Full Endoscopy Combined with Allogeneic Bone Grafting for Benign Spinal Lesions: Technical Notes and Preliminary Clinical Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Preparations
2.2. Surgical Technique
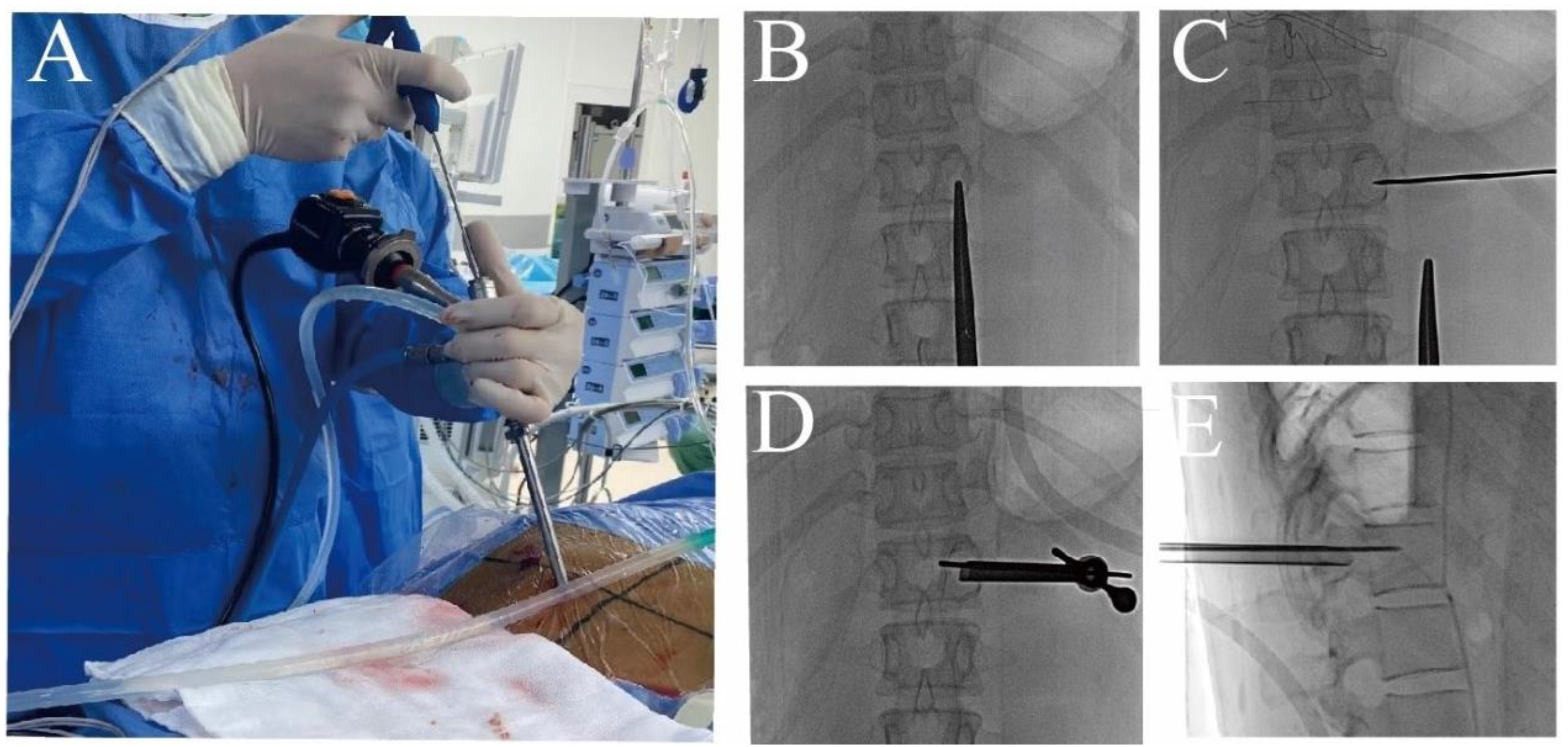
2.2.1. Surgical Instruments
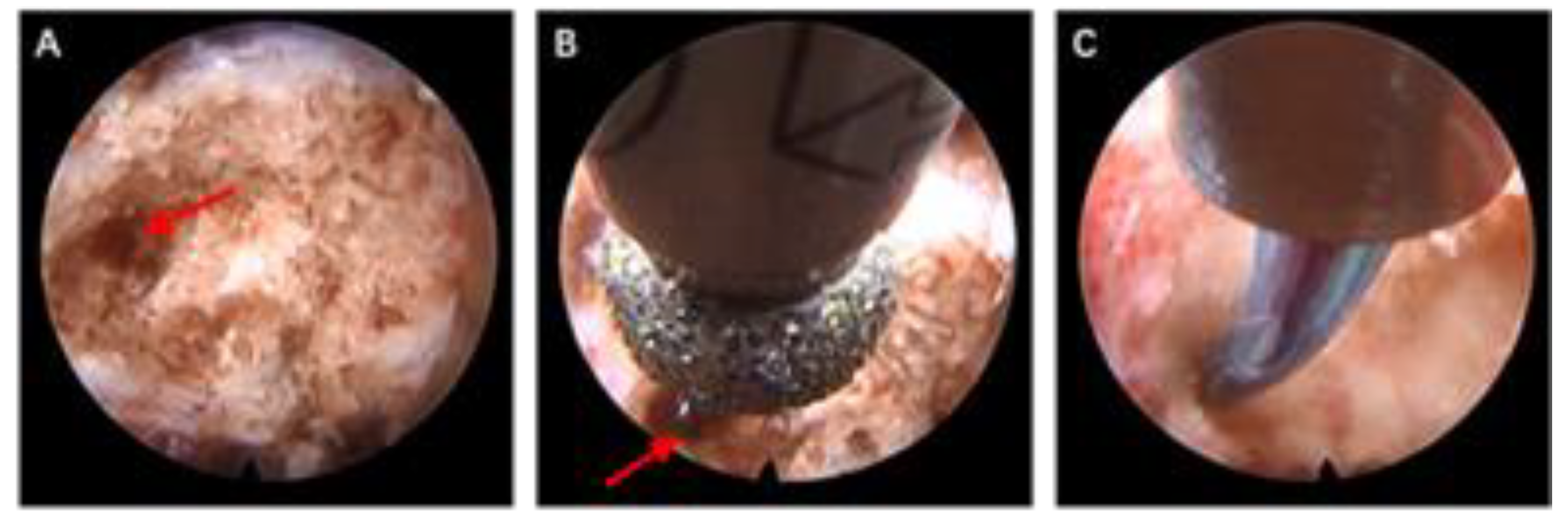
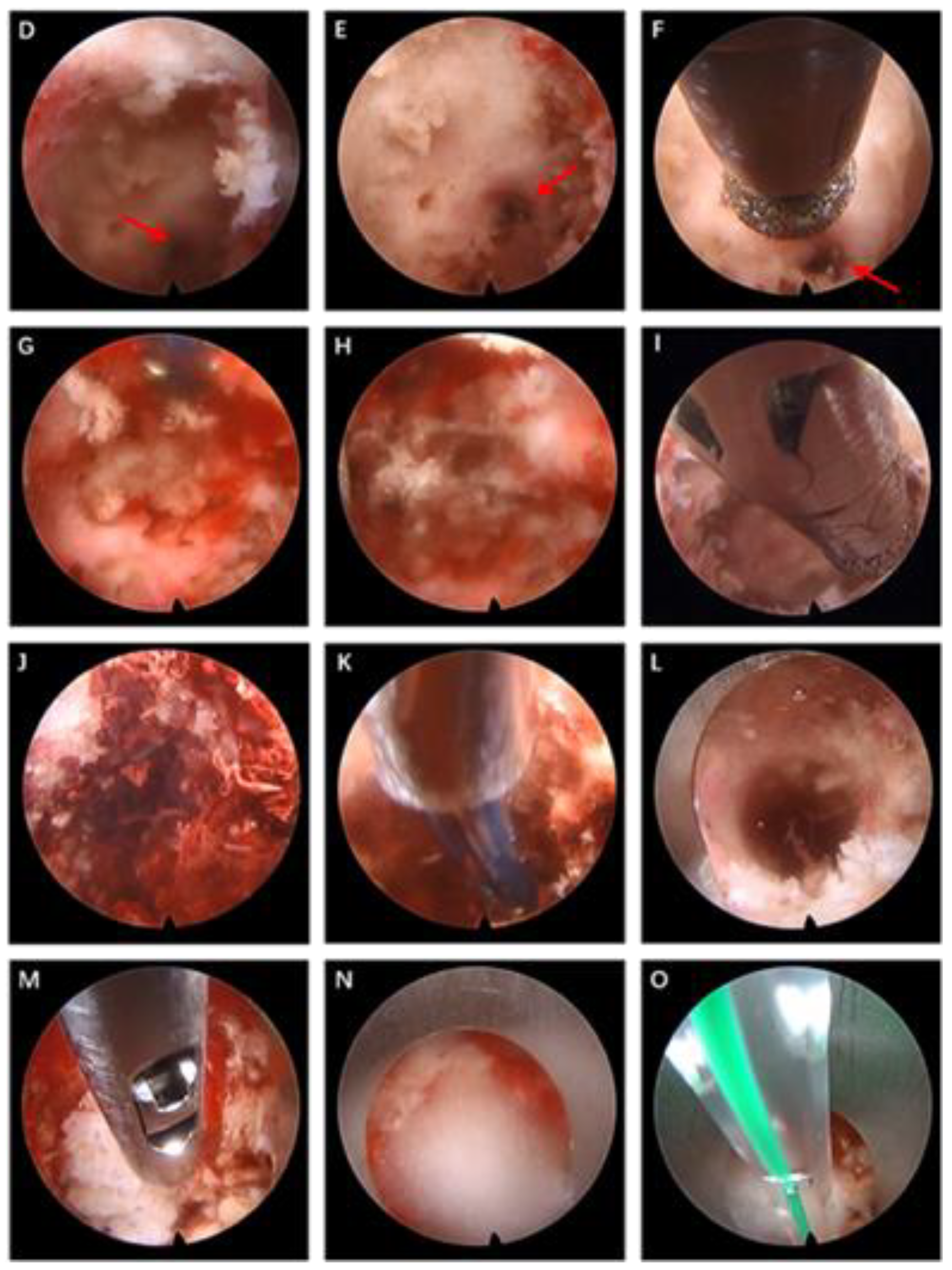
2.2.2. Surgical Procedure
2.2.3. Postoperative Management and Follow-Up
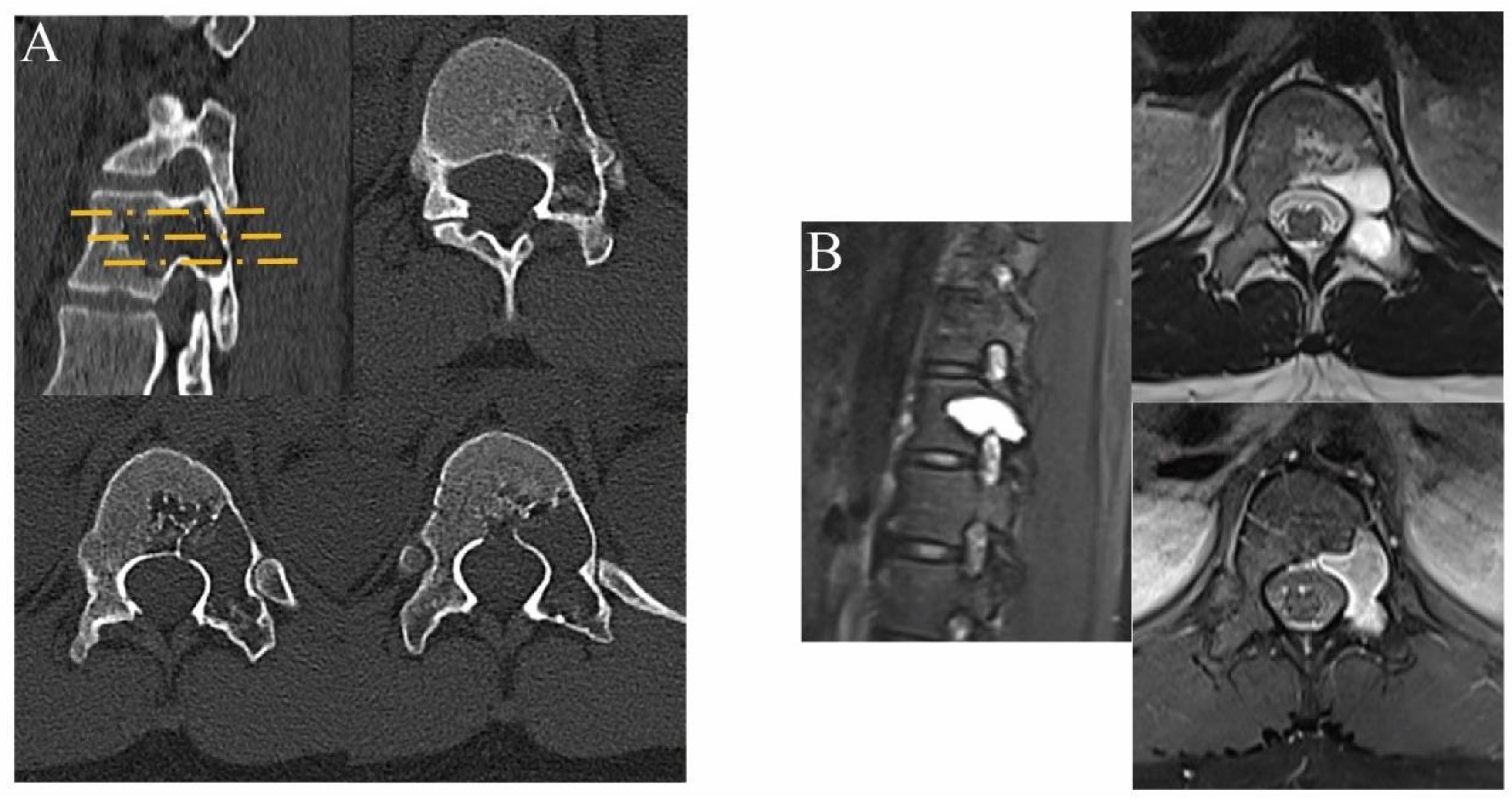
2.3. Analysis of Clinical Results
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.-H.; Xiao, L.-F.; Zhang, C.; Lei, J.; Deng, Z.-M. The minimally invasive endoscopic technique for the treatment of symptomatic benign bone lesions: Preliminary results from a retrospective study. J. Bone Oncol. 2020, 24, 100313. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.A.; Daniels, A.H.; Schiller, J.; Valdes, M.A.; Czerwein, J.K.; Schiller, A.; Esmende, S.; Terek, R.M. Benign tumors of the spine. J. Am. Acad. Orthop. Surg. 2012, 20, 715–724. [Google Scholar] [CrossRef]
- Regev, G.J.; Salame, K.; Keynan, O.; Lidar, Z. Resection of benign vertebral tumors by minimally invasive techniques. Spine J. Off. J. N. Am. Spine Soc. 2015, 15, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; Weinstein, J.N.; Biagini, R. Primary bone tumors