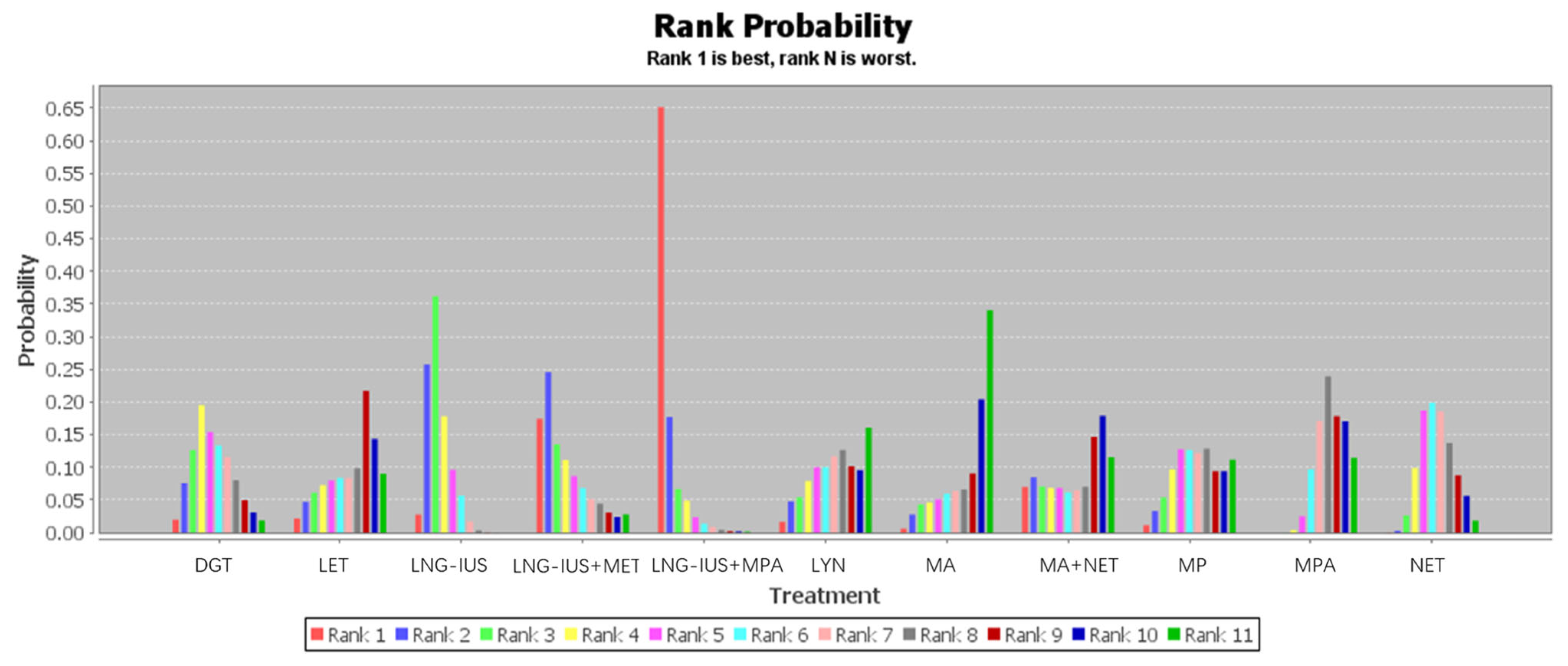
Twenty-six studies involving 2268 patients contributed to our network meta-analysis of regression rates after treatment with one of 11 regimens (Table 2 and Figure 6). The rank probability of regression across all patients showed the following trend: LNG-IUS+MPA > LNG-IUS+MET > LNG-IUS > DGT > NET > MP > MPA > LYN > LET > MA+MET > MA. The LNG-IUS was ranked higher than any of the oral medications on their own, and combining the LNG-IUS with oral MPA or MET shifted the LNG-IUS to the two highest rank positions. Among the oral medications on their own, DGT was ranked at the top.

Table 2.
Network meta-analysis of regression rates in patients with endometrial hyperplasia after the indicated treatments.

