Supine MDS-UPDRS-III Assessment: An Explorative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.3. Measurements
2.4. Video Ratings
2.5. Accelerometry Analysis
2.6. Statistical Analysis
2.6.1. Primary Endpoint
2.6.2. Secondary Endpoints
3. Results
3.1. Primary Endpoint: MDS-UPDRS-III Agreement in Sitting and Supine Positions
3.2. Secondary Endpoints
3.2.1. Inter-Rater Reliability
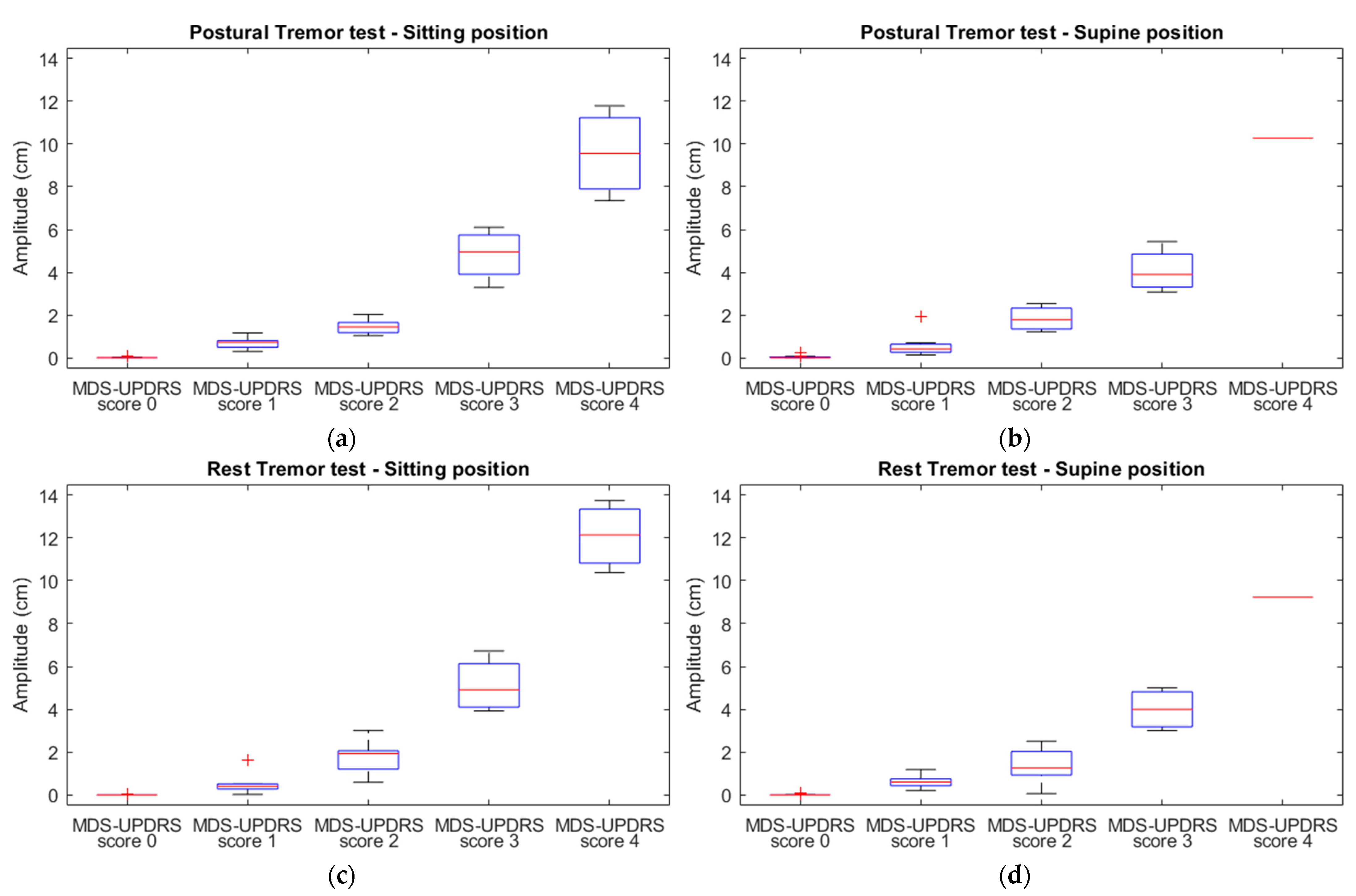
3.2.2. The Relationship between MDS-UPDRS-III Scores and Accelerometric Amplitude
4. Discussion
4.1. Limitations
4.2. Recommendations
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goetz, C.G.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stebbins, G.T.; Stern, M.B.; Tilley, B.C.; Dodel, R.; Dubois, B.; et al. The MDS-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale. Available online: www.movementdisorders.org (accessed on 26 September 2022).
