Pelvic Floor Muscle Training versus Functional Magnetic Stimulation for Stress Urinary Incontinence in Women: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Participants
2.4. Outcome Measures
- The resting vaginal pressure (mmHg) was registered when a perineometer was inserted into the vagina without contracting PFM.
- PFM strength was calculated as the mean of three isolated maximal voluntary contractions (mmHg). The patient was asked to contract their pelvic floor muscles to a maximum without holding their breath three times with a 5 s rest between trials.
- PFM endurance was calculated as the mean of three endurance trials (s). Participants were asked to hold an isolated maximal voluntary PFM contraction for as long as they could without holding their breath. The trial was stopped when the squeeze pressure dropped by 2 mm. There was a 10 s rest between the three trials.
2.5. Interventions
2.6. Statistical Analysis
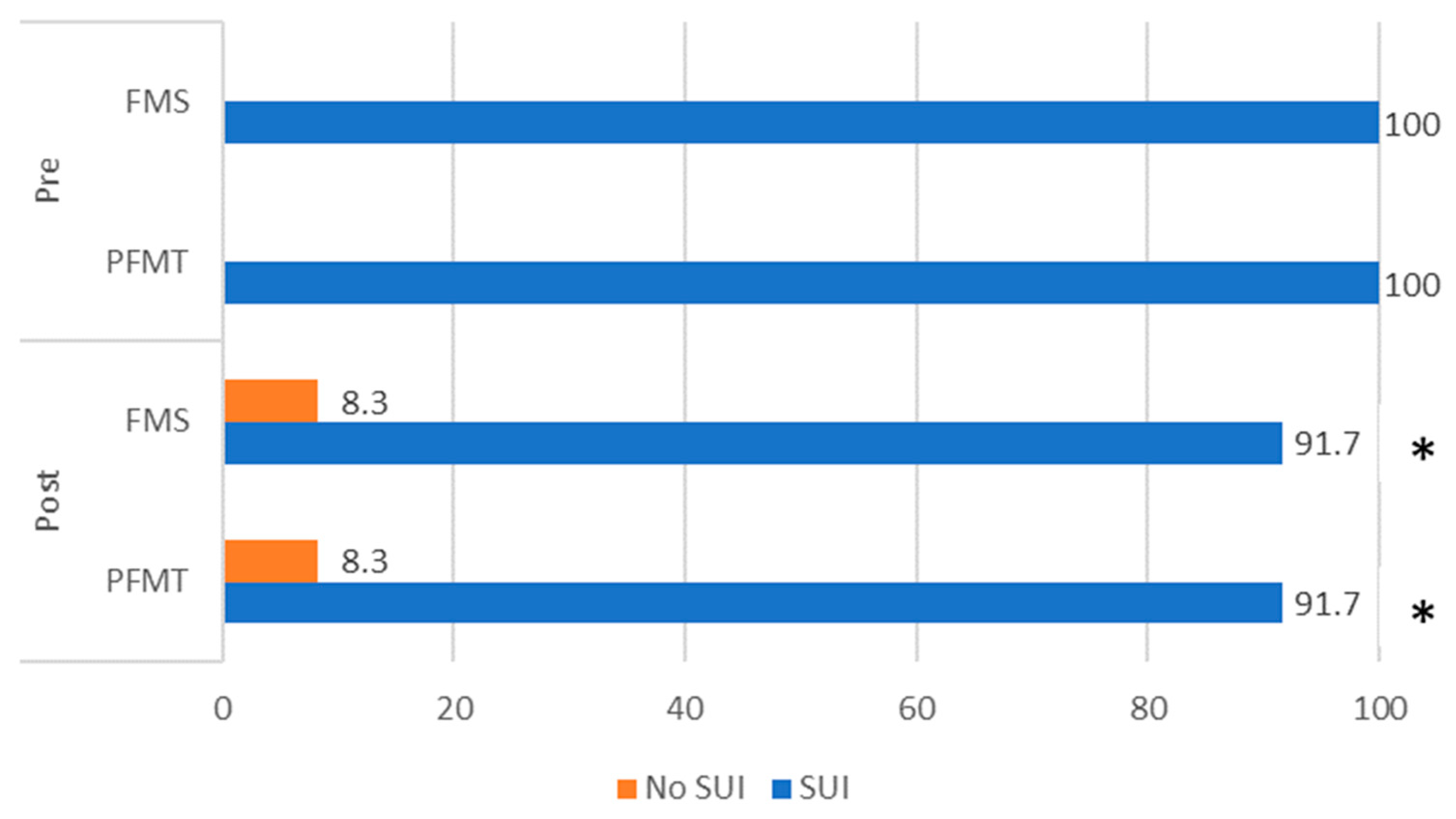
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilçioğlu, K.; Şahin, S.; Özerdoğan, N.; Ünsal, A. Evaluation of urinary incontinence and quality of life in married women aged between 20 and 49 years (Sakarya, Turkey). Turk. J. Med. Sci. 2018, 48, 100–109. [Google Scholar] [CrossRef]
- Goforth, J.; Langaker, M. Urinary incontinence in women. N. C. Med. J. 2016, 77, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Kołomańska-Bogucka, D.; Nowakowski, C.; Tim, S. Urinary incontinence in women: Modern methods of physiotherapy as a support for surgical treatment or independent therapy. J. Clin. Med. 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Moossdorff-Steinhauser, H.F.; Berghmans, B.C.; Spaanderman, M.E.; Bols, E.M. Prevalence, incidence and bothersomeness of urinary incontinence between 6 weeks and 1 year post-partum: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 1675–1693. [Google Scholar] [CrossRef] [PubMed]
- D’Ancona, C.; Haylen, B.; Oelke, M.; Abranches-Monteiro, L.; Arnold, E.; Goldman, H.; Hamid, R.; Homma, Y.; Marcelissen, T.; Rademakers, K.; et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol. Urodyn. 2019, 38, 433–477. [Google Scholar] [CrossRef]
- Milsom, I.; Gyhagen, M. The prevalence of urinary incontinence. Climacteric 2019, 22, 217–222. [Google Scholar] [CrossRef]
- Chisholm, L.; Delpe, S.; Priest, T.; Reynolds, W.S. Physical activity and stress incontinence in women. Curr. Bladder Dysfunct. Rep. 2019, 14, 174–179. [Google Scholar] [CrossRef]
- Khowailed, I.A.; Pinjuv-Turney, J.; Lu, C.; Lee, H. Stress incontinence during different high-impact exercises in women: A pilot survey. Int. J. Environ. Res. Public Health 2020, 17, 8372. [Google Scholar] [CrossRef]
- Turner, C.E.; Young, J.M.; Solomon, M.J.; Ludlow, J.; Benness, C. Incidence and etiology of pelvic floor dysfunction and mode of delivery: An overview. Dis. Colon Rectum 2009, 52, 1186–1195. [Google Scholar] [CrossRef]