of the spine. Terminol. Surg. Staging. Spine 1997, 22, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.E.; Cahill, K.S.; Hanna, J.W.; McCarthy, E.F.; Gokaslan, Z.L.; Chi, J.H. Primary vertebral tumors: A review of epidemiologic, histological, and imaging findings, Part I: Benign tumors. Neurosurgery 2011, 69, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Ren, Q.; Chu, L.; Shi, L.; Yu, Q.; Yan, Z.; Yu, K.; Liu, C.; Wu, W.; Xiong, Y.; et al. Modified posterior percutaneous endoscopic cervical discectomy for lateral cervical disc herniation: The vertical anchoring technique. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2018, 27, 1460–1468. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Q.; Gao, X.; Wang, K.; Zhang, X.; Du, Y.; Chen, L. Posterior percutaneous endoscopic lumbar discectomy combined with the vertical anchoring technique for lumbar disc herniation with distant upward migration. J. Orthop. Surg. Res. 2019, 14, 467. [Google Scholar] [CrossRef]
- Tomasian, A.; Cazzato, R.L.; Sharma, K.; Gangi, A.; Jennings, J.W. Benign Bone Tumors: State of the Art in Minimally Invasive Percutaneous Interventions. Radiogr. A Rev. Publ. Radiol. Soc. N. Am. Inc 2023, 43, e220041. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Boriani, S.; Fourney, D.R.; Biagini, R.; Dekutoski, M.B.; Fehlings, M.G.; Ryken, T.C.; Gokaslan, Z.L.; Vrionis, F.D.; Harrop, J.S.; et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009, 34, 384–391. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Tomasian, A.; Wallace, A.N.; Jennings, J.W. Benign Spine Lesions: Advances in Techniques for Minimally Invasive Percutaneous Treatment. AJNR. Am. J. Neuroradiol. 2017, 38, 852–861. [Google Scholar] [CrossRef]
- Yang, J.S.; Chu, L.; Deng, R.; Chen, C.M.; Wang, X.F.; Xie, P.G.; Yu, K.X.; Rong, L.M.; Hao, D.J.; Wei, J.M.; et al. Treatment of Single-Level Thoracic Tuberculosis by Percutaneous Endoscopic Débridement and Allograft via the Transforaminal Approach Combined with Percutaneous Pedicle Screw Fixation: A Multicenter Study with a Median Follow-Up of 36 Months. World Neurosurg. 2019, 122, e1472–e1481. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.C.; Ok, W.K.; Baik, S.H.; Kim, H.J.; Kwon, O.S.; Kim, K.H. Removal of a vertebral metastatic tumor compressing the spinal nerve roots via a single-port, transforaminal, endoscopic approach under monitored anesthesia care. Pain Physician 2012, 15, 297–302. [Google Scholar] [PubMed]
- Telfeian, A.E.; Choi, D.B.; Aghion, D.M. Transforaminal endoscopic surgery under local analgesia for ventral epidural thoracic spinal tumor: Case report. Clin. Neurol. Neurosurg. 2015, 134, 1–3. [Google Scholar] [CrossRef]