- Lange, S.F.; Kremer, N.I.; van Laar, T.; Lange, F.; Steendam-Oldekamp, T.E.; Oterdoom, D.L.M.; Absalom, A.R.; van Dijk, J.M.C.; Drost, G. The Intraoperative Microlesion Effect Positively Correlates with the Short-Term Clinical Effect of Deep Brain Stimulation in Parkinson’s Disease. Neuromodulation 2021, 26, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Smid, A.; Elting, J.W.J.; van Dijk, J.M.C.; Otten, B.; Oterdoom, D.L.M.; Tamasi, K.; Heida, T.; van Laar, T.; Drost, G. Intraoperative Quantification of MDS-UPDRS Tremor Measurements Using 3D Accelerometry: A Pilot Study. J. Clin. Med. 2022, 11, 2275. [Google Scholar] [CrossRef] [PubMed]
- Krauss, P.; Oertel, M.F.; Baumann-Vogel, H.; Imbach, L.; Baumann, C.R.; Sarnthein, J.; Regli, L.; Stieglitz, L.H. Intraoperative Neurophysiologic Assessment in Deep Brain Stimulation Surgery and Its Impact on Lead Placement. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, N.; Yu, Y.; Li, H.; Wu, F.; Yang, Y.; Lin, J.; Han, J.; Liang, S. Intraoperative Quantitative Measurements for Bradykinesia Evaluation during Deep Brain Stimulation Surgery Using Leap Motion Controller: A Pilot Study. Parkinsons Dis. 2021, 2021, 6639762. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Yu, Y.; Lin, J.; Yang, Y.; Wu, J.; Liang, S.; Wu, J.; Han, J. A Non-Contact System for Intraoperative Quantitative Assessment of Bradykinesia in Deep Brain Stimulation Surgery. Comput. Methods Programs Biomed. 2022, 225, 107005. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M. Interrater Reliability—The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.; Aggarwal, R. Common Pitfalls in Statistical Analysis: Measures of Agreement. Perspect. Clin. Res. 2017, 8, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Elble, R.J.; Pullman, S.L.; Matsumoto, J.Y.; Raethjen, J.; Deuschl, G.; Tintner, R. Tremor Amplitude Is Logarithmically Related to 4- and 5-Point Tremor Rating Scales. Brain 2006, 129, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Shiller, D.M.; Ostry, D.J.; Gribble, P.L. Effects of gravitational load on jaw movements in speech. J. Neurosci. 1999, 19, 9073–9080. [Google Scholar] [CrossRef] [PubMed]