- Hilde, G.; Stær-Jensen, J.; Siafarikas, F.; Engh, M.E.; Brækken, I.H.; Bø, K. Impact of childbirth and mode of delivery on vaginal resting pressure and on pelvic floor muscle strength and endurance. Am. J. Obstet. Gynecol. 2013, 208, 50.e1–50.e7. [Google Scholar] [CrossRef]
- Barca, J.A.; Bravo, C.; Pintado-Recarte, M.P.; Asúnsolo, Á.; Cueto-Hernández, I.; Ruiz-Labarta, J.; Buján, J.; Ortega, M.A.; De León-Luis, J.A. Pelvic floor morbidity following vaginal delivery versus cesarean delivery: Systematic review and meta-analysis. J. Clin. Med. 2021, 10, 1652. [Google Scholar] [CrossRef]
- Aoki, Y.; Brown, H.W.; Brubaker, L.; Cornu, J.N.; Daly, J.O.; Cartwright, R. Urinary incontinence in women. Nat. Rev. Dis. Primers 2017, 3, 17041. [Google Scholar] [CrossRef] [PubMed]
- Steibliene, V.; Aniuliene, R.; Aniulis, P.; Raskauskiene, N.; Adomaitiene, V. Affective symptoms and health-related quality of life among women with stress urinary incontinence: Cross-sectional study. Neuropsychiatr. Dis. Treat. 2020, 16, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Krhut, J.; Gärtner, M.; Mokris, J.; Horcicka, L.; Svabik, K.; Zachoval, R.; Martan, A.; Zvara, P. Effect of severity of urinary incontinence on quality of life in women. Neurourol. Urodyn. 2018, 37, 1925–1930. [Google Scholar] [CrossRef]
- Lim, R.; Liong, M.L.; Leong, W.S.; Khan, N.A.K.; Yuen, K.H. Effect of stress urinary incontinence on the sexual function of couples and the quality of life of patients. J. Urol. 2016, 196, 153–158. [Google Scholar] [CrossRef]
- Pizzol, D.; Demurtas, J.; Celotto, S.; Maggi, S.; Smith, L.; Angiolelli, G.; Trott, M.; Yang, L.; Veronese, N. Urinary incontinence and quality of life: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Castronovo, F.; Caccia, G.; Papadia, A.; Regusci, L.; Torella, M.; Salvatore, S.; Scancarello, C.; Ghezzi, F.; Serati, M. Efficacy of 3 Tesla Functional Magnetic Stimulation for the Treatment of Female Urinary Incontinence. J. Clin. Med. 2022, 11, 2805. [Google Scholar] [CrossRef]
- Dumoulin, C.; Cacciari, L.P.; Hay-Smith, E.J.C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst. Rev. 2018, 10, CD005654. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, M.; Azevedo, M.J.; Firmino-Machado, J.; Santos, P.C. Pelvic floor muscle training protocol for stress urinary incontinence in women: A systematic review. Rev. Assoc. Med. Bras. 2017, 63, 642–650. [Google Scholar] [CrossRef]
- Fitz, F.F.; Gimenez, M.M.; de Azevedo Ferreira, L.; Matias, M.M.P.; Bortolini, M.A.T.; Castro, R.A. Pelvic floor muscle training for female stress urinary incontinence: A randomised control trial comparing home and outpatient training. Int. Urogynecol. J. 2020, 31, 989–998. [Google Scholar] [CrossRef]
- Felicíssimo, M.F.; Carneiro, M.M.; Saleme, C.S.; Pinto, R.Z.; da Fonseca, A.M.R.M.; da Silva-Filho, A.L. Intensive supervised versus unsupervised pelvic floor muscle training for the treatment of stress urinary incontinence: A randomized comparative trial. Int. Urogynecol. J. 2010, 21, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Titman, S.C.; Radley, S.C.; Gray, T.G. Self-management in women with stress incontinence: Strategies, outcomes and integration into clinical care. Res. Rep. Urol. 2019, 11, 111. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, D.; Wu, G.; Wang, T.; Wu, J.; Ren, H.; Cui, Y. Efficacy of magnetic stimulation for female stress urinary incontinence: A meta-analysis. Ther. Adv. Urol. 2021, 13, 17562872211032485. [Google Scholar] [CrossRef]