- Zileli, M.; Isik, H.S.; Ogut, F.E.; Is, M.; Cagli, S.; Calli, C. Aneurysmal bone cysts of the spine. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2013, 22, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, I.; Dezawa, A.; Urayama, S.; Nakamura, S. Surgical treatment of a lumbar aneurysmal bone cyst using percutaneous endoscopic lumbar discectomy. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2018, 27, 368–374. [Google Scholar] [CrossRef]
- Xie, T.; Xiu, P.; Song, Y.; Zeng, J.; Huang, S. Percutaneous Endoscopic Excision and Ablation of Osteoid Osteoma of the Lumbar Spine and Sacrum: A Technical Note and Outcomes. World Neurosurg. 2020, 133, 121–126. [Google Scholar] [CrossRef]
- Kotheeranurak, V.; Jitpakdee, K.; Rujiramongkolchai, N.; Atikankul, T.; Singhatanadgige, W.; Limthongkul, W.; Tejapongvorachai, T.; Kim, J.S. Remodeling of the Lumbar Facet Joint After Full Endoscopic Resection for Lumbar Osteoid Osteoma: Case Report and Literature Review. Int. J. Spine Surg. 2022, 16, 378–383. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, F.; Zheng, H.; Yao, X.; Yan, Z.; Liu, Y.; Wang, L.; Zhang, X.; Chen, L. Full Endoscopic Posterolateral Transarticular Lumbar Interbody Fusion Using Transparent Plastic Working Tubes: Technical Note and Preliminary Clinical Results. Front. Surg. 2022, 9, 884794. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Du, Y.; Yan, Z.; Chen, L.; Wang, L. Efficacy Analysis of Percutaneous Endoscopic Spinal Surgery for Young Patients with Discogenic Low Back Pain. J. Pain Res. 2022, 15, 665–674. [Google Scholar] [CrossRef]
- Gao, X.; Tang, K.; Xia, Y.; Zhang, X.; Wang, K.; Yan, Z.; Du, Y.; Chen, L. Efficacy Analysis of Percutaneous Endoscopic Lumbar Discectomy Combined with PEEK Rods for Giant Lumbar Disc Herniation: A Randomized Controlled Study. Pain Res. Manag. 2020, 2020, 3401605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.M.; Yu, K.X.; Deng, R.; Long, Q.Y.; Chu, L.; Xiong, Y.; Sun, B.; Chen, L.; Yan, Z.J.; Deng, Z.L. Modified K-Hole Percutaneous Endoscopic Surgery for Cervical Foraminal Stenosis: Partial Pediculectomy Approach. Pain Physician 2019, 22, E407–E416. [Google Scholar] [PubMed]
- Liu, C.; Liu, K.; Chu, L.; Chen, L.; Deng, Z. Posterior percutaneous endoscopic cervical discectomy through lamina-hole approach for cervical intervertebral disc herniation. Int. J. Neurosci. 2019, 129, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.P., Jr.; Hefele, M.C.; Peabody, T.D.; Montag, A.G.; Aithal, V.; Simon, M.A. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J. Bone Jt. Surg. Am. Vol. 1999, 81, 1671–1678. [Google Scholar] [CrossRef]