Figure 6.
Ranking of the 11 treatments for endometrial hyperplasia based on regression rate. Rank 1 indicates the best regression rate and rank 11 the worst.
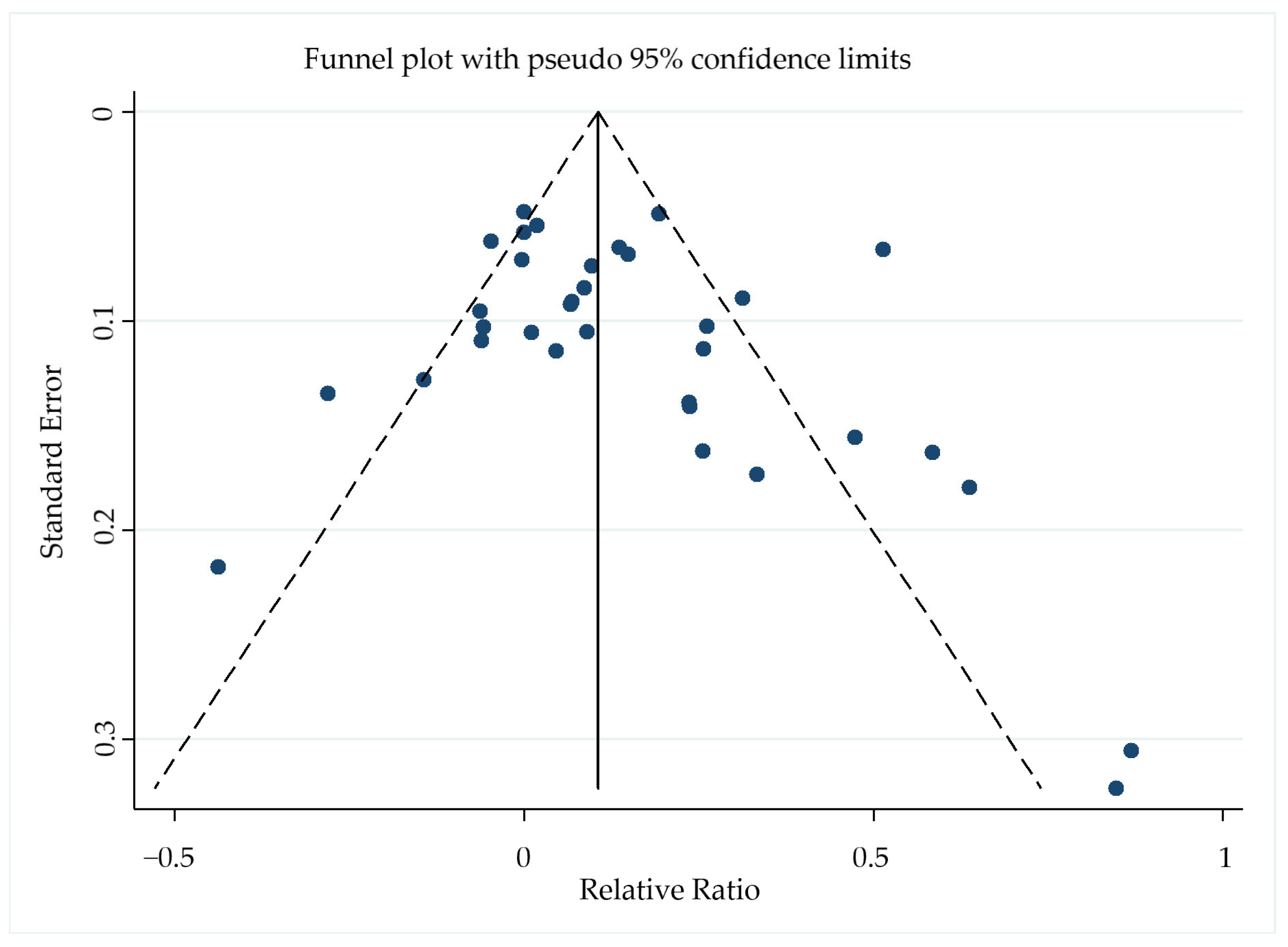
3.6. Publication Bias
The Begg–Mazumdar rank correlation test showed no evidence of publication bias in the meta-analysis of regression rates (p = 0.152), and the funnel plot was symmetrical (Figure 7).

