Value of NT-proBNP and Galectin-3 as Biomarkers in the Follow-Up of Asymptomatic Elderly Patients with Severe Aortic Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Study
2.2. Clinical Data
2.3. Echocardiography Information
2.4. Biochemical Analysis
2.5. Study Population Follow-Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart survey on valvular heart disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Zilberszac, R.; Schemper, M.; Czerny, M.; Mundigler, G.; Graf, S.; Bergler-Klein, J.; Grimm, M.; Gabriel, H.; Maurer, G. Natural history of very severe aortic stenosis. Circulation 2010, 121, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Leyhe, T.; Reynolds, C.F., 3rd; Melcher, T.; Linnemann, C.; Klöppel, S.; Blennow, K.; Zetterberg, H.; Dubois, B.; Lista, S.; Hampel, H. A common challenge in older adults: Classification, overlap, and therapy of depression and dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2017, 13, 59–71. [Google Scholar] [CrossRef]
- Aiello, G.; Cuocina, M.; La Via, L.; Messina, S.; Attaguile, G.A.; Cantarella, G.; Sanfilippo, F.; Bernardini, R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 435. [Google Scholar] [CrossRef]
- Ramos, M.; Quezada, D.M.; Ayala, R.; Gómez-Pavón, F.J.; Jaramillo, J.; Toro, R. Aortic stenosis prognosis in older patients: Frailty is a strong marker of early congestive heart failure admissions. Eur. Geriatr. Med. 2019, 10, 483–491. [Google Scholar] [CrossRef]
- Lim, P.; Monin, J.; Monchi, M.; Garot, J.; Pasquet, A.; Hittinger, L.; Vanoverschelde, J.; Carayon, A.; Gueret, P. Predictors of outcome in patients with severe aortic stenosis and normal left ventricular function: Role of B-type natriuretic peptide. Eur. Heart J. 2004, 25, 2048–2053. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moonen, M.; Magne, J.; O’Connor, K.; Cosyns, B.; Attena, E.; Donal, E.; Pierard, L. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am. J. Cardiol. 2010, 105, 383–388. [Google Scholar] [CrossRef]
- Cimadevilla, C.; Cueff, C.; Hekimian, G.; Dehoux, M.; Lepage, L.; Iung, B.; Duval, X.; Huart, V.; Tubach, F.; Vahanian, A.; et al. Prognostic value of B-type natriuretic peptide in elderly patients with aortic valve stenosis: The COFRASA–GENERAC study. Heart 2013, 99, 461–467. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Lopez-Andrès, N.; Rossignol, P.; Iraqi, W.; Fay, R.; Nuée, J.; Ghio, S.; Cleland, J.G.F.; Zannad, F.; Lacolley, P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) trial. Eur. J. Heart Fail. 2012, 14, 74–81. [Google Scholar] [CrossRef]
- Sádaba, J.R.; Martínez-Martínez, E.; Arrieta, V.; Álvarez, V.; Fernández-Celis, A.; Ibarrola, J.; Melero, A.; Rossignol, P.; Cachofeiro, V.; López-Andrés, N. Role for galectin-3 in calcific aortic valve stenosis. J. Am. Heart Assoc. 2016, 5, e004360. [Google Scholar] [CrossRef] [PubMed]