- Post, B.; Merkus, M.P.; de Bie, R.M.A.; de Haan, R.J.; Speelman, J.D. Unified Parkinson’s Disease Rating Scale Motor Examination: Are Ratings of Nurses, Residents in Neurology, and Movement Disorders Specialists Interchangeable? Mov. Disord. 2005, 20, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xiong, W.X.; Liu, F.T.; Sun, Y.M.; Luo, S.; Ding, Z.T.; Wu, J.J.; Wang, J. Objective and Quantitative Assessment of Motor Function in Parkinson’s Disease-from the Perspective of Practical Applications. Ann. Transl. Med. 2016, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Evers, L.J.W.; Krijthe, J.H.; Meinders, M.J.; Bloem, B.R.; Heskes, T.M. Measuring Parkinson’s Disease over Time: The Real-World within-Subject Reliability of the MDS-UPDRS. Mov. Disord. 2019, 34, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Rovini, E.; Maremmani, C.; Cavallo, F. How Wearable Sensors Can Support Parkinson’s Disease Diagnosis and Treatment: A Systematic Review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Xu, Y.; Li, X.; Fan, Y.; Zeng, W.; Tan, Y.; Ren, K.; Chen, W.; Cao, X. Evaluation of Wearable Sensor Devices in Parkinson’s Disease: A Review of Current Status and Future Prospects. Parkinsons Dis. 2020, 2020, 4693019. [Google Scholar] [CrossRef] [PubMed]
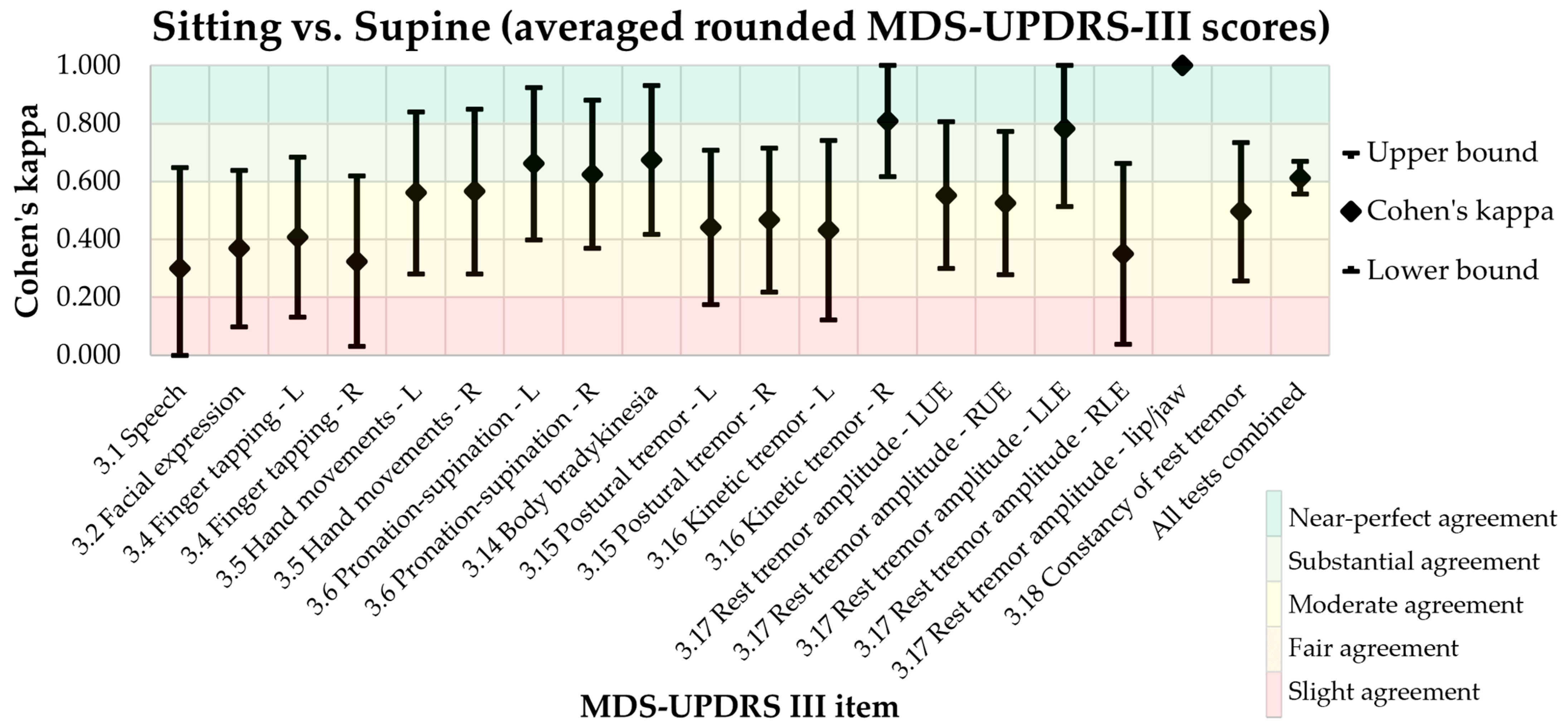
| MDS-UDPRS-III Test | Site | Cohen’s Kappa | 95% CI | p |
|---|---|---|---|---|
| 3.1 Speech | 0.300 | −0.049, 0.649 | 0.073 | |
| 3.2 Facial expression | 0.369 | 0.099, 0.639 | <0.001 | |
| 3.4 Finger tapping | Left | 0.407 | 0.131, 0.683 | <0.001 |
| Right | 0.325 | 0.031, 0.619 | 0.007 | |
| 3.5 Hand movements | Left | 0.561 | 0.281, 0.841 | <0.001 |
| Right | 0.566 | 0.282, 0.850 | <0.001 | |
| 3.6 Pronation/supination | Left | 0.662 | 0.399, 0.925 | <0.001 |
| Right | 0.625 | 0.370, 0.880 | <0.001 | |
| 3.14 Body bradykinesia | 0.674 | 0.417, 0.931 | <0.001 | |
| 3.15 Postural tremor | Left | 0.441 | 0.174, 0.708 | <0.001 |
| Right | 0.467 | 0.218, 0.716 | <0.001 | |
| 3.16 Kinetic tremor | Left | 0.432 | 0.122, 0.742 | <0.001 |
| Right | 0.810 | 0.616, 1.004 | <0.001 | |