Figure 7.
Funnel plot of the 26 included studies with pseudo−95% confidence limits based on the relative ratio for regression rates.
4. Discussion
In this network meta-analysis, we evaluated the effectiveness of different medications in the treatment of EH with or without atypia. Among the oral medications used individually, DGT may be superior to other progestins or non-progestins. Among any of the medications used individually, the LNG-IUS seems to be associated with a higher regression rate than oral progestins or non-progestins. Consistently, two previous studies suggested that the LNG-IUS was more effective than other oral progestins [10,50]. The present review substantially extends those findings by examining a much larger sample and by comparing the LNG-IUS and non-progestins.
Among patients with EH without atypia, the present meta-analysis showed that the LNG-IUS was associated with a significantly higher regression rate than other treatments, similar to another meta-review involving fewer studies, only a few of which were also included in the present analysis [50]. In contrast, another meta-analysis, which involved only single-arm studies that conducted purely indirect comparisons of the LNG-IUS and oral medications, found no significant difference in regression rates between oral medications and the LNG-IUS [51]. Our network meta-analysis suggests that the combinations of the LNG-IUS with MPA or MET are superior to the LNG-IUS or MPA on their own. Our work supports the use of the LNG-IUS+MPA for young women with early-stage endometrial carcinoma who want to preserve their fertility [52,53]. Our work also supports previous studies that concluded that adding MET or LET can increase the efficacy of the LNG-IUS or oral progestins [45,54]. Non-progestins may increase the efficacy of progestins on their own by upregulating the progesterone receptor in the endometrium [55].
Our findings should be interpreted with caution because of its limitations. First, the samples were quite small for some treatment regimens, especially the LNG-IUS+MPA (27 of 2268 patients), LET (92), LYN (55), LNG-IUS+MET (25), MA+MET (73), and MP (61) treatments. Second, our meta-analysis pooled data from RCTs and non-randomized studies, which differed substantially in size and potentially in heterogeneity. Indeed, the included studies differed markedly in their medication dose and the usage method of drugs, as well as the duration of follow-up. Patient compliance also plays an important role in the treatment of EH, while women treated with the LNG-IUS need to go to the hospital regularly to make sure the location of the IUD and women treated with oral medication need to take their medicine according to their physician’s orders. Third, our study did not compare the safety of the different treatments. For patients receiving the LNG-IUS, the IUD may migrate to any place other than the uterine cavity. Patients with EH who take oral progestins for a long time may have bloating, nausea, headaches, and mood swings or even depression, and the use of oral progestins may lead to liver function damage. Our study also did not compare the curative effect and safety between oral progestins and non-progestins as a result of lacking relevant data. More studies are needed. Fourth, the included studies did not uniformly report sufficient data for us to compare the treatments in terms of other clinically important outcomes, such as menstrual blood loss or other symptomatic improvements. The same was true for patient characteristics such as obesity or the presence of diabetes mellitus, whose potential influence we could not assess in the subgroup analyses [50,56]. We thought that the same characteristics as the risk factors of EH were related to the treatment effect. Fifth, most of the patients in the included studies were younger than 50 years old, and there were no suitable studies about the therapies of EH with the women aged around 50–60, even though these women are more susceptible to EH [5]. Although we did not get enough information about the race of the women included, we did find that the LNG-IUS is more used in economically advanced countries based on the countries of each study. Sixth, the treatment of patients with EH is a long-term treatment, and our study did not compare the recurrence rate and resistant rate of different therapies. Seventh, we observed the high heterogeneity in regression rate between LNG-IUS and MPA groups, LNG-IUS and oral medications groups, LNG-IUS and NET groups, MA and MA+MET groups, and we found the potential sources by using subgroup analyses, sensitivity analyses and Galbraith plots. After removing those studies, we obtained similar results, this suggests that even our more heterogeneous meta-analyses are reliable. We also observed the high heterogeneity in regression rate between MA and MA+MET groups, and we thought the reason might be there were only two studies and too few patients. More research is needed to support whether MET can increase the efficacy of MA. Lastly, we may have introduced bias by including only English-language studies, yet our analysis suggests a low risk of such bias.