- Filipe, M.D.; Meijers, W.C.; Rogier van der Velde, A.; de Boer, R.A. Galectin-3 and heart failure: Prognosis, prediction & clinical utility. Clin. Chim. Acta 2015, 443, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.; Barthel, D. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European association of cardiovascular imaging and the American society of echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- McCullough, P.A.; Olobatoke, A.; Vanhecke, T.E. Galectin-3: A novel blood test for the evaluation and management of patients with heart failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Feola, M.; Testa, M.; Leto, L.; Cardone, M.; Sola, M.; Rosso, G.L. Role of galectin-3 and plasma B type-natriuretic peptide in predicting prognosis in discharged chronic heart failure patients. Medicine 2016, 95, e4014. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Dumesnil, J.G.; Capoulade, R.; Mathieu, P.; Sénéchal, M.; Pibarot, P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.P.; Neumann, F.J.; Jander, N. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010, 96, 1463–1468. [Google Scholar] [CrossRef]
- White, M.; Baral, R.; Ryding, A.; Tsampasian, V.; Ravindrarajah, T.; Garg, P.; Koskinas, K.C.; Clark, A.; Vassiliou, V.S. Biomarkers associated with mortality in aortic stenosis: A systematic review and meta-analysis. Med. Sci. 2021, 9, 29. [Google Scholar] [CrossRef]
- Agoston-Coldea, L.; Bheecarry, K.; Petra, C.; Strambu, L.; Ober, C.; Revnic, R.; Lupu, S.; Mocan, T.; Fodor, D. The value of global longitudinal strain and galectin-3 for predicting cardiovascular events in patients with severe aortic stenosis. Med. Ultrason. 2018, 20, 205–212. [Google Scholar] [CrossRef]
- Toro, R.; Mangas, A.; Gómez, F. Enfermedad de la válvula aórtica calcificada. Su asociación con la arteriosclerosis. Med. Clín. 2011, 136, 588–593. [Google Scholar] [CrossRef]
- Martínez-Martínez, E.; López-Ándres, N.; Jurado-López, R.; Rousseau, E.; Bartolomé, M.V.; Fernández-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S.; et al. Galectin-3 participates in cardiovascular remodeling associated with obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef]
- Arangalage, D.; Nguyen, V.; Robert, T.; Melissopoulou, M.; Mathieu, T.; Estellat, C.; Codogno, I.; Huart, V.; Duval, X.; Cimadevilla, C.; et al. Determinants and prognostic value of Galectin-3 in patients with aortic valve stenosis. Heart 2016, 102, 862–868. [Google Scholar] [CrossRef]
- Bobrowska, B.; Wieczorek-Surdacka, E.; Kruszelnicka, O.; Chyrchel, B.; Surdacki, A.; Dudek, D. Clinical correlates and prognostic value of plasma galectin-3 levels in degenerative aortic stenosis: A single-center prospective study of patients referred for invasive treatment. Int. J. Mol. Sci. 2017, 18, 947. [Google Scholar] [CrossRef] [PubMed]