- Bø, K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J. Urol. 2012, 30, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Bø, K.; Herbert, R.D. There is not yet strong evidence that exercise regimens other than pelvic floor muscle training can reduce stress urinary incontinence in women: A systematic review. J. Physiother. 2013, 59, 159–168. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K.; Peng, L.; Lai, J.; Li, H.; Luo, D.; Wang, K. An effective meta-analysis of magnetic stimulation therapy for urinary incontinence. Sci. Rep. 2019, 9, 9077. [Google Scholar] [CrossRef]
- Bo, K.; Frawley, H.C.; Haylen, B.T.; Abramov, Y.; Almeida, F.G.; Berghmans, B.; Bortolini, M.; Dumoulin, C.; Gomes, M.; McClurg, D.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int. Urogynecol. J. 2017, 28, 191–213. [Google Scholar] [CrossRef]
- Lim, R.; Liong, M.L.; Leong, W.S.; Khan, N.A.K.; Yuen, K.H. Effect of pulsed magnetic stimulation on quality of life of female patients with stress urinary incontinence: An IDEAL-D stage 2b study. Int. Urogynecol. J. 2018, 29, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Suzuki, T.; Sato, R.; Kaga, K.; Kaga, M.; Fuse, M. Effects of magnetic stimulation on urodynamic stress incontinence refractory to pelvic floor muscle training in a randomized sham-controlled study. Low. Urin. Tract Symptoms 2019, 11, 61–65. [Google Scholar] [CrossRef]
- Galloway, N.T.M.; El-Galley, R.E.; Sand, P.K.; Appell, R.A.; Russell, H.W.; Carlan, S.J. Extracorporeal magnetic innervation therapy for stress urinary incontinence. Urology 1999, 53, 1108–1111. [Google Scholar] [CrossRef]
- Vadala, M.; Palmieri, B.; Malagoli, A.; Laurino, C. High-power Magnetotherapy: A New Weapon in Urinary Incontinence? Low. Urin. Tract. Symptoms 2018, 10, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Liong, M.L.; Leong, W.S.; Yuen, K.H. Which outcome measures should be used in stress urinary incontinence trials? BJU Int. 2018, 121, 805–810. [Google Scholar] [CrossRef]
- Revicki, D.; Hays, R.D.; Cella, D.; Sloan, J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J. Clin. Epidemiol. 2008, 61, 102–109. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, X.; Shen, H.; Luo, D. Magnetic stimulation for female patients with stress urinary incontinence, a meta-analysis of studies with short-term follow-up. Medicine 2019, 98, e15572. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.; Apostolidis, A.; Birder, L.; Bliss, D.; Brubaker, L.; Cardozo, L.; Castro, D.; O’Connell, P.R.; Cottenden, A.; et al. 6th International Consultation on Incontinence. Recommendations of the International Scientific Committee: Evaluation and treatment of urinary incontinence, pelvic organ prolapse and faecal incontinence. Neurourol. Urodyn. 2018, 37, 2271–2272. [Google Scholar] [CrossRef] [PubMed]