Nowadays, people are beginning to pay more attention to their own health, and when there is a sign something’s wrong, such as abnormal uterine bleeding, many people will go to hospitals for professional instruction and treatment. Additionally, with the development of medical technology, the diagnosis rate of EH has been increasing gradually in recent years, especially in perimenopausal women and postmenopausal women. Some studies have suggested that calculating the endometrial thickness of postmenopausal women using an endovaginal color dopple has similar diagnostic accuracy compared with a histopathologic diagnosis [57]. The diagnosis methods of EH include dilatation and curettage (D&C) and an endometrial biopsy with a hysteroscopy [58,59]. The treatment of EH should be individualized according to age, fertility demands, personal conditions, and other factors. For postmenopausal women with atypical EH, hysterectomy is the most suitable treatment. For premenopausal women with EH who want to preserve their fertility, the LNG-IUS and oral medication are more suitable [8]. Our study has compared the therapeutic effect to EH with or without atypia between the LNG-IUS and oral medication, and we hope the outcomes of our study can help clinicians find the most appropriate treatment for these patients.
In spite of these limitations, the present work appears to be the first network meta-analysis suggesting that the LNG-IUS may be the most appropriate choice for patients with EH with or without atypia, and that combining the LNG-IUS with MPA or MET may further increase the regression rate. Our work also suggests that among the oral medications, DGT may be the most appropriate choice for women who are unwilling to use the LNG-IUS or who cannot tolerate its side effects.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm12082980/s1, Figure S1: Galbraith radial plot depicting sources of heterogeneity in regression rates between LNG-IUS and MPA group [26,27,28,32,33,36,37,38,41,42,44,49]; Figure S2: Galbraith radial plot depicting sources of heterogeneity in regression rates between LNG-IUS and oral medications in patients with EH without Atypia [24,25,27,28,29,32,33,41]; Figure S3: Forest plot of the meta-analysis of regression rates in LNG-IUS and NET groups after removal of one study [32] potentially contributing substantially to heterogeneity in the pooled data [24,25,41]; Figure S4: Galbraith radial plot depicting sources of heterogeneity in regression rates between MPA and NET groups [32,35,39,41].
Author Contributions
Conceptualization, Y.-F.Z.; methodology, Y.-F.Z., Y.F. and Y.M.; literature search, Y.-F.Z. and Y.F.; literature screening, Y.-F.Z. and Y.F.; study quality assessment, Y.-F.Z. and Y.F.; data extraction, Y.-F.Z. and Y.F.; meta-analysis, Y.-F.Z. and Y.M.; writing—original draft preparation, Y.-F.Z.; writing—review and editing, J.-K.L.; supervision, J.-K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Science and Technology Department of Sichuan Province, China (2017SZ0118, 2021YJ0124).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Si-Yu Cao and Jia-Ying Ruan for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Auclair, M.H.; Yong, P.J.; Salvador, S.; Thurston, J.; Colgan, T.T.J.; Sebastianelli, A. Guideline No. 390-Classification and Management of Endometrial Hyperplasia. J. Obstet. Gynaecol. Can. 2019, 41, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Beckmann, M.W.; Schmidt, D.; Mallmann, P. New who classification of endometrial hyperplasias. Geburtshilfe. Frauenheilkd. 