| 3.17 Rest tremor amplitude | LUE | 0.553 | 0.300, 0.806 | <0.001 |
| RUE | 0.525 | 0.278, 0.772 | <0.001 | |
| LLE | 0.782 | 0.513, 1.051 | <0.001 | |
| RLE | 0.350 | 0.038, 0.662 | 0.030 | |
| Lip/jaw | 1.000 | 1.000, 1.000 | <0.001 | |
| 3.18 Constancy of rest tremor | 0.496 | 0.257, 0.735 | <0.001 | |
| All tests combined | 0.613 | 0.556, 0.670 | <0.001 |
| MDS-UDPRS-III Test | Site | Cohen’s Kappa | 95% CI | p |
|---|---|---|---|---|
| 3.1 Speech | −0.196 | −0.435, 0.043 | 0.132 | |
| 3.2 Facial expression | 0.228 | −0.021, 0.477 | 0.029 | |
| 3.4 Finger tapping | Left | 0.378 | 0.119, 0.637 | <0.001 |
| Right | 0.346 | 0.091, 0.601 | 0.002 | |
| 3.5 Hand movements | Left | 0.051 | −0.169, 0.271 | 0.598 |
| Right | −0.040 | −0.297, 0.217 | 0.744 | |
| 3.6 Pronation-supination | Left | 0.111 | −0.093, 0.315 | 0.160 |
| Right | 0.122 | −0.117, 0.361 | 0.216 | |
| 3.7 Toe tapping | Left | 0.501 | 0.242, 0.760 | <0.001 |
| Right | 0.141 | −0.071, 0.353 | 0.183 | |
| 3.8 Leg agility | Left | 0.351 | 0.084, 0.618 | 0.002 |
| Right | 0.237 | −0.004, 0.478 | 0.024 | |
| 3.9 Arising from chair | 0.704 | 0.388, 1.020 | <0.001 | |
| 3.10 Gait | 0.473 | 0.154, 0.792 | 0.005 | |
| 3.11 Freezing of gait | 0.000 | 0.000, 0.000 | <0.001 | |
| 3.12 Postural stability | 0.629 | 0.384, 0.874 | <0.001 | |
| 3.13 Posture | 0.385 | 0.124, 0.646 | <0.001 | |
| 3.14 Body bradykinesia | 0.075 | −0.168, 0.318 | 0.462 | |
| 3.15 Postural tremor | Left | 0.550 | 0.291, 0.809 | <0.001 |
| Right | 0.786 | 0.574, 0.998 | <0.001 | |
| 3.16 Kinetic tremor | Left | 0.398 | 0.071, 0.725 | 0.003 |
| Right | 0.339 | 0.092, 0.586 | 0.009 | |
| 3.17 Rest tremor amplitude | LUE | 0.574 | 0.343, 0.805 | <0.001 |
| RUE | 0.742 | 0.526, 0.958 | <0.001 | |
| LLE | 0.429 | 0.100, 0.758 | 0.005 | |
| RLE | −0.045 | −0.112, 0.022 | 0.766 | |
| Lip/jaw | 1.000 | 1.000, 1.000 | <0.001 | |
| 3.18 Constancy of rest tremor | 0.468 | 0.239, 0.697 | <0.001 | |
| All tests combined | 0.431 | 0.382, 0.480 | <0.001 |
| MDS-UDPRS-III Test | Site | Cohen’s Kappa | 95% CI | p |
|---|---|---|---|---|
| 3.1 Speech | −0.135 | −0.284, 0.014 | 0.104 | |
| 3.2 Facial expression | 0.140 | −0.081, 0.361 | 0.140 | |