- Ulici, A.; Florea, D.C.; Carp, M.; Ladaru, A.; Tevanov, I. Treatment of the aneurysmal bone cyst by percutaneous intracystic sclerotherapy using ethanol ninety five percent in children. Int. Orthop. 2018, 42, 1413–1419. [Google Scholar] [CrossRef]
- Salame, K.; Lidar, Z.; Khashan, M.; Ofir, D.; Regev, G.J. Minimally Invasive Resection of Benign Osseous Tumors of the Spinal Column: 10 Years’ Experience and Long-Term Outcomes of a Specialized Center. Medicina 2022, 58, 1840. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Deluiz, D.; Tinoco, E.M.B. Horizontal Alveolar Ridge Augmentation with Allogeneic Bone Block Graft Compared with Autogenous Bone Block Graft: A Systematic Review. J. Oral Maxillofac. Res. 2020, 11, e1. [Google Scholar] [CrossRef]

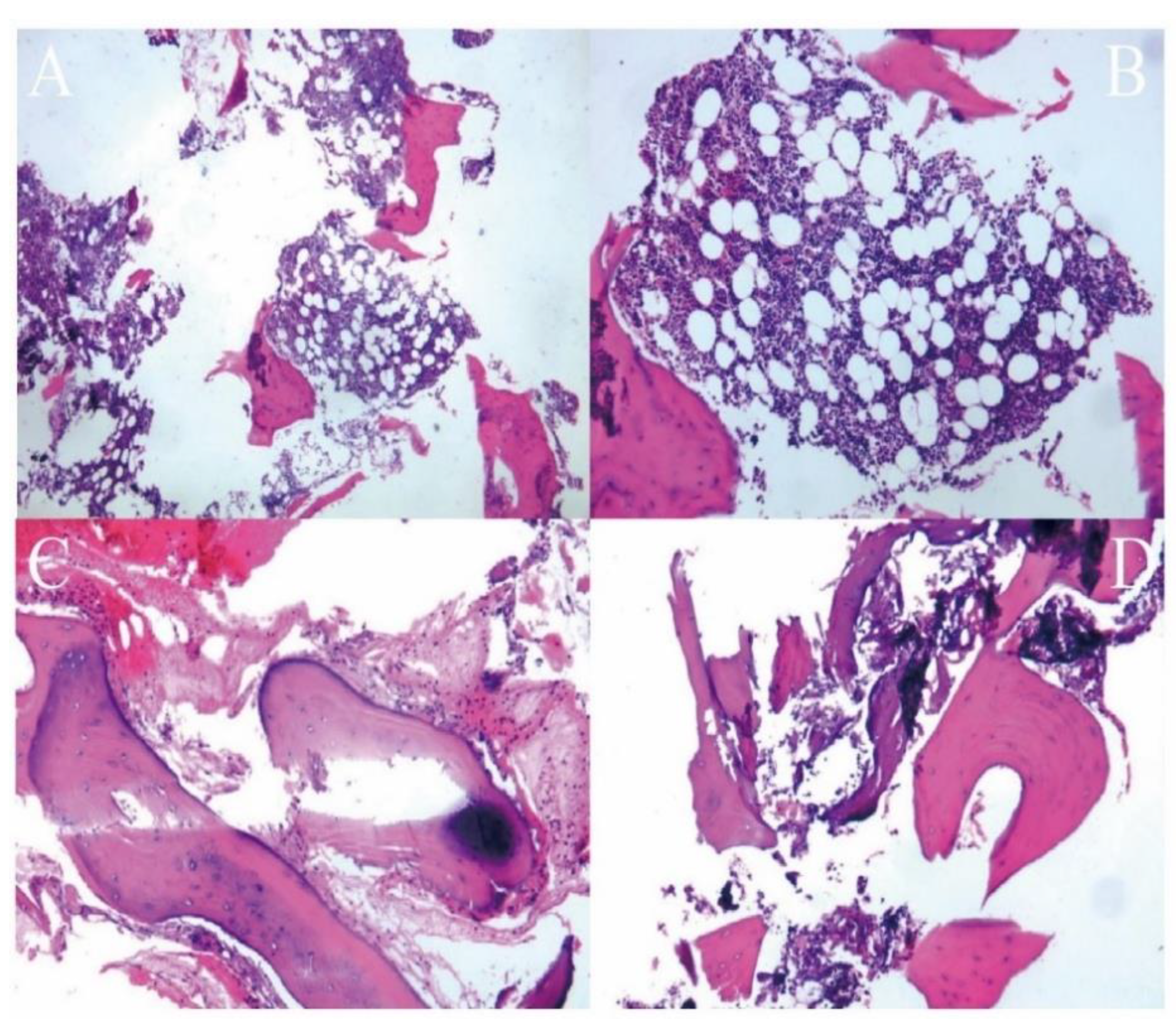
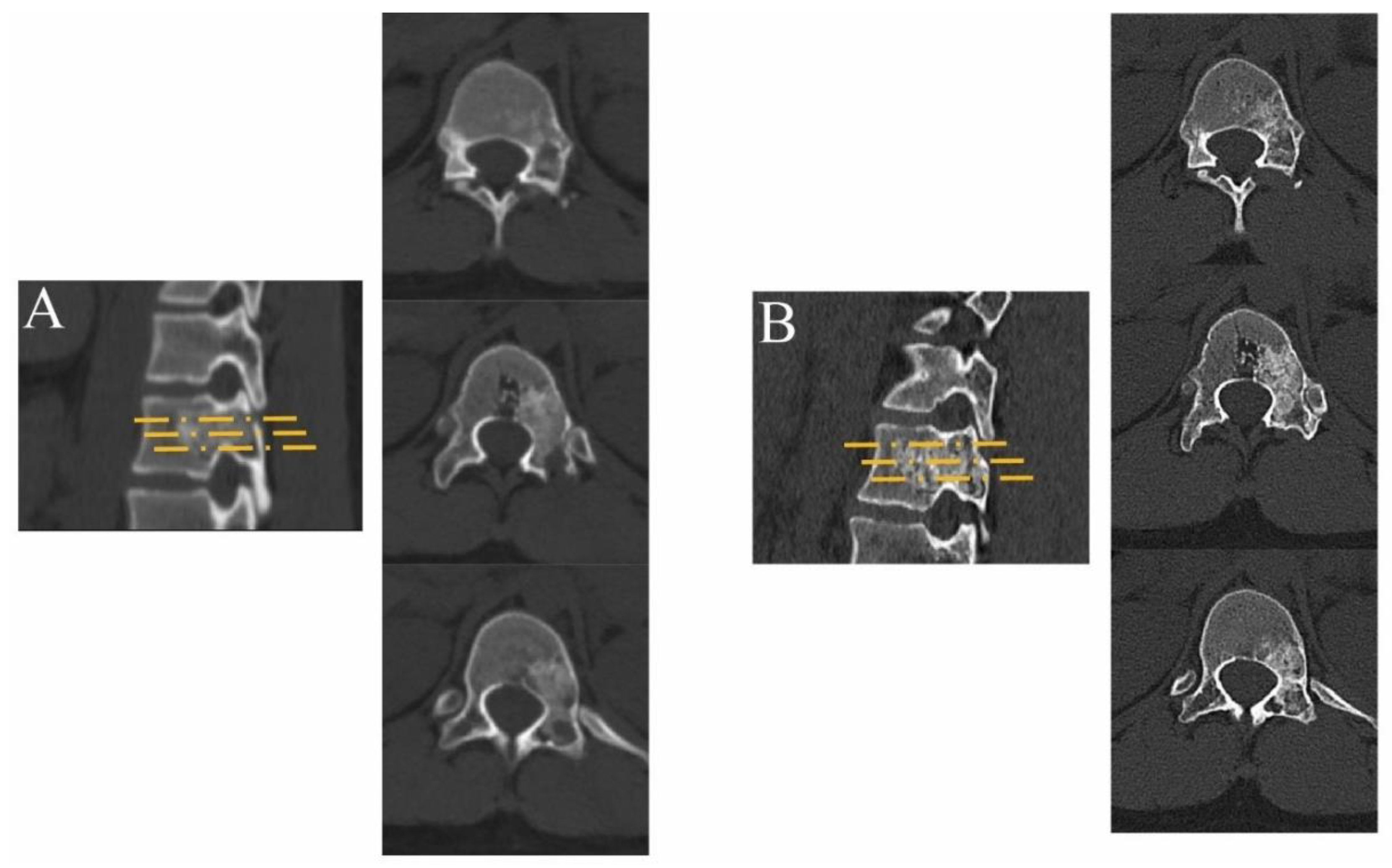
| Characteristic | Value |
|---|---|
| Mean age (years) | 21.8 ± 4.3 |
| Sex | |
| M | 6 |
| F | 9 |
| Mean follow-up period (months) | 18.0 ± 5.1 |
| Diagnosis | |
| aneurysmal bone cyst | 3 |
| osteoid osteoma | 8 |
| fibrous dysplasia | 4 |
| Mean estimated blood loss (mL) | 16.7 ± 6.9 |
| Mean operative time (min) | 63.3 ± 7.2 |
| Postoperative complications | |
| Numbness | 0 |
