Intraoperative OCT for Lamellar Corneal Surgery: A User Guide
Abstract
:1. Intraoperative OCT: Technology and Characteristics
2. Intraoperative OCT Applications for Lamellar Corneal Surgery
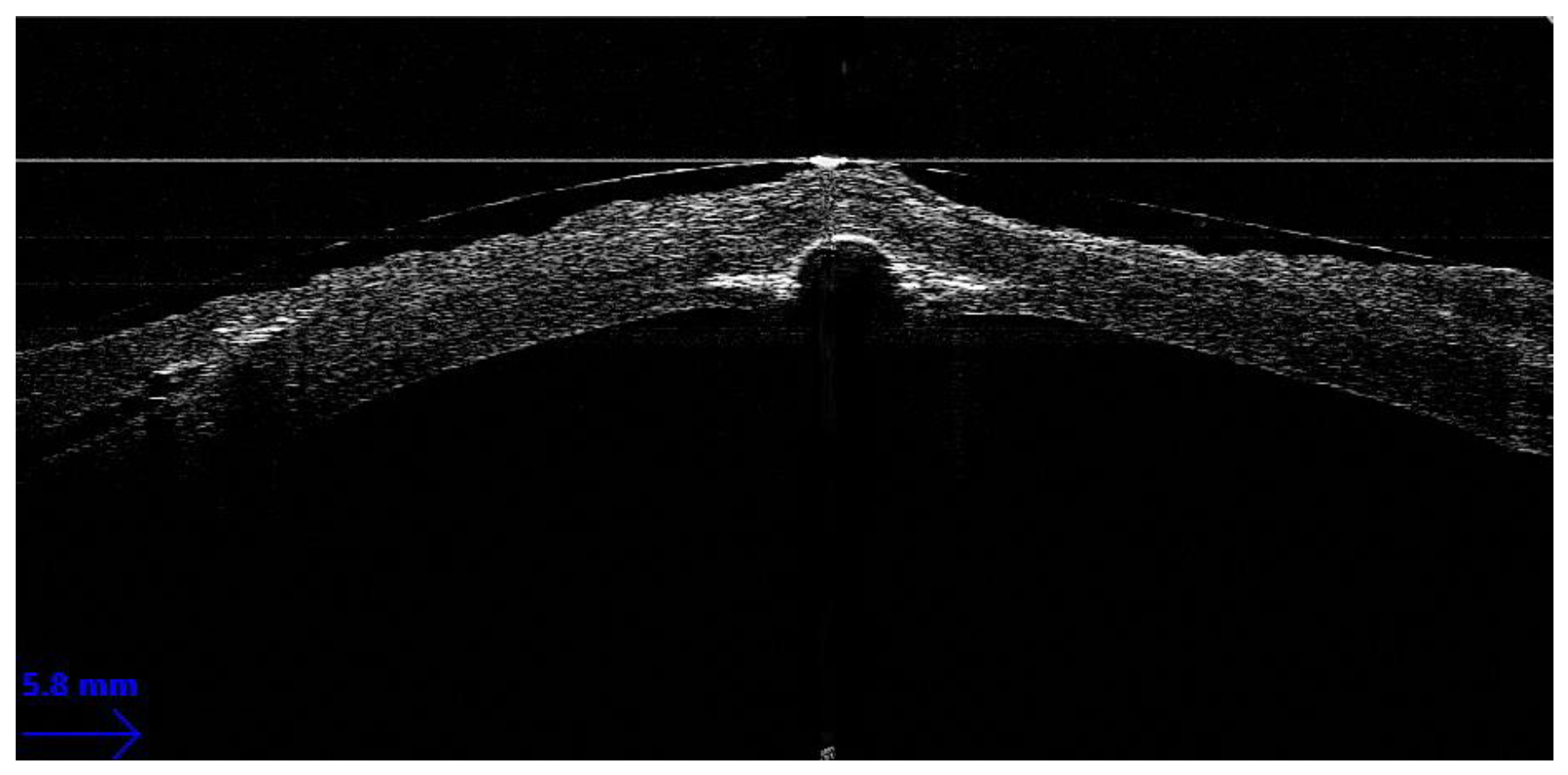
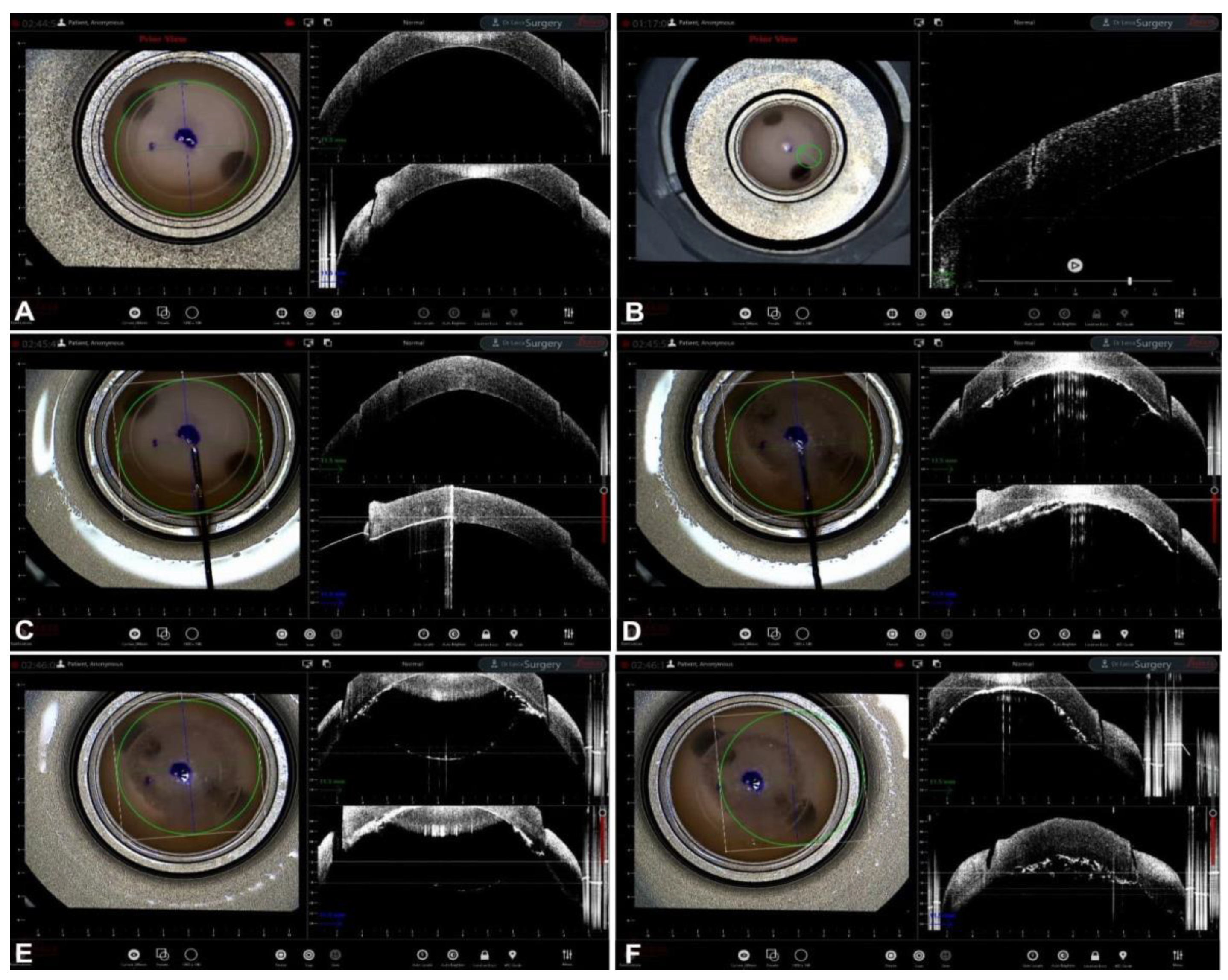
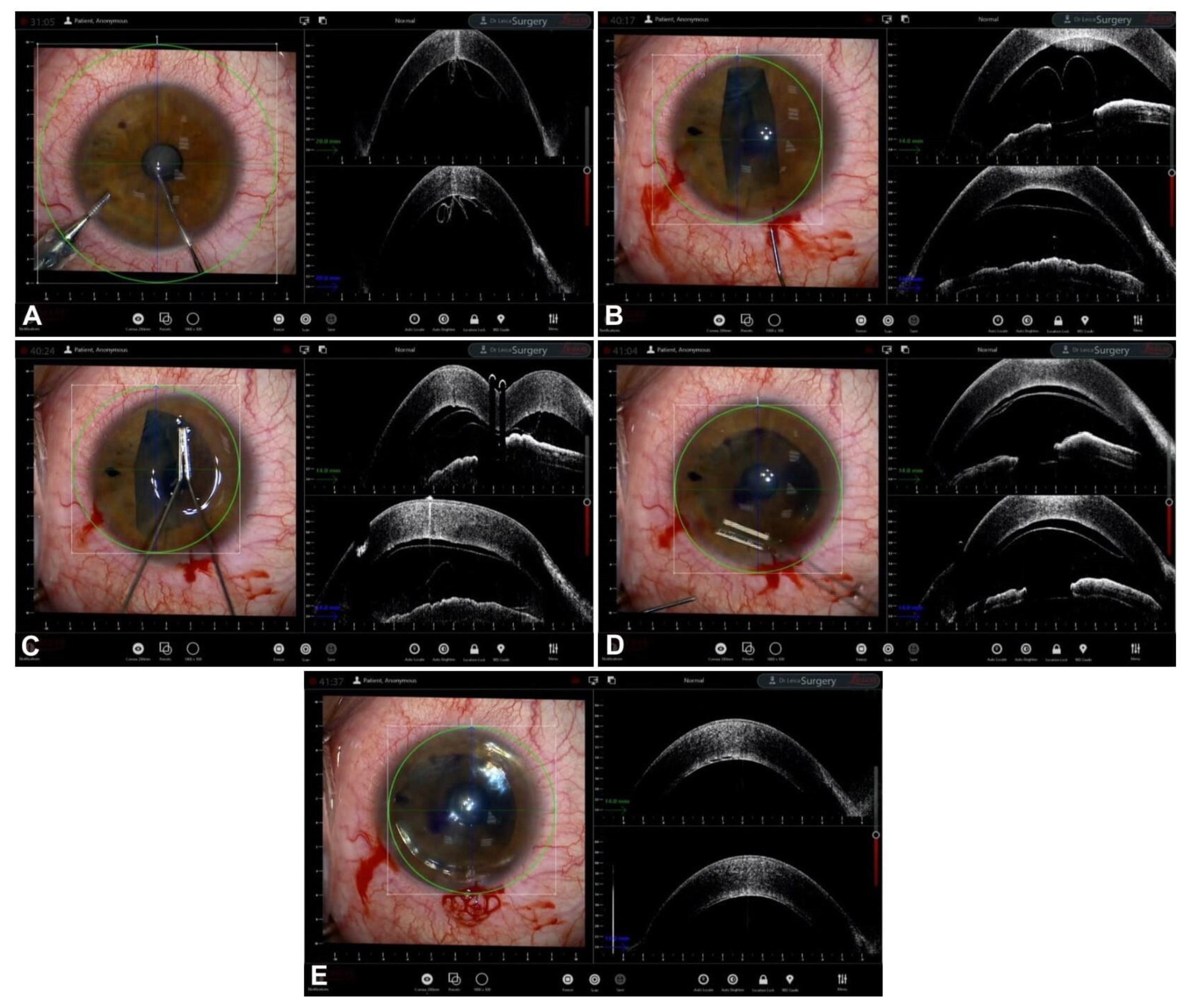
Guiding Big-Bubble Deep Anterior Lamellar Keratoplasty (BB-DALK)
3. Guiding Manual Stromal Dissection DALK
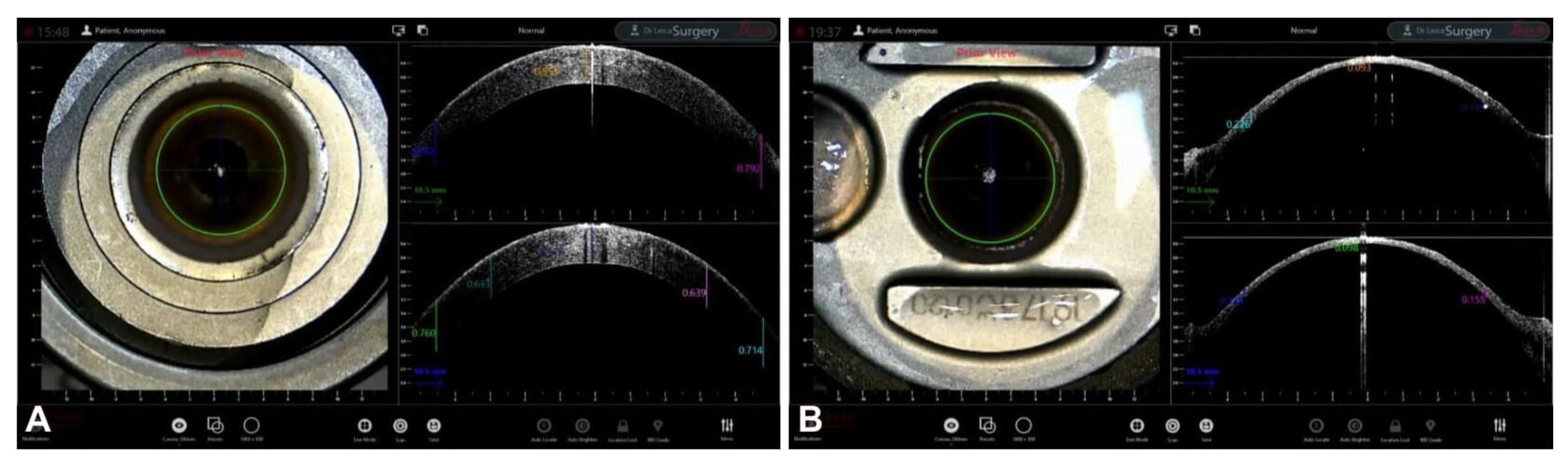
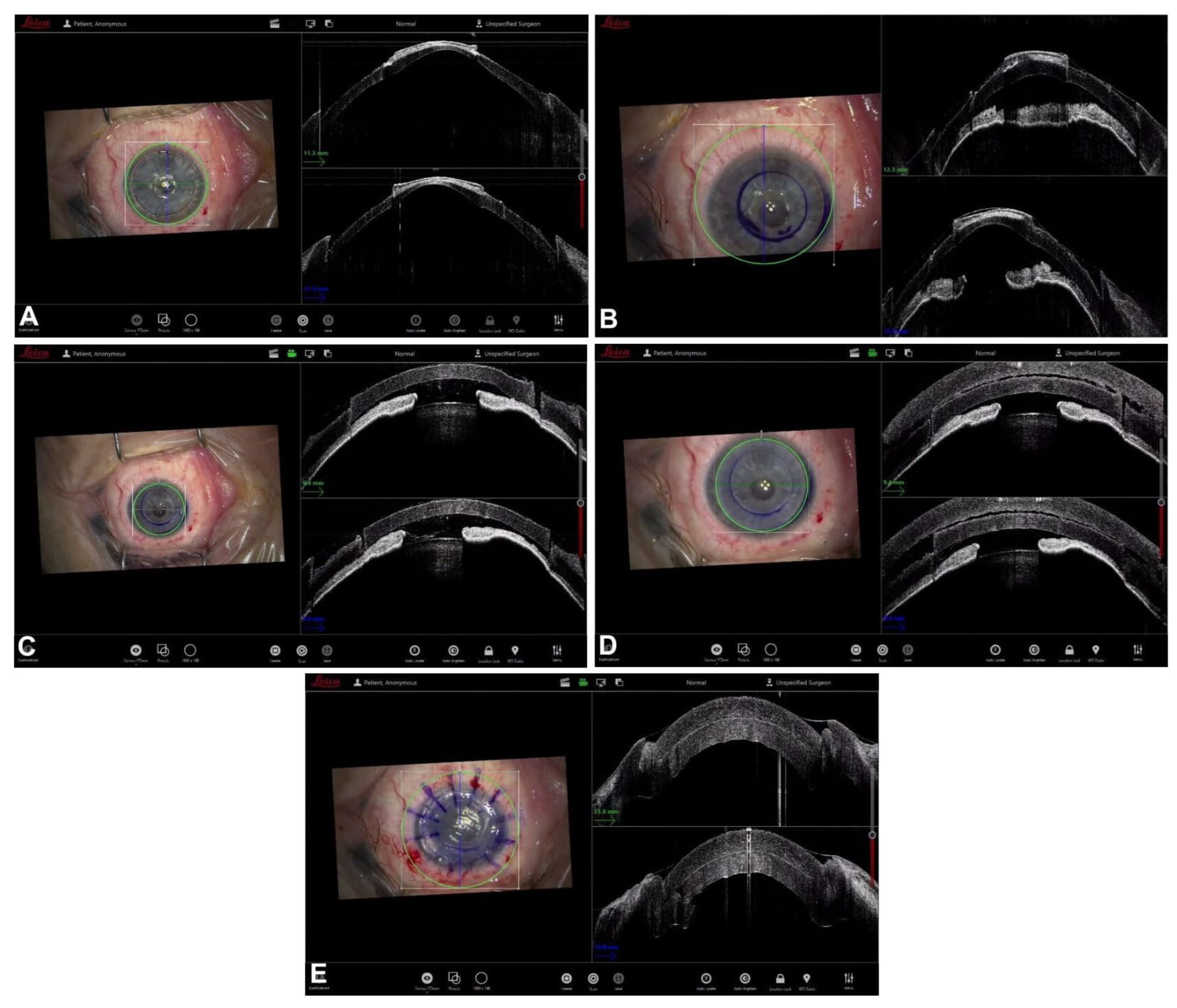
4. Guiding Ultra-Thin Descemet Stripping Automated Endothelial Keratoplasy
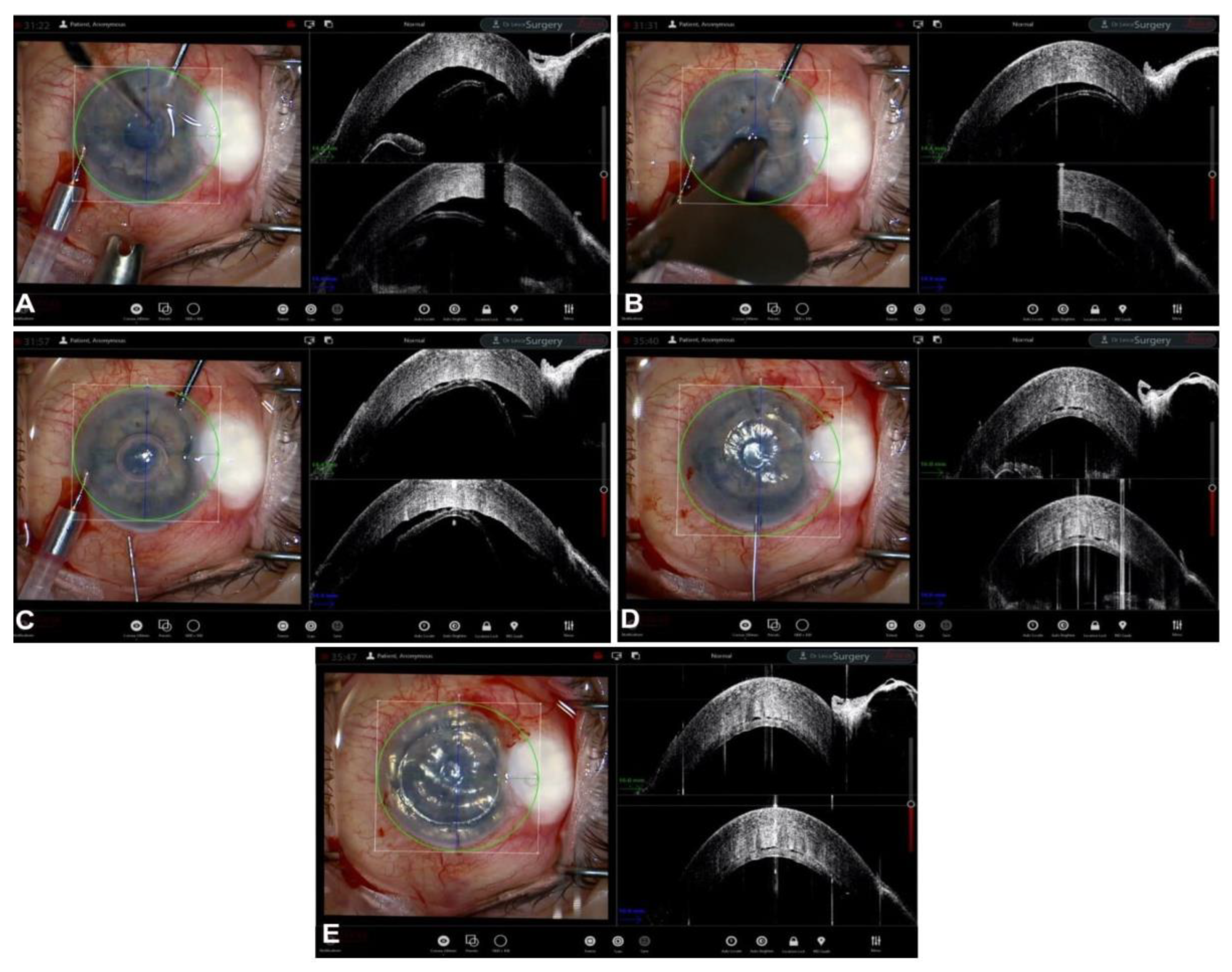
5. Guiding Descemet Membrane Endothelial Keratoplasty
6. Guiding Mushroom Penetrating Keratoplasty
7. Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-Scale Resolution Imaging of the Anterior Eye In Vivo with Optical Coherence Tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Mallipatna, A.; Vinekar, A.; Jayadev, C.; Dabir, S.; Sivakumar, M.; Krishnan, N.; Mehta, P.; Berendschot, T.; Yadav, N.K. The use of handheld spectral domain optical coherence tomography in pediatric ophthalmology practice: Our experience of 975 infants and children. Indian J. Ophthalmol. 2015, 63, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Dubis, A.M.; Wilkins, M.R. Descemet Membrane Endothelial Keratoplasty: Intraoperative and Postoperative Imaging Spectral-Domain Optical Coherence Tomography. Case Rep. Ophthalmol. Med. 2015, 2015, 506251. [Google Scholar] [CrossRef] [PubMed]
- Branchini, L.A.; Gurley, K.; Duker, J.S.; Reichel, E. Use of Handheld Intraoperative Spectral-Domain Optical Coherence Tomography in a Variety of Vitreoretinal Diseases. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Khademi, M.R.; Riazi-Esfahani, M.; Mazloumi, M.; Khodabandeh, A.; Riazi-Esfahani, H. Macular surgery using intraoperative spectral domain optical coherence tomography. J. Ophthalmic Vis. Res. 2015, 10, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Mendez, N.; Nayak, N.V.; Kolomeyer, A.M.; Szirth, B.C.; Khouri, A.S. Feasibility of Spectral Domain Optical Coherence Tomography Acquisition Using a Handheld Versus Conventional Tabletop Unit. J. Diabetes Sci. Technol. 2015, 10, 277–281. [Google Scholar] [CrossRef] [PubMed]
- De Benito-Llopis, L.; Mehta, J.S.; Angunawela, R.I.; Ang, M.; Tan, D.T. Intraoperative Anterior Segment Optical Coherence Tomography: A Novel Assessment Tool during Deep Anterior Lamellar Keratoplasty. Am. J. Ophthalmol. 2013, 157, 334–341.e3. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Barañano, D.E.; Fortun, J.A.; Schwent, B.J.; Cribbs, B.E.; Bergstrom, C.S.; Hubbard, G.B.; Srivastava, S.K. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology 2011, 118, 2212–2217. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am. J. Ophthalmol. 2014, 158, 999–1007.e1. [Google Scholar] [CrossRef]
- Ehlers, J.P. Intraoperative optical coherence tomography: Past, present, and future. Eye 2015, 30, 193–201. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027. [Google Scholar] [CrossRef]