| 3.4 Finger tapping | Left | 0.371 | 0.102, 0.640 | <0.001 |
| Right | 0.303 | 0.031, 0.575 | 0.008 | |
| 3.5 Hand movements | Left | 0.061 | −0.172, 0.294 | 0.547 |
| Right | 0.168 | −0.079, 0.415 | 0.138 | |
| 3.6 Pronation-supination | Left | 0.212 | 0.000, 0.424 | 0.011 |
| Right | −0.005 | −0.164, 0.154 | 0.954 | |
| 3.14 Body bradykinesia | 0.250 | −0.013, 0.513 | 0.033 | |
| 3.15 Postural tremor | Left | 0.512 | 0.242, 0.782 | <0.001 |
| Right | 0.717 | 0.462, 0.972 | <0.001 | |
| 3.16 Kinetic tremor | Left | 0.129 | −0.138, 0.396 | 0.198 |
| Right | 0.457 | 0.218, 0.696 | <0.001 | |
| 3.17 Rest tremor amplitude | LUE | 0.540 | 0.268, 0.812 | <0.001 |
| RUE | 0.509 | 0.256, 0.762 | <0.001 | |
| LLE | 0.416 | 0.153, 0.679 | 0.003 | |
| RLE | 0.361 | 0.057, 0.665 | 0.013 | |
| Lip/jaw | 1.000 | 1.000, 1.000 | <0.001 | |
| 3.18 Constancy of rest tremor | 0.604 | 0.392, 0.816 | <0.001 | |
| All tests combined | 0.385 | 0.326, 0.444 | <0.001 |
| MDS-UPDRS-III Test | Position | R | R2 * | Coefficient ** | 95% CI | p |
|---|---|---|---|---|---|---|
| Postural tremor | Sitting | 0.900 | 0.811 | 2.662 | 2.271, 3.052 | <0.001 |
| Supine | 0.862 | 0.743 | 2.873 | 2.360, 3.387 | <0.001 | |
| Rest tremor | Sitting | 0.905 | 0.818 | 3.604 | 3.088, 4.121 | <0.001 |
| Supine | 0.844 | 0.712 | 4.213 | 3.399, 5.027 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, N.I.; Smid, A.; Lange, S.F.; Mateus Marçal, I.; Tamasi, K.; van Dijk, J.M.C.; van Laar, T.; Drost, G. Supine MDS-UPDRS-III Assessment: An Explorative Study. J. Clin. Med. 2023, 12, 3108. https://doi.org/10.3390/jcm12093108
Kremer NI, Smid A, Lange SF, Mateus Marçal I, Tamasi K, van Dijk JMC, van Laar T, Drost G. Supine MDS-UPDRS-III Assessment: An Explorative Study. Journal of Clinical Medicine. 2023; 12(9):3108. https://doi.org/10.3390/jcm12093108
Chicago/Turabian StyleKremer, Naomi I., Annemarie Smid, Stèfan F. Lange, Iara Mateus Marçal, Katalin Tamasi, J. Marc C. van Dijk, Teus van Laar, and Gea Drost. 2023. "Supine MDS-UPDRS-III Assessment: An Explorative Study" Journal of Clinical Medicine 12, no. 9: 3108. https://doi.org/10.3390/jcm12093108