2015, 75, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Antonsen, S.L.; Ulrich, L.; Høgdall, C. Patients with atypical hyperplasia of the endometrium should be treated in oncological centers. Gynecol. Oncol. 2012, 125, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.D.; Newton, K.M.; Clinton, W.L.; Epplein, M.; Garcia, R.; Allison, K.; Voigt, L.F.; Weiss, N.S. Incidence of endometrial hyperplasia. Am. J. Obstet. Gynecol. 2009, 200, 678-e1. [Google Scholar] [CrossRef]
- Petersdorf, K.; Groettrup-Wolfers, E.; Overton, P.M.; Seitz, C.; Schulze-Rath, R. Endometrial hyperplasia in pre-menopausal women: A systematic review of incidence, prevalence, and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 158–171. [Google Scholar] [CrossRef]
- Lacey, J.V.; Chia, V.M. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas 2009, 63, 39–44. [Google Scholar] [CrossRef]
- Pennant, M.E.; Mehta, R.; Moody, P.; Hackett, G.; Prentice, A.; Sharp, S.J.; Lakshman, R. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG 2017, 124, 404–411. [Google Scholar] [CrossRef]
- Nees, L.K.; Heublein, S.; Steinmacher, S.; Juhasz-Böss, I.; Brucker, S.; Tempfer, C.B.; Wallwiener, M. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022, 306, 407–421. [Google Scholar] [CrossRef]
- Chandra, V.; Kim, J.J.; Benbrook, D.M.; Dwivedi, A.; Rai, R. Therapeutic options for management of endometrial hyperplasia. J. Gynecol. Oncol. 2016, 27, e8. [Google Scholar] [CrossRef]
- Gallos, I.D.; Yap, J.; Rajkhowa, M.; Luesley, D.M.; Coomarasamy, A.; Gupta, J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012, 207, 266.e1–266.e12. [Google Scholar] [CrossRef]
- Simpson, A.N.; Feigenberg, T.; Clarke, B.A.; Gien, L.T.; Ismiil, N.; Laframboise, S.; Massey, C.; Ferguson, S.E. Fertility sparing treatment of complex atypical hyperplasia and low grade endometrial cancer using oral progestin. Gynecol. Oncol. 2014, 133, 229–233. [Google Scholar] [CrossRef]
- Lacey, J.J.; Sherman, M.E.; Rush, B.B.; Ronnett, B.M.; Ioffe, O.B.; Duggan, M.A.; Glass, A.G.; Richesson, D.A.; Chatterjee, N.; Langholz, B. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol. 2010, 28, 788–792. [Google Scholar] [CrossRef]
- Mittermeier, T.; Farrant, C.; Wise, M.R. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst. Rev. 2020, 9, CD012658. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Ghayaty, E.; Rakhawy, M.E. Levonorgestrelreleasing intrauterine system vs. oral progestins for nonatypical endometrial hyperplasia: A systematic review and metaanalysis of randomised trials. Am. J. Obstet. Gynecol. 2015, 213, 469–478. [Google Scholar] [CrossRef]
- Lethaby, A.; Munawar, H.; Rishworth, J.R.; Rees, M.C. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2015, 30, CD002126. [Google Scholar] [CrossRef]
- Mitranovici, M.I.; Chiorean, D.M.; Sabău, A.H.; Cocuz, I.G.; Tinca, A.C.; Mărginean, M.C.; Popelea, M.C.; Irimia, T.; Moraru, R.; Mărginean, C.; et al. An Interesting Image of Transmural Migration of a Levonorgestrel-Releasing Intrauterine Device (LNg-IUD). Diagnostics 2022, 15, 2227. [Google Scholar] [CrossRef]