- Yee, P.; Sevgi, D.D.; Abraham, J.; Srivastava, S.K.; Le, T.K.; Uchida, A.; Figueiredo, N.; Rachitskaya, A.V.; Sharma, S.; Reese, J.; et al. iOCT-assisted macular hole surgery: Outcomes and utility from the DISCOVER study. Br. J. Ophthalmol. 2020, 105, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Berrocal, A.M.; Schefler, A.C.; Uhlhorn, S.R.; Ruggeri, M.; Hess, D. Intraoperative OCT of a Full-Thickness Macular Hole Before and After Internal Limiting Membrane Peeling. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Sevgi, D.D.; Srivastava, S.K.; Reese, J.; Ehlers, J.P. Vitreomacular Traction Surgery from the DISCOVER Study: Intraoperative OCT Utility, Ellipsoid Zone Dynamics, and Outcomes. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, 544–550. [Google Scholar] [CrossRef]
- Falkner-Radler, C.I.; Glittenberg, C.; Gabriel, M.; Binder, S. Intrasurgical microscopeintegrated spectral domain optical coherence Tomography-Assisted membrane peeling. Retina 2015, 35, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, E.; Philippakis, E.; Dupas, B.; Nguyen-Kim, P.; Tadayoni, R.; Couturier, A. Benefit of intraoperative optical coherence tomography for vitreomacular surgery in highly myopic eyes. Retina 2018, 38, 2035–2044. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Khan, M.; Petkovsek, D.; Stiegel, L.; Kaiser, P.K.; Singh, R.P.; Reese, J.L.; Srivastava, S.K. Outcomes of Intraoperative OCT–Assisted Epiretinal Membrane Surgery from the PIONEER Study. Ophthalmol. Retin. 2018, 2, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.R.; Srivastava, S.K.; Le, T.K.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study: Impact and Outcomes. Ophthalmol. Retin. 2020, 4, 378–383. [Google Scholar] [CrossRef]
- Abraham, J.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT Features and Postoperative Ellipsoid Mapping in Primary Macula-Involving Retinal Detachments from the PIONEER Study. Ophthalmol. Retin. 2018, 3, 252–257. [Google Scholar] [CrossRef]
- Heindl, L.M.; Siebelmann, S.; Dietlein, T.; Hüttmann, G.; Lankenau, E.; Cursiefen, C.; Steven, P. Future Prospects: Assessment of Intraoperative Optical Coherence Tomography in Ab Interno Glaucoma Surgery. Curr. Eye Res. 2014, 40, 1288–1291. [Google Scholar] [CrossRef]
- Zaldivar, R.; Zaldivar, R.; Adamek, P.; Cerviño, A. Intraoperative adjustment of implantable collamer lens vault by lens rotation aided by intraoperative OCT. J. Cataract. Refract. Surg. 2022, 48, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Milan, S.; Tognetto, D.; Rejdak, R.; Costagliola, C.; Zweifel, S.A.; Posarelli, C.; Figus, M.; Rejdak, M.; Avitabile, T.; et al. Intraoperative Anterior Segment Optical Coherence Tomography in the Management of Cataract Surgery: State of the Art. J. Clin. Med. 2022, 11, 3867. [Google Scholar] [CrossRef] [PubMed]
- Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Gambini, G.; Caporossi, T.; De Vico, U.; Mosca, L.; Guccione, L.; Baldascino, A.; Rizzo, C.; et al. An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer? Diagnostics 2022, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Hotta, F.; Kusaka, S.; Shimomura, Y. Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: A Literature Review and Proposal of Novel Applications. J. Ophthalmol. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.-V.; Aiello, F.; Day, A.C.; Allan, B.D. Deep Anterior Lamellar Keratoplasty for Keratoconus: Multisurgeon Results. Am. J. Ophthalmol. 2019, 201, 54–62. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.-V.; Aiello, F.; Day, A.C.; Gore, D.M.; Allan, B.D. Femtosecond Laser–Assisted Deep Anterior Lamellar Keratoplasty for Keratoconus: Multi-surgeon Results. Am. J. Ophthalmol. 2020, 220, 191–202. [Google Scholar] [CrossRef]
- Han, D.C.; Mehta, J.S.; Por, Y.M.; Htoon, H.M.; Tan, D.T. Comparison of Outcomes of Lamellar Keratoplasty and Penetrating Keratoplasty in Keratoconus. Am. J. Ophthalmol. 2009, 148, 744–751.e1. [Google Scholar] [CrossRef]
- Riss, S.; Heindl, L.M.; Bachmann, B.O.; Kruse, F.E.; Cursiefen, C. Pentacam-Based Big Bubble Deep Anterior Lamellar Keratoplasty in Patients with Keratoconus. Cornea 2012, 31, 627–632. [Google Scholar] [CrossRef]
- Santorum, P.; Yu, A.C.; Bertelli, E.; Busin, M. Microscope-Integrated Intraoperative Optical Coherence Tomography–Guided Big-Bubble Deep Anterior Lamellar Keratoplasty. Cornea 2021, 41, 125–129. [Google Scholar] [CrossRef]
- Titiyal, J.S.; Kaur, M.; Nair, S.; Sharma, N. Intraoperative optical coherence tomography in anterior segment surgery. Surv. Ophthalmol. 2020, 66, 308–326. [Google Scholar] [CrossRef]
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior Segment Optical Coherence Tomography–Guided Big-Bubble Technique. Ophthalmology 2013, 120, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Myerscough, J.; Friehmann, A.; Busin, M.; Goor, D. Successful Visualization of a Big Bubble during Deep Anterior Lamellar Keratoplasty using Intraoperative OCT. Ophthalmology 2019, 126, 1062. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.P.; Goshe, J.; Dupps, W.; Kaiser, P.; Singh, R.P.; Gans, R.; Eisengart, J.; Srivastava, S.K. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015, 133, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Altaan, S.L.; Termote, K.; Elalfy, M.S.; Hogan, E.; Werkmeister, R.; Schmetterer, L.; Holland, S.; Dua, H.S. Optical coherence tomography characteristics of different types of big bubbles seen in deep anterior lamellar keratoplasty by the big bubble technique. Eye 2016, 30, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Le Blanc, C.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huettmann, G.; Cursiefen, C. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). Br. J. Ophthalmol. 2014, 98, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Au, J.; Goshe, J.; Dupps, W.J.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography for Enhanced Depth Visualization in Deep Anterior Lamellar Keratoplasty from the PIONEER Study. Cornea 2015, 34, 1039–1043. [Google Scholar] [CrossRef]
