Cardiometabolic Index (CMI) and Visceral Adiposity Index (VAI) Highlight a Higher Risk of Metabolic Syndrome in Women with Severe Obesity
Abstract
:1. Introduction
2. Study Design and Methods
2.1. Subjects
2.2. Measurements
2.2.1. Physical Characteristics and Body Composition
2.2.2. Blood Pressure Measurements
2.3. Laboratory Analyses
2.4. Anthropometric Indices
2.5. Statistical Analyses
3. Results
3.1. Physical Characteristics of Subjects
3.2. Correlations
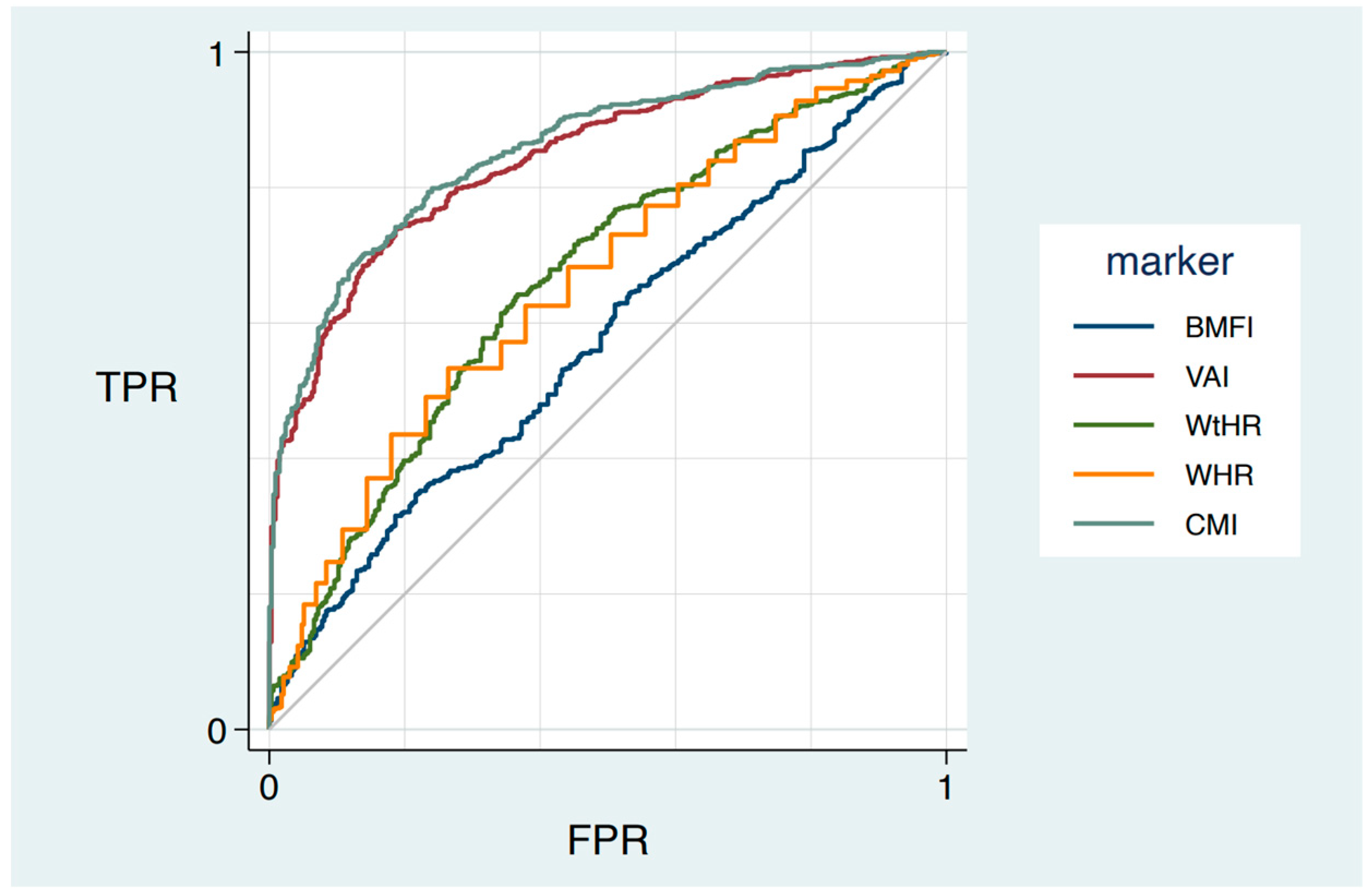
3.3. ROC Curve of Anthropometric Indexes to Predict MetS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, Y.; Qin, B.; Poti, J.; Sokol, R.; Gordon-Larsen, P. Epidemiology of Obesity in Adults: Latest Trends. Curr. Obes. Rep. 2018, 7, 276–288. [Google Scholar] [CrossRef]
- Wang, H.; Liu, A.; Zhao, T.; Gong, X.; Pang, T.; Zhou, Y.; Xiao, Y.; Yan, Y.; Fan, C.; Teng, W.; et al. Comparison of Anthropometric Indices for Predicting the Risk of Metabolic Syndrome and Its Components in Chinese Adults: A Prospective, Longitudinal Study. BMJ Open 2017, 7, e016062. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; And International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and Gender-Related Prevalence, Cardiovascular Risk and Therapeutic Approach in Metabolic Syndrome: A Review of the Literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Chedraui, P.; Pérez-López, F.R. Metabolic Syndrome during Female Midlife: What Are the Risks? Climacteric 2019, 22, 127–132. [Google Scholar] [CrossRef]
- Stefanescu, A.; Revilla, L.; Lopez, T.; Sanchez, S.E.; Williams, M.A.; Gelaye, B. Using A Body Shape Index (ABSI) and Body Roundness Index (BRI) to Predict Risk of Metabolic Syndrome in Peruvian Adults. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Casanueva, F.; Haluzik, M.; van Hulsteijn, L.; Ledoux, S.; Monteiro, M.P.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guideline: Endocrine Work-up in Obesity. Eur. J. Endocrinol. 2020, 182, G1–G32. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Yoon, J.W.; Ahn, H.Y.; Kim, S.Y.; Lee, K.H.; Shin, H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Android Fat Depot Is More Closely Associated with Metabolic Syndrome than Abdominal Visceral Fat in Elderly People. PLoS ONE 2011, 6, e27694. [Google Scholar] [CrossRef]
- Xu, H.; Jin, C.; Guan, Q. Causal Effects of Overall and Abdominal Obesity on Insulin Resistance and the Risk of Type 2 Diabetes Mellitus: A Two-Sample Mendelian Randomization Study. Front. Genet. 2020, 11, 603. [Google Scholar] [CrossRef]