- Ramos-Rivera, M.; Averbach, S.; Selvaduray, P.; Gibson, A.; Ngo, L.L. Complications after interval postpartum intrau-terine device insertion. Am. J. Obstet. Gynecol. 2022, 226, 95-e1. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 28, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Van Valkenhoef, G.; Tervonen, T.; Zwinkels, T.; de Brock, B.; Hillege, H. ADDIS: A decision support system for evidence-based medicine. Decis. Support Syst. 2013, 55, 459–475. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Abosrie, M. Levonorgestrel-releasing intrauterine system is An Efficient Therapeutic Modality for Simple Endometrial Hyperplasia. Am. J. Sci. 2013, 9, 417–424. [Google Scholar]
- Abu Hashim, H.; Zayed, A.; Ghayaty, E.; El Rakhawy, M. LNG-IUS treatment of non-atypical endometrial hyperplasia in perimenopausal women: A randomized controlled trial. J. Gynecol. Oncol. 2013, 24, 128–134. [Google Scholar] [CrossRef]
- Behnamfar, F.; Ghahiri, A.; Tavakoli, M. Levonorgestrel-releasing intrauterine system (Mirena) in compare to medroxyprogesterone acetate as a therapy for endometrial hyperplasia. J. Res. Med. Sci. 2014, 19, 686–690. [Google Scholar]
- Campo, G.; Rebecchi, A.; Vanni, V.S.; Pagliardini, L.; Patruno, C.; Papaleo, E.; Candiani, M.; Giardina, P. Levonorgestrel-releasing intrauterine system versus oral medroxyprogesterone acetate in infertile women with endometrial hyperplasia without atypia. Reprod. Biomed. Online 2021, 43, 864–870. [Google Scholar] [CrossRef]
- Dolapcioglu, K.; Boz, A.; Baloglu, A. The efficacy of intrauterine versus oral progestin for the treatment of endometrial hyperplasia. A prospective randomized comparative study. Clin. Exp. Obstet. Gynecol. 2013, 40, 122–126. [Google Scholar]
- El Behery, M.M.; Saleh, H.S.; Ibrahiem, M.A.; Kamal, E.M.; Kassem, G.A.; Mohamed Mel, S. Levonorgestrel-releasing intrauterine device versus dydrogesterone for management of endometrial hyperplasia without atypia. Reprod. Sci. 2015, 22, 329–334. [Google Scholar] [CrossRef]
- Fang, F.; Xu, H.; Wu, L.; Hu, L.; Liu, Y.; Li, Y.; Zhang, C. LNG-IUS combined with progesterone ameliorates endometrial thickness and pregnancy outcomes of patients with early-stage endometrial cancer or atypical hyperplasia. Am. J. Transl. Res. 2021, 13, 5412–5419. [Google Scholar]
- Gharabaghi, P.M.; Azadi, A.; Tabrizi, A.D.; Ouladsahebmadarek, E.; Tasbihi, P.; Shoari, N. Effect of letrozole on endometrial histology in patients with disordered proliferative endometrium and simple hyperplasia. Int. J. Women’s Health Reprod. Sci. 2014, 2, 73–79. [Google Scholar]
- Ismail, M.T.; Fahmy, D.M.; Elshmaa, N.S. Efficacy of levonorgestrel-releasing intrauterine system versus oral progestins in treatment of simple endometrial hyperplasia without atypia. Reprod. Sci. 2013, 20, 45–50. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Dehghani-Firoozabadi, R.; Tabatabaie, A.; Dehghani-Firoozabadi, Z.; Teimoori, S.; Chiti, Z.; Miratashi-Yazdi, A.; Dehghani, A. A comparison of the effect of levonorgestrel IUD with oral medroxyprogesterone acetate on abnormal uterine bleeding with simple endometrial hyperplasia and fertility preservation. Clin. Exp. Obstet. Gynecol. 2013, 40, 421–424. [Google Scholar]
- Moradan, S.; Nikkhah, N.; Mirmohammadkhanai, M. Comparing the Administration of Letrozole and Megestrol Acetate in the Treatment of Women with Simple Endometrial Hyperplasia without Atypia: A Randomized Clinical Trial. Adv. Ther. 2017, 34, 1211–1220. [Google Scholar] [CrossRef]
- Nooh, A.M.; Abdeldayem, H.M.; Girbash, E.F.; Arafa, E.M.; Atwa, K.; Abdel-Raouf, S.M. Depo-Provera Versus Norethisterone Acetate in Management of Endometrial Hyperplasia Without Atypia. Reprod. Sci. 2016, 23, 448–454. [Google Scholar] [CrossRef]