- Heywood, J.T.; Fonarow, G.C.; Costanzo, M.R.; Mathur, V.S.; Wigneswaran, J.R.; Wynne, J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: A report from the ADHERE database. J. Card. Fail. 2007, 13, 422–430. [Google Scholar] [CrossRef]
- de Boer, R.A.; Lok, D.J.A.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.L.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; De Meester de Ravenstein, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.-C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Malouf, J.; Michelena, H.I.; Suri, R.M.; Jaffe, A.S.; Mahoney, D.W.; Enriquez-Sarano, M. B-type natriuretic peptide clinical activation in aortic stenosis. J. Am. Coll. Cardiol. 2014, 63, 2016–2025. [Google Scholar] [CrossRef]
- Henri, C.; Dulgheru, R.; Magne, J.; Caballero, L.; Laaraibi, S.; Davin, L.; Kou, S.; Voilliot, D.; Nchimi, A.; Oury, C.; et al. Impact of serial B-type natriuretic peptide changes for predicting outcome in asymptomatic patients with aortic stenosis. Can. J. Cardiol. 2016, 32, 183–189. [Google Scholar] [CrossRef]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef]
- Detaint, D.; Messika-Zeitoun, D.; Chen, H.H.; Rossi, A.; Avierinos, J.-F.; Scott, C.; Burnett, J.C.; Enriquez-Sarano, M. Association of B-type natriuretic peptide activation to left ventricular end-systolic remodeling in organic and functional mitral regurgitation. Am. J. Cardiol. 2006, 97, 1029–1034. [Google Scholar] [CrossRef]
- Weber, M.; Arnold, R.; Rau, M.; Brandt, R.; Berkovitsch, A.; Mitrovic, V.; Hamm, C. Relation of N-terminal pro–B-type natriuretic peptide to severity of valvular aortic stenosis. Am. J. Cardiol. 2004, 94, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Maréchaux, S.; Hattabi, M.; Juthier, F.; Neicu, D.V.; Richardson, M.; Carpentier, E.; Bouabdallaoui, N.; Delelis, F.; Banfi, C.; Breyne, J.; et al. Clinical and echocardiographic correlates of plasma B-type natriuretic peptide levels in patients with aortic valve stenosis and normal left ventricular ejection fraction. Echocardiography 2011, 28, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Leip, E.P.; Benjamin, E.J.; Wilson, P.W.F.; Sutherland, P.; Omland, T.; Vasan, R.S. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002, 90, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.; Niewiara, Ł.; Podolec, J.; Siedliński, M.; Józefczuk, E.; Bernacik, A.; Badacz, R.; Przewłocki, T.; Pieniążek, P.; Żmudka, K.; et al. Serum and vascular stiffness biomarkers associated with the severity of degenerative aortic valve stenosis and cardiovascular outcomes. J. Cardiovasc. Dev. Dis. 2022, 9, 193. [Google Scholar] [CrossRef]
- Baldenhofer, G.; Laule, M.; Mockel, M.; Sanad, W.; Knebel, F.; Dreger, H.; Leonhardt, F.; Sander, M.; Grubitzsch, H.; Baumann, G.; et al. Mid-regional pro-adrenomedullin (MR-proADM) and mid-regional pro-atrial natriuretic peptide (MR-proANP) in severe aortic valve stenosis: Association with outcome after transcatheter aortic valve implantation (TAVI). Clin. Chem. Lab. Med. 2016, 55, 275–283. [Google Scholar] [CrossRef]