- Bener, A.; Yousafzai, M.T.; Darwish, S.; Al-Hamaq, A.O.A.A.; Nasralla, E.A.; Abdul-Ghani, M. Obesity Index That Better Predict Metabolic Syndrome: Body Mass Index, Waist Circumference, Waist Hip Ratio, or Waist Height Ratio. J. Obes. 2013, 2013, 269038. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A Systematic Review of Waist-to-Height Ratio as a Screening Tool for the Prediction of Cardiovascular Disease and Diabetes: 05 Could Be a Suitable Global Boundary Value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Sartorio, A. Indexes of Adiposity and Body Composition in the Prediction of Metabolic Syndrome in Obese Children and Adolescents: Which Is the Best? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1189–1196. [Google Scholar] [CrossRef]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Tamini, S.; Cicolini, S.; Sartorio, A. The Role of Different Indexes of Adiposity and Body Composition for the Identification of Metabolic Syndrome in Women with Obesity. J. Clin. Med. 2021, 10, 1975. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Daimon, T. The “Cardiometabolic Index” as a New Marker Determined by Adiposity and Blood Lipids for Discrimination of Diabetes Mellitus. Clin. Chim. Acta 2015, 438, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, I.; Alkhalidy, H.; Alnaser, K.; Mukattash, T.L.; al Hourani, H.; Alzboun, T.; Orabi, A.; Liu, D. Assessing Metabolic Syndrome Prediction Quality Using Seven Anthropometric Indices among Jordanian Adults: A Cross-Sectional Study. Sci. Rep. 2022, 12, 21043. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Weststrate, J.A.; Seidell, J.C. Body Mass Index as a Measure of Body Fatness: Age- and Sex-Specific Prediction Formulas. Br. J. Nutr. 1991, 65, 105–114. [Google Scholar] [CrossRef]
- Kagawa, M.; Byrne, N.M.; Hills, A.P. Comparison of Body Fat Estimation Using Waist:Height Ratio Using Different “waist” Measurements in Australian Adults. Br. J. Nutr. 2008, 100, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Methods for the Assessment of Human Body Composition: Traditional and New. Am. J. Clin. Nutr. 1987, 46, 537–556. [Google Scholar] [CrossRef]
- Bedogni, G.; Agosti, F.; de Col, A.; Marazzi, N.; Tagliaferri, A.; Sartorio, A. Comparison of Dual-Energy X-Ray Absorptiometry, Air Displacement Plethysmography and Bioelectrical Impedance Analysis for the Assessment of Body Composition in Morbidly Obese Women. Eur. J. Clin. Nutr. 2013, 67, 1129–1132. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Park, H. Metabolic Disorders in Menopause. Metabolites 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat Mass Changes during Menopause: A Metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Gortan Cappellari, G.; Semolic, A.; Ius, M.; Zanetti, M.; Gabrielli, A.; Vinci, P.; Guarnieri, G.; Simon, G. Central Adiposity Markers, Plasma Lipid Profile and Cardiometabolic Risk Prediction in Overweight-Obese Individuals. Clin. Nutr. 2019, 38, 1171–1179. [Google Scholar] [CrossRef]