- Novikova, O.V.; Nosov, V.B.; Panov, V.A.; Novikova, E.G.; Krasnopolskaya, K.V.; Andreeva, Y.Y.; Shevchuk, A.S. Live births and maintenance with levonorgestrel IUD improve disease-free survival after fertility-sparing treatment of atypical hyperplasia and early endometrial cancer. Gynecol. Oncol. 2021, 161, 152–159. [Google Scholar] [CrossRef]
- Orbo, A.; Arnes, M.; Pettersen, I.; Larsen, K.; Hanssen, K.; Moe, B. Down-regulated progesterone receptor A and B coinciding with successful treatment of endometrial hyperplasia by the levonorgestrel impregnated intrauterine system. Acta. Obstet. Gynecol. Scand. 2010, 89, 1438–1446. [Google Scholar] [CrossRef]
- Orbo, A.; Vereide, A.; Arnes, M.; Pettersen, I.; Straume, B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: A national multicentre randomised trial. BJOG 2014, 121, 477–486. [Google Scholar] [CrossRef]
- Ozdegirmenci, O.; Kayikcioglu, F.; Bozkurt, U.; Akgul, M.A.; Haberal, A. Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecol. Obstet. Investig. 2011, 72, 10–14. [Google Scholar] [CrossRef]
- Ravi, R.D.; Kalra, J.; Srinivasan, R.; Bagga, R.; Jain, V.; Suri, V.; Sachdeva, N. A Randomized Clinical Trial of Levonorgestrel Intrauterine System with or without Metformin for Treatment of Endometrial Hyperplasia without Atypia in Indian Women. Asian Pac. J. Cancer Prev. 2021, 22, 983–989. [Google Scholar] [CrossRef]
- Rezk, M.; Kandil, M.; Saleh, S.; Shaheen, A. Comparison of levonorgestrel–releasing intrauterine system, medroxyprogesterone and norethisterone for treatment of endometrial hyperplasia without atypia: A randomized clinical trial. Obstet. Gynecol. Int. J. 2016, 5, 353–356. [Google Scholar] [CrossRef]
- Rizvi, S.; Ghaffar, S.; Haider, R.; Jafri, A. Levonorgestril intrauterine system (LNG-IUS) versus medroxyprogesterone acetate for the treatment of endometrial hyperplasia: A randomized control trial. Pak. J. Med. Health Sci. 2018, 12, 675–678. [Google Scholar]
- Sayyah-Melli, M.; Pourazad, S.; Gharebaghi, P.M.; Ouladsahebmadarek, E.; Jafari-Shobeiri, M.; Rahmani, V. The comparative effect of combination of metformin and megestrol acetate with megestrol acetate alone on endometrial growth disorders. Int. J. Women’s Health Reprod. Sci. 2018, 6, 211–215. [Google Scholar] [CrossRef]
- Sletten, E.T.; Arnes, M.; Lysa, L.M.; Moe, B.T.; Straume, B.; Orbo, A. Prediction of Relapse After Therapy Withdrawal in Women with Endometrial Hyperplasia: A Long-term Follow-up Study. Anticancer Res. 2017, 37, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, A.; Karimi Zarchi, M.; Dehghani-Tafti, M.; Miratashi-Yazdi, A.; Teimoori, S.; Dehghani, A. Comparing letrozole with medroxyprogesterone acetate (MPA) as hormonal therapy for simple endometrial hyperplasia without atypia in adult and middle-aged women. Eur. J. Gynaecol. Oncol. 2013, 34, 552–555. [Google Scholar]
- Tasci, Y.; Polat, O.G.; Ozdogan, S.; Karcaaltincaba, D.; Seckin, L.; Erkaya, S. Comparison of the efficacy of micronized progesterone and lynestrenol in treatment of simple endometrial hyperplasia without atypia. Arch. Gynecol. Obstet. 2014, 290, 83–86. [Google Scholar] [CrossRef]
- Tehranian, A.; Ghahghaei-Nezamabadi, A.; Arab, M.; Khalagi, K.; Aghajani, R.; Sadeghi, S. The impact of adjunctive metformin to progesterone for the treatment of non-atypical endometrial hyperplasia in a randomized fashion, a placebo-controlled, double blind clinical trial. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101863. [Google Scholar] [CrossRef]