- Hajebrahimi, S.; Corcos, J.; Lemieux, M.C. International consultation on incontinence questionnaire short form: Comparison of physician versus patient completion and immediate and delayed self-administration. Urology 2004, 63, 1076–1078. [Google Scholar] [CrossRef]
- Skorupska, K.; Grzybowska, M.E.; Kubik-Komar, A.; Rechberger, T.; Miotla, P. Identification of the Urogenital Distress Inventory-6 and the Incontinence Impact Questionnaire-7 cutoff scores in urinary incontinent women. Health Qual. Life Outcomes 2021, 19, 87. [Google Scholar] [CrossRef]
- Monticone, M.; Ferriero, G.; Giordano, A.; Foti, C.; Franchignoni, F. Rasch analysis of the Incontinence Impact Questionnaire short version (IIQ-7) in women with urinary incontinence. Int. J. Rehabil. Res. 2020, 43, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, J.S.; Wyman, J.F.; Shumaker, S.A.; McClish, D.K. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol. Urodyn. 1995, 14, 131–139. [Google Scholar] [CrossRef]
- Bø, K.; Næss, K.; Stær-Jensen, J.; Siafarikas, F.; Ellström, E.M.; Hilde, G. Recovery of pelvic floor muscle strength and endurance 6 and 12 months postpartum in primiparous women—A prospective cohort study. Int. Urogynecol. J. 2022, 33, 3455–3464. [Google Scholar] [CrossRef]
- Walton, L.M.; Raigangar, V.; Abraham, M.S.; Buddy, C.; Hernandez, M.; Krivak, G.; Caceras, R. Effects of an 8-week pelvic core stability and nutrition community programme on maternal health outcomes. Physiother. Res. Int. 2019, 24, e1780. [Google Scholar] [CrossRef] [PubMed]
- Monz, B.; Chartier-Kastler, E.; Hampel, C.; Samsioe, G.; Hunskaar, S.; Espuna-Pons, M.; Wagg, A.; Quail, D.; Castro, R.; Chinn, C. Patient characteristics associated with quality of life in European women seeking treatment for urinary incontinence: Results from PURE. Eur. Urol. 2007, 51, 1073–1082. [Google Scholar] [CrossRef]
- Cacciari, L.P.; Dumoulin, C.; Hay-Smith, E.J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: A cochrane systematic review abridged republication. Braz. J. Phys. Ther. 2019, 23, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Bertotto, A.; Schvartzman, R.; Uchôa, S.; Wender, M.C.O. Effect of electromyographic biofeedback as an add-on to pelvic floor muscle exercises on neuromuscular outcomes and quality of life in postmenopausal women with stress urinary incontinence: A randomized controlled trial. Neurourol. Urodyn. 2017, 36, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, E.; Ávila-Gandía, V.; López-Román, J.; Martínez-Rodríguez, A.; Rubio-Arias, J.Á. What pelvic floor muscle training load is optimal in minimizing urine loss in women with stress urinary incontinence? A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 4358. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.P.; Sand, P.K. Electromagnetic pelvic floor stimulation for urinary incontinence and bladder disease. Int. Urogynecol. J. 2001, 12, 401–404. [Google Scholar] [CrossRef]