| Characteristics | AS, n (%) | Control, n (%) | p |
|---|---|---|---|
| Sex (Female) | 32 (64) | 32 (64) | 1 |
| Age | 82.86 ± 7.2 | 80.48 ± 6.42 | 0.084 |
| Body surface | 1.68 ± 0.19 | 1.67 ± 0.18 | 0.82 |
| Functional class | |||
| I | 19 (38) | 44 (88) | <0.001 |
| II | 31 (62) | 6 (12) | |
| Coronary heart disease | 6 (12) | 0 (0) | 0.027 |
| AF | 14 (28) | 11 (22) | 0.488 |
| DM | 18 (36) | 11 (22) | 0.122 |
| Dyslipidemia | 27 (54) | 29 (58) | 0.68 |
| HBP | 40 (80) | 38 (76) | 0.629 |
| COPD | 11 (22) | 4 (8) | 0.0499 |
| CVD | 5 (10) | 6 (12) | 0.74 |
| Dementia | 7 (14) | 3 (6) | 0.31 |
| Degree of dependence | |||
| Independent | 28 (56) | 42 (84) | 0.0123 |
| Mild | 12 (24) | 5 (10) | |
| Moderate | 6 (12) | 3 (6) | |
| Severe | 4 (8) | 0 (0) | |
| Barthel index | 91.86 ± 16.88 | 97.86 ± 6.02 | 0.0199 |
| Comorbidity according to Charlson index | |||
| No comorbidity | 27 (54) | 39 (78) | |
| Low comorbidity | 14 (28) | 8 (16) | 0.033 |
| High comorbidity | 9 (18) | 3 (6) | |
| Charlson index | 1.36 ± 1.31 | 0.84 ± 0.89 | 0.022 |
| Medication | |||
| ACE inhibitors/ARBs | 34 (68) | 29 (58) | 0.3004 |
| Calcium channel blockers | 12 (24) | 9 (18) | 0.461 |
| Potassium-sparing diuretics | 1 (2) | 3 (6) | 0.617 |
| Statins | 21 (42) | 25 (50) | 0.422 |
| Beta-blockers | 15 (30) | 17 (34) | 0.668 |
| Loop diuretics | 7 (14) | 1 (2) | 0.071 |
| Thiazides | 14 (28) | 14 (28) | 0.132 |
| Echocardiography | |||
| LVH | |||
| No | 2 (4) | 19 (38) | |
| Mild (11–13 mm) | 15 (30) | 29 (28) | <0.001 |
| Moderate (13–15 mm) | 20 (40) | 2 (4) | |
| Severe > 15 mm | 13 (26) | 0 (0) | |
| PHT | |||
| Normal | 15 (30) | 27 (54) | |
| Mild (35–40) | 12 (24) | 5 (10) | |
| Moderate (45–65) | 6 (12) | 1 (2) | 0.0199 |
| Severe > 65 | 1 (2) | 0 (0) | |
| Not estimated | 16 (32) | 17 (34) | |
| IVS | 13.7 ± 1.66 | 11.1 ± 1.22 | <0.001 |
| Peak gradient (mmHg) | 57.7 ± 20.32 | 9.35 ± 3.77 | <0.001 |
| Mean gradient (mmHg) | 33.3 ± 12.9 | 4.65 ± 1.83 | <0.001 |
| Peak velocity (m/s) | 3.7 ± 0.69 | 1.48 ± 0.31 | <0.001 |
| AVA (cm2) | 0.82 ± 0.26 | 2.1 ± 0.39 | <0.001 |
| AVA indexed | 0.49 ± 0.16 | 1.3 ± 0.28 | <0.001 |
| Integral relation | 0.25 ± 0.08 | 0.66 ± 0.13 | <0.001 |
| LVEF Teicholz (%) | 67.9 ± 9.8 | 71.2 ± 7.64 | 0.07 |
| Indexed stroke volume | 39.5 ± 10.68 | 39.4 ± 9.31 | 0.966 |
| GLS | 18.67 ± 3.25 | 19.48 ± 1.78 | 0.136 |
| GLS < 18% | 19 (38.8) | 7 (14) | 0.0051 |
| Lab results | |||
| GFR (mL/min) | 57.96 ± 8.9 | 59.6 ± 8.49 | 0.53 |
| GFR < 50 mL/min | 15 (30) | 7 (14) | 0.045 |
| NTproBNP | 1117.28 ± 1528.49 | 271.9 ± 350.8 | <0.001 |
| NTproBNP > 450 | 28 (56) | 6 (12) | <0.001 |
| NTproBNP > 435 | 26 (52) | 5 (10) | <0.001 |
| Galectin-3 | |||