- Muijzer, M.B.; Schellekens, P.A.; Beckers, H.J.M.; de Boer, J.H.; Imhof, S.M.; Wisse, R.P.L. Clinical applications for intraoperative optical coherence tomography: A systematic review. Eye 2021, 36, 379–391. [Google Scholar] [CrossRef]
- Eguchi, H.; Kusaka, S.; Arimura-Koike, E.; Tachibana, K.; Tsujioka, D.; Fukuda, M.; Shimomura, Y. Intraoperative optical coherence tomography (RESCAN®700) for detecting iris incarceration and iridocorneal adhesion during keratoplasty. Int. Ophthalmol. 2016, 37, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Aron, N.; Kakkar, P.; Titiyal, J.S. Continuous intraoperative OCT guided management of post-deep anterior lamellar keratoplasty descemet’s membrane detachment. Saudi J. Ophthalmol. 2016, 30, 133–136. [Google Scholar] [CrossRef]
- Busin, M.; Albè, E. Does thickness matter: Ultrathin Descemet stripping automated endothelial keratoplasty. Curr. Opin. Ophthalmol. 2014, 25, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Bahar, I.; Kaiserman, I.; Levinger, E.; Sansanayudh, W.; Slomovic, A.R.; Rootman, D.S. Retrospective Contralateral Study Comparing Descemet Stripping Automated Endothelial Keratoplasty with Penetrating Keratoplasty. Cornea 2009, 28, 485–488. [Google Scholar] [CrossRef]
- Fenech, M.T.; Coco, G.; Pagano, L.; Gadhvi, K.A.; Titley, M.; Levis, H.J.; Parekh, M.; Kaye, S.B.; Romano, V. Thinning rate over 24 months in ultrathin DSAEK. Eye 2022, 37, 655–659. [Google Scholar] [CrossRef]
- Romano, V.; Parekh, M.; Virgili, G.; Coco, G.; Leon, P.; Islein, K.; Ponzin, D.; Ferrari, S.; Fasolo, A.; Yu, A.C.; et al. Gender Matching Did Not Affect 2-year Rejection or Failure Rates Following DSAEK for Fuchs Endothelial Corneal Dystrophy. Am. J. Ophthalmol. 2021, 235, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Price, F.W. Intraoperative Optical Coherence Tomography: Game-Changing Technology. Cornea 2020, 40, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Steger, B.; Myneni, J.; Batterbury, M.; Willoughby, C.E.; Kaye, S.B. Preparation of ultrathin grafts for Descemet-stripping endothelial keratoplasty with a single microkeratome pass. J. Cataract. Refract. Surg. 2017, 43, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Pagano, L.; Gadhvi, K.A.; Coco, G.; Titley, M.; Fenech, M.T.; Ferrari, S.; Levis, H.J.; Parekh, M.; Kaye, S. Clinical outcomes of pre-loaded ultra-thin DSAEK and pre-loaded DMEK. BMJ Open Ophthalmol. 2020, 5, e000546. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Yokogawa, H.; Mori, N.; Sugiyama, K. Visualization of precut DSAEK and pre-stripped DMEK donor corneas by intraoperative optical coherence tomography using the RESCAN 700. BMC Ophthalmol. 2016, 16, 135. [Google Scholar] [CrossRef]
- Agarwal, R.; Shakarwal, C.; Sharma, N.; Titiyal, J. Intraoperative optical coherence tomography-guided donor corneal tissue assessment and preparation. Indian J. Ophthalmol. 2022, 70, 3496. [Google Scholar] [CrossRef]