- Duan, S.; Yang, D.; Xia, H.; Ren, Z.; Chen, J.; Yao, S. Cardiometabolic Index: A New Predictor for Metabolic Associated Fatty Liver Disease in Chinese Adults. Front. Endocrinol. 2022, 13, 1004855. [Google Scholar] [CrossRef]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic Fatty Liver, Steatohepatitis, and the Metabolic Syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Bellarosa, C.; Bedogni, G.; Bianco, A.; Cicolini, S.; Caroli, D.; Tiribelli, C.; Sartorio, A. Association of Serum Bilirubin Level with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study of 1672 Obese Children. J. Clin. Med. 2021, 10, 2812. [Google Scholar] [CrossRef]
- Ismaiel, A.; Jaaouani, A.; Leucuta, D.C.; Popa, S.L.; Dumitrascu, D.L. The Visceral Adiposity Index in Non-Alcoholic Fatty Liver Disease and Liver Fibrosis—Systematic Review and Meta-Analysis. Biomedicines 2021, 9, 1890. [Google Scholar] [CrossRef]
| All | MetS− | MetS+ | p | |
|---|---|---|---|---|
| Number of Subjects | 876 | 334 | 542 | |
| Age (y) | 52.1 ± 13.8 | 47.1 ± 15.2 | 55.2 ± 11.8 | 0.001 |
| Stature (m) | 1.57 ± 0.06 | 1.58 ± 0.08 | 1.57 ± 0.07 | 0.108 |
| Body mass (kg) | 107.8 ± 17.1 | 106.4 ± 15.8 | 108.7 ± 17.7 | 0.341 |
| BMI (kg m−2) | 43.6 ± 6.1 | 42.7 ± 5.1 | 44.2 ± 6.6 | 0.006 |
| WC (cm) | 121.0 ± 12.7 | 116.7 ± 11.8 | 123.6 ± 12.5 | 0.001 |
| HC (cm) | 133.0 ± 13.1 | 132.5 ± 11.5 | 133.1 ± 14.0 | 0.982 |
| Fat-free mass (kg) | 52.2 ± 4.8 | 51.6 ± 4.3 | 52.6 ± 5.0 | 0.021 |
| Fat mass (kg) | 55.6 ± 14.2 | 54.8 ± 13.3 | 56.1 ± 14.7 | 0.761 |
| Fat-free mass (%) | 49.1 ± 5.5 | 49.2 ± 5.4 | 49.1 ± 5.5 | 0.967 |
| Fat mass (%) | 50.9 ± 5.5 | 50.9 ± 5.6 | 51.0 ± 5.5 | 0.805 |
| SBP (mm Hg−1) | 127.4 ± 14.3 | 124.0 ± 14.3 | 130.0 ± 13.8 | 0.001 |
| DBP (mm Hg−1) | 76.8 ± 8.0 | 75.8 ± 8.0 | 77.3 ± 7.8 | 0.006 |
| TG (mg dL−1) | 131.6 ± 63.4 | 96.4 ± 31.0 | 153.3 ± 68.4 | 0.001 |
| HDL-C (mg dL−1) | 50.1 ± 12.4 | 56.7 ± 12.0 | 46.1 ± 11.0 | 0.001 |
| Glycemia (mmol L−1) | 97.0 ± 30.5 | 82.2 ± 10.7 | 106.0 ± 35.0 | 0.001 |
| WHR | 0.91 ± 0.08 | 0.88 ± 0.07 | 0.93 ± 0.08 | 0.001 |
| WtHR | 0.77 ± 0.08 | 0.74 ± 0.07 | 0.79 ± 0.08 | 0.001 |
| BMFI (kg/m) | 27.6 ± 9.2 | 26.0 ± 7.7 | 28.7 ± 9.8 | 0.001 |
| VAI (cm2) | 2.44 ± 1.64 | 1.46 ± 0.61 | 3.04 ± 1.79 | 0.001 |