| Mild risk ≤ 17.8 | 27 (54) | 43 (86) | 0.0011 |
| Moderate risk > 17.8–25.9 | 21 (42) | 7 (14) | |
| High risk > 25.9 | 2 (4) | 0 | |
| Galectin-3 | 16.91 ± 4.57 | 12.7 ± 4.74 | <0.001 |
| Galectin-3 > 14.3 | 35 (70) | 15 (30) | <0.001 |
| ECG | |||
| Heart rate | 69.8 ± 12.13 | 69.22 ± 12.04 | 0.81 |
| Rhythm | |||
| Sinusal | 42 (84) | 46 (92) | |
| AF | 6 (12) | 4 (8) | 0.275 |
| Pacemaker | 2 (4) | 0 (0) | |
| LVH criteria | 7 (14) | 2 (4) | 0.16 |
| Age | Mean Gradient | AVA | GLS | IVS | ||
|---|---|---|---|---|---|---|
| NT-proBNP | S | 0.419 | 0.455 | −0.474 | −0.181 | 0.574 |
| p | <0.0001 | <0.0001 | <0.0001 | 0.07 | <0.0001 | |
| Galectin-3 | S | 0.183 | 0.339 | −0.366 | −0.029 | 0.369 |
| p | 0.069 | 0.0006 | 0.0002 | 0.77 | 0.0002 |
| Features | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| Maximum pressure gradient | 1.03 (1.00; 1.05) | 0.0277 | ||
| MPG | 1.06 (1.02; 1.10) | 0.0045 | ||
| Vmax | 2.48 (1.17; 5.27) | 0.0184 | ||
| AVA | 0.02 (0.00; 0.23) | 0.0018 | ||
| AVA indexed | 0.001 (0.000; 0.088) | 0.0019 | ||
| Integral relation | 0.000 (0.000; 0.002) | 0.0003 | ||
| LVEF Teichholz | 0.94 (0.89; 1.00) | 0.0329 | ||
| Indexed stroke volume | 0.95 (0.90; 0.99) | 0.0242 | ||
| GLS | 0.90 (0.77; 1.05) | 0.1882 | ||
| NT-proBNP | 1.000 (1.000; 1.000) | 0.0125 | 3.45 (1.32; 9.03) | 0.011 |
| Galectin-3 | 0.98 (0.89; 1.09) | 0.7679 | 0.97 (0.87; 1.08) | 0.609 |
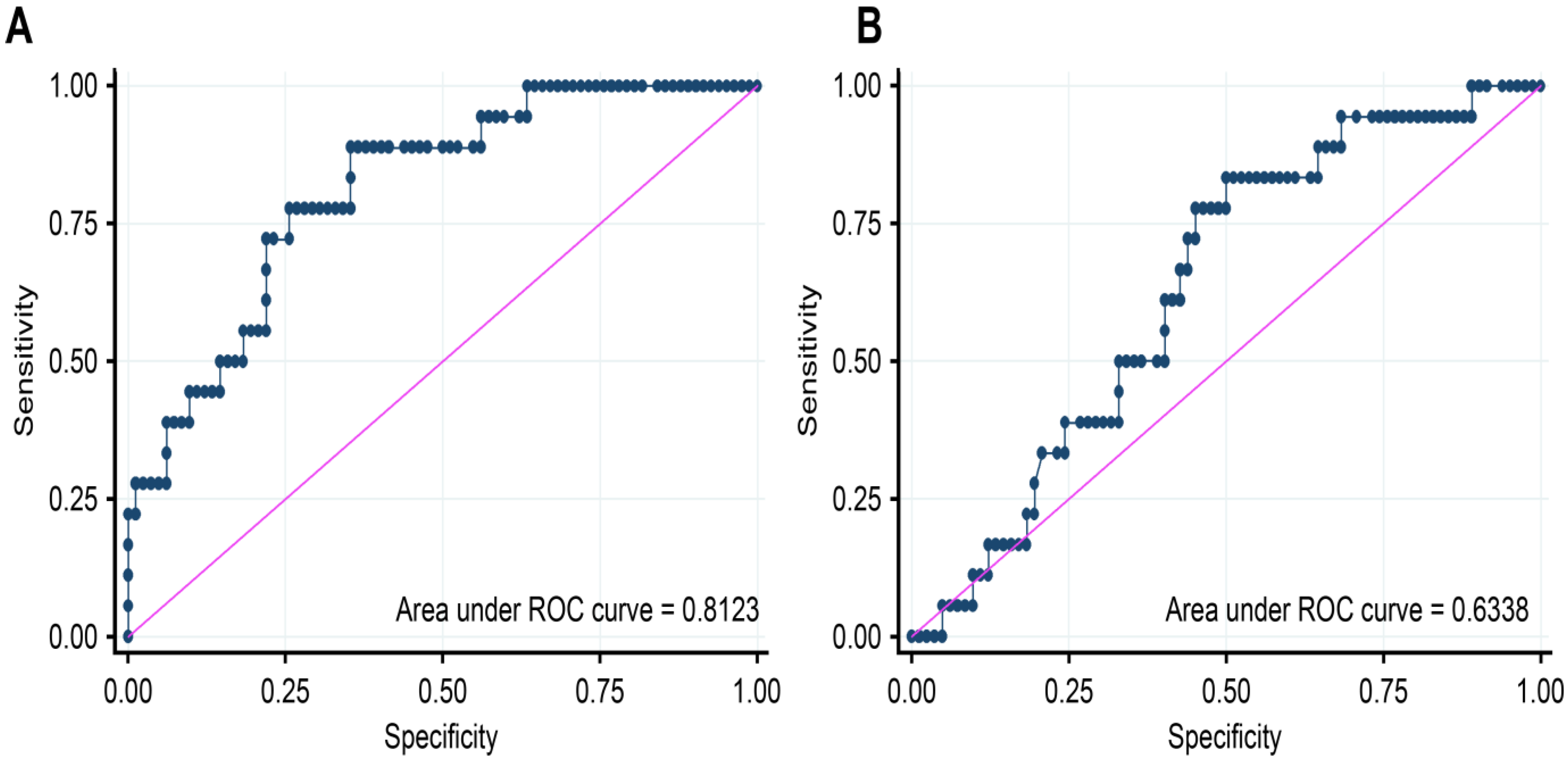
| ROC AREA | Youden Index | Sensitivity | Specificity | PPV | NPV | Cut Point AUC | |
|---|---|---|---|---|---|---|---|
| NT-proBNP | 0.8123 | 0.5352 | 88.8889 | 64.6341 | 35.5556 | 96.3636 | 435.00 |
| Galectin-3 | 0.6338 | 0.3333 | 83.3333 | 50.0000 | 26.7857 | 93.1818 | 14.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Quezada-Feijoó, M.; Ayala, R.; Manzano, A.; Gómez-Pavón, F.J.; Jaramillo, J.; Herrera, C.; López Vazquez de la Torre, M.; Toro, R. Value of NT-proBNP and Galectin-3 as Biomarkers in the Follow-Up of Asymptomatic Elderly Patients with Severe Aortic Stenosis. J. Clin. Med. 2023, 12, 2987. https://doi.org/10.3390/jcm12082987
Ramos M, Quezada-Feijoó M, Ayala R, Manzano A, Gómez-Pavón FJ, Jaramillo J, Herrera C, López Vazquez de la Torre M, Toro R. Value of NT-proBNP and Galectin-3 as Biomarkers in the Follow-Up of Asymptomatic Elderly Patients with Severe Aortic Stenosis. Journal of Clinical Medicine. 2023; 12(8):2987. https://doi.org/10.3390/jcm12082987
Chicago/Turabian StyleRamos, Mónica, Maribel Quezada-Feijoó, Rocío Ayala, Ascensión Manzano, Francisco Javier Gómez-Pavón, Javier Jaramillo, Cristina Herrera, Mariola López Vazquez de la Torre, and Rocío Toro. 2023. "Value of NT-proBNP and Galectin-3 as Biomarkers in the Follow-Up of Asymptomatic Elderly Patients with Severe Aortic Stenosis" Journal of Clinical Medicine 12, no. 8: 2987. https://doi.org/10.3390/jcm12082987