- Ruzza, A.; Parekh, M.; Avoni, L.; Wojcik, G.; Ferrari, S.; Desneux, L.; Ponzin, D.; Levis, H.J.; Romano, V. Ultra-thin DSAEK using an innovative artificial anterior chamber pressuriser: A proof-of-concept study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1871–1877. [Google Scholar] [CrossRef]
- Pagano, L.; Gadhvi, K.A.; Parekh, M.; Coco, G.; Levis, H.J.; Ponzin, D.; Ferrari, S.; Virgili, G.; Kaye, S.B.; Edwards, R.T.; et al. Cost analysis of eye bank versus surgeon prepared endothelial grafts. BMC Health Serv. Res. 2021, 21, 801. [Google Scholar] [CrossRef]
- Romano, V.; Tey, A.; Hill, N.M.E.; Ahmad, S.; Britten, C.; Batterbury, M.; Willoughby, C.; Kaye, S.B. Influence of graft size on graft survival following Descemet stripping automated endothelial keratoplasty. Br. J. Ophthalmol. 2015, 99, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, N.D.; Shieh, C.; Carrasco-Zevallos, O.; Keller, B.; Izatt, J.A.; Toth, C.A.; Kuo, A.N. Real-Time Microscope-Integrated OCT to Improve Visualization in DSAEK for Advanced Bullous Keratopathy. Cornea 2015, 34, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Steverink, J.G.; Wisse, R.P.L. Intraoperative optical coherence tomography in descemet stripping automated endothelial keratoplasty: Pilot experiences. Int. Ophthalmol. 2016, 37, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Shazly, T.A.; To, L.K.; Conner, I.P.; Espandar, L. Intraoperative Optical Coherence Tomography-Assisted Descemet Stripping Automated Endothelial Keratoplasty for Anterior Chamber Fibrous Ingrowth. Cornea 2017, 36, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Nowinska, A.; Wylegala, E.; Wroblewska-Czajka, E.; Janiszewska, D. Donor disc attachment assessment with intraoperative spectral optical coherence tomography during descemet stripping automated endothelial keratoplasty. Indian J. Ophthalmol. 2013, 61, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Juthani, V.V.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Association Between Transient Interface Fluid on Intraoperative OCT and Textural Interface Opacity After DSAEK Surgery in the PIONEER Study. Cornea 2014, 33, 887–892. [Google Scholar] [CrossRef]
- Hallahan, K.M.; Cost, B.; Goshe, J.M.; Dupps, W.J.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Interface Fluid Dynamics and Clinical Outcomes for Intraoperative Optical Coherence Tomography–Assisted Descemet Stripping Automated Endothelial Keratoplasty From the PIONEER Study. Am. J. Ophthalmol. 2016, 173, 16–22. [Google Scholar] [CrossRef]
- Yokogawa, H.; Kobayashi, A.; Mori, N.; Nishino, T.; Nozaki, H.; Sugiyama, K. Intraoperative optical coherence tomography-guided nanothin Descemet stripping automated endothelial keratoplasty in a patient with a remarkably thickened cornea. Am. J. Ophthalmol. Case Rep. 2022, 25, 101414. [Google Scholar] [CrossRef]
- Kurji, K.H.; Cheung, A.Y.; Eslani, M.; Rolfes, E.J.; Chachare, D.Y.; Auteri, N.J.; Nordlund, M.L.; Holland, E.J. Comparison of Visual Acuity Outcomes Between Nanothin Descemet Stripping Automated Endothelial Keratoplasty and Descemet Membrane Endothelial Keratoplasty. Cornea 2018, 37, 1226–1231. [Google Scholar] [CrossRef]