| Diagnosis | Location/WBB Classification | Age/ Gender | Conservative Treatment Time | Visual Analog Scale | Operative Time | Estimated Blood Loss | Follow-Up Period | Recurrence | Visual Analog Scale Score at Last Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| ABC | T12/sectors4-5, layer C | 17/F | 3M | 4 | 55 | 10 | 18M | N | 1 |
| ABC | T10/sectors4-6, layer C | 28/F | 4M | 3 | 70 | 15 | 24M | N | 0 |
| ABC | T1/sectors7-8, layer C | 20/M | 2M | 4 | 50 | 15 | 18M | N | 1 |
| OO | T11/sectors4-5, layer C | 22/F | 2M | 3 | 70 | 10 | 18M | N | 0 |
| OO | T12/sectors7-9, layer C | 18/M | 3M | 2 | 65 | 10 | 18M | N | 0 |
| OO | T8/sectors7-8, layer C | 21/F | 5M | 3 | 55 | 10 | 12M | N | 1 |
| OO | L2/sectors4-5, layer C | 25/F | 8M | 4 | 60 | 20 | 18M | N | 0 |
| OO | L2/sectors4-5, layer C | 30/F | 2M | 3 | 65 | 20 | 12M | N | 1 |
| OO | L3/sectors4-6, layer C | 19/M | 12M | 3 | 75 | 20 | 24M | N | 0 |
| OO | L4/sectors5-6, layer C | 19/F | 12M | 2 | 65 | 30 | 12M | N | 0 |
| OO | L4/sectors4-5, layer C | 22/M | 14M | 2 | 70 | 30 | 24M | N | 0 |
| FD | T8/sectors4-5, layer C | 23/M | 10M | 3 | 55 | 10 | 18M | N | 0 |
| FD | T11/sectors4-5, layer C | 19/F | 5M | 3 | 60 | 10 | 12M | N | 0 |
| FD | L3/sectors4-5, layer C | 16/F | 3M | 4 | 65 | 20 | 18M | N | 0 |