- Uysal, G.; Acmaz, G.; Madendag, Y.; Cagli, F.; Akkaya, H.; Madendag, I.; Karakilic, E.U. The Efficacy of Dienogest in the Treatment of Simple Endometrial Hyperplasia without Atypia. Gynecol. Obstet. Investig. 2018, 83, 151–155. [Google Scholar] [CrossRef]
- Vereide, A.B.; Kaino, T.; Sager, G.; Arnes, M.; Ørbo, A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol. Oncol. 2006, 101, 214–223. [Google Scholar] [CrossRef]
- Yuk, J.S.; Song, J.Y.; Lee, J.H.; Park, W.I.; Ahn, H.S.; Kim, H.J. Levonorgestrel-Releasing Intrauterine Systems Versus Oral Cyclic Medroxyprogesterone Acetate in Endometrial Hyperplasia Therapy: A Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 1322–1329. [Google Scholar] [CrossRef]
- Gallos, I.D.; Shehmar, M.; Thangaratinam, S.; Papapostolou, T.K.; Coomarasamy, A.; Gupta, J.K. Oral progestogens vs. levonorgestrelreleasing intrauterine system for endometrial hyperplasia: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2010, 203, 547.e1–547.e10. [Google Scholar] [CrossRef]
- Kim, M.K.; Seong, S.J.; Kang, S.B.; Bae, D.S.; Kim, J.W.; Nam, J.H.; Lim, M.C.; Lee, T.S.; Kim, S.; Paek, J. Six months response rate of combined oral medroxyprogesterone/levonorgestrel-intrauterine system for early-stage endometrial cancer in young women: A Korean Gynecologic-Oncology Group Study. J. Gynecol. Oncol. 2019, 30, e47. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, D.H.; Bae, H.S.; Kim, M.L.; Jung, Y.W.; Yun, B.S.; Seong, S.J.; Shin, E.; Kim, M.K. Combined Oral Medroxyprogesterone/Levonorgestrel-Intrauterine System Treatment for Women with Grade 2 Stage IA Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 738–742. [Google Scholar] [CrossRef]
- Yang, B.Y.; Gulinazi, Y.; Du, Y.; Ning, C.C.; Cheng, Y.L.; Shan, W.W.; Luo, X.Z.; Zhang, H.W.; Zhu, Q.; Ma, F.H.; et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: A randomised controlled trial. BJOG 2020, 127, 848–857. [Google Scholar] [CrossRef]
- Li, H.Z.; Chen, X.N.; Qiao, J. Letrozole as primary therapy for endometrial hyperplasia in young women. Int. J. Gynaecol. Obstet. 2008, 100, 10–12. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Saccone, G.; Di Maio, A.; Mollo, A.; Mascolo, M.; De Rosa, R.; De Placido, G.; Insabato, L.; Zullo, F. Diabetes mellitus and responsiveness of endometrial hyperplasia and early endometrial cancer to conservative treatment. Gynecol. Endocrinol. 2019, 35, 932–937. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Haimovich, S.; Carugno, J.; Alonso, P.L.; Perez-Medina, T.; Parry, J.P.; Török, P.; Tesarik, J.; Della, C.L.; et al. Risk of endometrial cancer in asymptomatic postmenopausal women in relation to ultrasonographic endometrial thickness: Systematic review and diagnostic test accuracy meta-analysis. Am. J. Obstet. Gynecol. 2023, 228, 22–35. [Google Scholar] [CrossRef]
- De Franciscis, P.; Riemma, G.; Schiattarella, A.; Cobellis, L.; Guadagno, M.; Vitale, S.G.; Mosca, L.; Cianci, A.; Colacurci, N. Con-cordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics 2019, 7, 142. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Chiofalo, B.; Vilos, G.A.; Cianci, S.; Budak, M.S.; Lasmar, B.P.; Raffone, A.; Kahramanoglu, I. Hysteroscopy in the management of endometrial hyperplasia and cancer in reproductive aged women: New de-velopments and current perspectives. Transl. Cancer Res. 2020, 9, 7767–7777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).