- Parekh, M.M.; Ruzza, A.B.; Romano, V.; Favaro, E.B.; Baruzzo, M.B.; Salvalaio, G.R.; Grassetto, A.M.; Ferrari, S.; Ponzin, D. Descemet Membrane Endothelial Keratoplasty Learning Curve for Graft Preparation in an Eye Bank Using 645 Donor Corneas. Cornea 2018, 37, 767–771. [Google Scholar] [CrossRef]
- Moramarco, A.; Romano, V.; Modugno, R.L.; Coco, G.; Viola, P.; Fontana, L.M. Yogurt Technique for Descemet Membrane Endothelial Keratoplasty Graft Preparation: Early Clinical Outcomes. Cornea 2022, 42, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; de Lossada, C.R.; Parekh, M.; Gadhvi, K.; Bonzano, C.; Romano, V.; Levis, H.J.; Tzamalis, A.; Steger, B.; Rechichi, M.; et al. Tips, Tricks, and Guides in Descemet Membrane Endothelial Keratoplasty Learning Curve. J. Ophthalmol. 2021, 2021, 1819454. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Le Blanc, C.; Velten, K.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Hüttmann, G.; Cursiefen, C. Optimizing Descemet Membrane Endothelial Keratoplasty Using Intraoperative Optical Coherence Tomography. JAMA Ophthalmol. 2013, 131, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sahay, P.; Maharana, P.K.; Kumar, P.; Ahsan, S.; Titiyal, J.S. Microscope Integrated Intraoperative Optical Coherence Tomography-Guided DMEK in Corneas with Poor Visualization. Clin. Ophthalmol. 2020, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Goshe, J.M.; Srivastava, S.K.; Ehlers, J.P. Intraoperative Optical Coherence Tomography–Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study: First 100 Cases. Am. J. Ophthalmol. 2019, 210, 167–173. [Google Scholar] [CrossRef]
- Muijzer, M.B.; Soeters, N.; Godefrooij, D.A.; van Luijk, C.M.; Wisse, R.P.L. Intraoperative Optical Coherence Tomography–Assisted Descemet Membrane Endothelial Keratoplasty: Toward More Efficient, Safer Surgery. Cornea 2020, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Cost, B.; Goshe, J.M.; Srivastava, S.; Ehlers, J.P. Intraoperative Optical Coherence Tomography–Assisted Descemet Membrane Endothelial Keratoplasty in the DISCOVER Study. Am. J. Ophthalmol. 2015, 160, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Muijzer, M.B.; Heslinga, F.G.; Couwenberg, F.; Noordmans, H.-J.; Oahalou, A.; Pluim, J.P.W.; Veta, M.; Wisse, R.P.L. Automatic evaluation of graft orientation during Descemet membrane endothelial keratoplasty using intraoperative OCT. Biomed. Opt. Express 2022, 13, 2683. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Myerscough, J.; Spena, R.; Fusco, F.; Socea, S.; Furiosi, L.; De Rosa, L.; Bovone, C.; Busin, M. Three-Year Outcomes of Tri-Folded Endothelium-In Descemet Membrane Endothelial Keratoplasty with Pull-Through Technique. Am. J. Ophthalmol. 2020, 219, 121–131. [Google Scholar] [CrossRef]
- Price, M.; Lisek, M.; Kelley, M.; Feng, M.T.; Price, F.W. Endothelium-in Versus Endothelium-out Insertion with Descemet Membrane Endothelial Keratoplasty. Cornea 2018, 37, 1098–1101. [Google Scholar] [CrossRef]