| FD | L5/sectors8-9, layer C | 28/M | 2M | 3 | 70 | 20 | 24M | N | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-G.; He, W.-G.; Li, Q.-C.; Ren, Q.; Zhang, J.-N.; He, L.-J.; Zhang, X.-J.; Chen, L. Full Endoscopy Combined with Allogeneic Bone Grafting for Benign Spinal Lesions: Technical Notes and Preliminary Clinical Results. J. Clin. Med. 2023, 12, 2990. https://doi.org/10.3390/jcm12082990
Liao C-G, He W-G, Li Q-C, Ren Q, Zhang J-N, He L-J, Zhang X-J, Chen L. Full Endoscopy Combined with Allogeneic Bone Grafting for Benign Spinal Lesions: Technical Notes and Preliminary Clinical Results. Journal of Clinical Medicine. 2023; 12(8):2990. https://doi.org/10.3390/jcm12082990
Chicago/Turabian StyleLiao, Cong-Gang, Wen-Ge He, Qi-Chang Li, Qiang Ren, Jia-Nan Zhang, Liang-Jie He, Xiao-Juan Zhang, and Liang Chen. 2023. "Full Endoscopy Combined with Allogeneic Bone Grafting for Benign Spinal Lesions: Technical Notes and Preliminary Clinical Results" Journal of Clinical Medicine 12, no. 8: 2990. https://doi.org/10.3390/jcm12082990
APA StyleLiao, C.-G., He, W.-G., Li, Q.-C., Ren, Q., Zhang, J.-N., He, L.-J., Zhang, X.-J., & Chen, L. (2023). Full Endoscopy Combined with Allogeneic Bone Grafting for Benign Spinal Lesions: Technical Notes and Preliminary Clinical Results. Journal of Clinical Medicine, 12(8), 2990. https://doi.org/10.3390/jcm12082990