| CMI | 0.98 ± 0.65 | 0.58 ± 0.24 | 1.23 ± 0.70 | 0.001 |
| SBP (mm Hg−1) | DBP (mm Hg−1) | HDL-C (mg dL−1) | Glycemia (mg dL−1) | TG (mg dL−1) | |
|---|---|---|---|---|---|
| WHR | R2 = 0.014 | R2 = 0.001 | R2 = 0.031 | R2 = 0.064 | R2 = 0.050 |
| p = 0.001 | p = 0.253 | p = 0.001 | p = 0.001 | p = 0.001 | |
| WtHR | R2 = 0.065 | R2 = 0.038 | R2 = 0.011 | R2 = 0.050 | R2 = 0.008 |
| p = 0.001 | p = 0.253 | p = 0.002 | p = 0.001 | p = 0.007 | |
| BMFI (kg m−1) | R2 = 0.040 | R2 = 0.042 | R2 = 0.006 | R2 = 0.006 | R2 = 0.001 |
| p = 0.001 | p = 0.001 | p = 0.030 | p = 0.026 | p = 0.821 | |
| VAI (cm2) | R2 = 0.001 | R2 = 0.002 | R2 = 0.333 | R2 = 0.084 | R2 = 0.827 |
| p = 0.887 | p = 0.631 | p = 0.001 | p = 0.001 | p = 0.001 | |
| CMI | R2 = 0.001 | R2 = 0.001 | R2 = 0.342 | R2 = 0.094 | R2 = 0.812 |
| p = 0.246 | p = 0.628 | p = 0.001 | p = 0.001 | p = 0.001 |
| ROC Area | 95% Confidence Interval | |
|---|---|---|
| WHR | 0.68 ° | (0.64–0.71) |
| WtHR | 0.67 ° | (0.63–0.70) |
| BMFI (kg m−1) | 0.57 * | (0.54–0.61) |
| VAI (cm2) | 0.84 * | (0.82–0.87) |
| CMI | 0.86 * | (0.83–0.88) |
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | Youden Index | |
|---|---|---|---|---|---|---|---|
| WHR | 57.2% | 65.8% | 73.1% | 48.6% | 1.67 | 0.62 | 0.27 |
| WtHR | 64.2% | 63.1% | 74.0% | 52% | 1.74 | 0.57 | 0.28 |
| BMFI (kg m−1) | 34.7% | 78.1% | 72% | 42.3% | 1.58 | 0.84 | 0.13 |
| VAI (cm2) | 73.8% | 81.4% | 86.6% | 65.6% | 3.96 | 0.32 | 0.55 |
| CMI | 68.6% | 87.7% | 90.1% | 63.2% | 5.57 | 0.36 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzer, S.; D’Alleva, M.; Isola, M.; De Martino, M.; Caroli, D.; Bondesan, A.; Marra, A.; Sartorio, A. Cardiometabolic Index (CMI) and Visceral Adiposity Index (VAI) Highlight a Higher Risk of Metabolic Syndrome in Women with Severe Obesity. J. Clin. Med. 2023, 12, 3055. https://doi.org/10.3390/jcm12093055
Lazzer S, D’Alleva M, Isola M, De Martino M, Caroli D, Bondesan A, Marra A, Sartorio A. Cardiometabolic Index (CMI) and Visceral Adiposity Index (VAI) Highlight a Higher Risk of Metabolic Syndrome in Women with Severe Obesity. Journal of Clinical Medicine. 2023; 12(9):3055. https://doi.org/10.3390/jcm12093055
Chicago/Turabian StyleLazzer, Stefano, Mattia D’Alleva, Miriam Isola, Maria De Martino, Diana Caroli, Adele Bondesan, Alice Marra, and Alessandro Sartorio. 2023. "Cardiometabolic Index (CMI) and Visceral Adiposity Index (VAI) Highlight a Higher Risk of Metabolic Syndrome in Women with Severe Obesity" Journal of Clinical Medicine 12, no. 9: 3055. https://doi.org/10.3390/jcm12093055