- Parekh, M.; Ruzza, A.; Ferrari, S.; Ahmad, S.; Kaye, S.; Ponzin, D.; Romano, V. Endothelium-in versus endothelium-out for Descemet membrane endothelial keratoplasty graft preparation and implantation. Acta Ophthalmol. 2016, 95, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Parekh, M.; Ruzza, A.; Willoughby, C.; Ferrari, S.; Ponzin, D.; Kaye, S.B.; Levis, H.J. Comparison of preservation and transportation protocols for preloaded Descemet membrane endothelial keratoplasty. Br. J. Ophthalmol. 2017, 102, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Tzamalis, A.; Vinciguerra, R.; Romano, V.; Arbabi, E.; Borroni, D.; Wojcik, G.; Ferrari, S.; Ziakas, N.; Kaye, S. The “Yogurt” Technique for Descemet Membrane Endothelial Keratoplasty Graft Preparation: A Novel Quick and Safe Method for Both Inexperienced and Senior Surgeons. Cornea 2020, 39, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Menassa, N.; Pagano, L.; Gadhvi, K.A.; Coco, G.; Kaye, S.B.; Levis, H.J.; Romano, V. Free-Floating DMEK in the Host Anterior Chamber: Surgical Management. Cornea 2020, 39, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.J.; Heinzelmann, S.; Böhringer, D.; Reinhard, T.; Maier, P. Indications for intraoperative anterior segment optical coherence tomography in corneal surgery. Int. Ophthalmol. 2020, 40, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Guilbert, E.; Grise-Dulac, A.; Sabatier, P.; Gatinel, D. Intraoperative OCT-Assisted DMEK: 14 Consecutive Cases. Cornea 2015, 34, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Famery, N.; Abdelmassih, Y.; El-Khoury, S.; Guindolet, D.; Cochereau, I.; Gabison, E.E. Artificial chamber and 3D printed iris: A new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2018, 97, e179–e183. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, A. The Different Techniques of Corneal Grafting and Their Indications. Am. J. Ophthalmol. 1955, 39, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Stocker, F.W. A new Technique for Corneal Mushroom Grafts. Am. J. Ophthalmol. 1959, 48, 27–30. [Google Scholar] [CrossRef]
- Busin, M.; Madi, S.; Scorcia, V.; Santorum, P.; Nahum, Y. A Two-Piece Microkeratome-Assisted Mushroom Keratoplasty Improves the Outcomes and Survival of Grafts Performed in Eyes with Diseased Stroma and Healthy Endothelium (An American Ophthalmological Society Thesis). Am. J. Ophthalmol. 2015, 113, T1. [Google Scholar]
- Yu, A.C.; Spena, R.; Fusco, F.; Dondi, R.; Myerscough, J.; Fabbri, F.; Bovone, C.; Busin, M. Long-Term Outcomes of Two-Piece Mushroom Keratoplasty for Traumatic Corneal Scars. Am. J. Ophthalmol. 2021, 236, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Saelens, I.E.Y.; Bartels, M.C.; Van Rij, G. Manual trephination of mushroom keratoplasty in advanced keratoconus. Cornea 2008, 27, 650–655. [Google Scholar] [CrossRef]
- Scorcia, V.; Busin, M. Survival of Mushroom Keratoplasty Performed in Corneas with Postinfectious Vascularized Scars. Am. J. Ophthalmol. 2012, 153, 44–50.e1. [Google Scholar] [CrossRef] [PubMed]
- Levinger, E.; Trivizki, O.; Levinger, S.; Kremer, I. Outcome of “Mushroom” Pattern Femtosecond Laser–Assisted Keratoplasty Versus Conventional Penetrating Keratoplasty in Patients with Keratoconus. Cornea 2014, 33, 481–485. [Google Scholar] [CrossRef]
- Busin, M.; Arffa, R.C. Microkeratome-assisted Mushroom Keratoplasty with Minimal Endothelial Replacement. Am. J. Ophthalmol. 2005, 140, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Canovetti, A.; Rossi, F.; Rossi, M.; Menabuoni, L.; Malandrini, A.; Pini, R.; Ferrara, P. Anvil-profiled penetrating keratoplasty: Load resistance evaluation. Biomech. Model. Mechanobiol. 2018, 18, 319–325. [Google Scholar] [CrossRef]
- Lee, H.P.; Zhuang, H. Biomechanical study on the edge shapes for penetrating keratoplasty. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 1071–1079. [Google Scholar] [CrossRef]
- Urkude, J.; Titiyal, J.S.; Sharma, N. Intraoperative Optical Coherence Tomography–Guided Management of Cap–Lenticule Adhesion During SMILE. J. Refract. Surg. 2017, 33, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Lally, D.R. High Resolution Spectral Domain Optical Coherence Tomography of Multiple Evanescent White Dot Syndrome. Retin. Cases Brief Rep. 2021. [Google Scholar] [CrossRef]
- Carrasco-Zevallos, O.M.; Viehland, C.; Keller, B.; Draelos, M.; Kuo, A.N.; Toth, C.A.; Izatt, J.A. Review of intraoperative optical coherence tomography: Technology and applications [Invited]. Biomed. Opt. Express 2017, 8, 1607–1637. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.; Carrasco-Zevallos, O.; Cunefare, D.; Migacz, J.; Farsiu, S.; Izatt, J.A.; Toth, C.A. Intrasurgical Human Retinal Imaging with Manual Instrument Tracking Using a Microscope-Integrated Spectral-Domain Optical Coherence Tomography Device. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Francone, A.A.; Gerber, M.J.; Lee, Y.-H.; Govetto, A.; Tsao, T.-C.; Hubschman, J.-P. Semiautomated optical coherence tomography-guided robotic surgery for porcine lens removal. J. Cataract. Refract. Surg. 2019, 45, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moramarco, A.; di Geronimo, N.; Airaldi, M.; Gardini, L.; Semeraro, F.; Iannetta, D.; Romano, V.; Fontana, L. Intraoperative OCT for Lamellar Corneal Surgery: A User Guide. J. Clin. Med. 2023, 12, 3048. https://doi.org/10.3390/jcm12093048
Moramarco A, di Geronimo N, Airaldi M, Gardini L, Semeraro F, Iannetta D, Romano V, Fontana L. Intraoperative OCT for Lamellar Corneal Surgery: A User Guide. Journal of Clinical Medicine. 2023; 12(9):3048. https://doi.org/10.3390/jcm12093048
Chicago/Turabian StyleMoramarco, Antonio, Natalie di Geronimo, Matteo Airaldi, Lorenzo Gardini, Francesco Semeraro, Danilo Iannetta, Vito Romano, and Luigi Fontana. 2023. "Intraoperative OCT for Lamellar Corneal Surgery: A User Guide" Journal of Clinical Medicine 12, no. 9: 3048. https://doi.org/10.3390/jcm12093048