- Quek, P. A critical review on magnetic stimulation: What is its role in the management of pelvic floor disorders? Curr. Opin. Urol. 2005, 15, 231–235. [Google Scholar] [CrossRef]
- Weber-Rajek, M.; Strączyńska, A.; Strojek, K.; Piekorz, Z.; Pilarska, B.; Podhorecka, M.; Sobieralska-Michalak, K.; Goch, A.; Radzimińska, A. Assessment of the effectiveness of pelvic floor muscle training (PFMT) and extracorporeal magnetic innervation (EXMI) in treatment of stress urinary incontinence in women: A randomized controlled trial. BioMed Res. Int. 2020, 2020, 1019872. [Google Scholar] [CrossRef]
- Sun, M.; Sun, R.; Chen, L. The therapeutic efficiency of extracorporeal magnetic innervation treatment in women with urinary tract dysfunction following radical hysterectomy. J. Obstet. Gynaecol. 2015, 35, 74–78. [Google Scholar] [CrossRef]
- Lukanović, D.; Kunič, T.; Batkoska, M.; Matjašič, M.; Barbič, M. Effectiveness of Magnetic Stimulation in the Treatment of Urinary Incontinence: A Systematic Review and Results of Our Study. J. Clin. Med. 2021, 10, 5210. [Google Scholar] [CrossRef]
- Wójcik, M.; Placek, K.; Goździewicz, T.; Plagens-Rotman, K.; Merks, P.; Mizgier, M.; Luwański, D.; Pisarska-Krawczyk, M.; Kędzia, W.; Jarząbek-Bielecka, G. The application of physiotherapy in urinary incontinence. Clin. Exp. Obstet. Gynecol. 2023, 50, 7. [Google Scholar] [CrossRef]



| PFMT Group (n = 24) Mean ± SD | FMS Group (n = 24) Mean ± SD | p between Groups (Student’s t Test) | |
|---|---|---|---|
| Age (years) | 37.58 ± 5.86 | 40.25 ± 6.49 | 0.142 |
| Weight (kg) | 69.79 ± 8.14 | 74.25 ± 10.64 | 0.062 |
| Height (cm) | 168.00 ± 4.00 | 168.50 ± 5.23 | 0.712 |
| Body mass index (kg/m2) | 23.44 ± 3.01 | 26.13 ± 3.43 | 0.371 |
| Physically active (%) | 66.67% | 75% | 0.535 |
| Frequency of physical activity (days/week) | 2.17 ± 2.28 | 2.50 ± 2.02 | 0.594 |
| Functional Magnetic Stimulation | Frequency of Stimulation | Time | Active Time | Pause Time | Duration of Session | Number of Sessions |
| 35 Hz | 12 s | 6 s | 6 s | 20 min. | 12 sessions | |
| 5 Hz | 12 s | 6 s | 6 s | 10 min | ||
| Pelvic Floor Muscle Training | Step 1 | Step 2 | Duration | Number of Sessions | ||
| Session 1–6 | Session 7–12 | 30 min. | 12 sessions | |||
| 6 exercises | 6 + 5 exercises | |||||
| Measurement | Group | Pre Mean ± SD | Post Mean ± SD | Mean Difference | p Inter- Group |
|---|---|---|---|---|---|
| Total ICIQ-SF score | PFMT | 11.00 ± 3.68 | 6.33 ± 3.07 | 4.67 ± 2.99 ** | 0.509 |
| FMS | 9.17 ± 3.33 | 5.08 ± 2.45 | 4.08 ± 3.08 ** | ||
| Total IIQ-7 score | PFMT | 33.25 ± 23.25 | 10.75 ± 11.54 | 22.50 ± 19.26 ** | 0.699 |
| FMS | 30.42 ± 14.36 | 9.33 ± 3.62 | 20.58 ± 14.57 ** | ||
| Resting vaginal pressure (mmHg) | PFMT | 4.67 ± 1.79 | 6.67 ± 1.27 | 2.00 ± 1.67 ** | 0.089 |
| FMS | 5.58 ± 2.19 | 6.92 ± 1.74 | 1.33 ± 0.87 ** | ||
| PFM strength (mmHg) | PFMT | 11.59 ± 5.72 | 15.36 ± 5.88 | 3.77 ± 4.43 ** | 0.458 |
| FMS | 15.08 ± 7.59 | 17.94 ± 8.32 | 2.86 ± 3.96 * | ||
| PFM endurance (s) | PFMT | 10.54 ± 9.28 | 18.97 ± 15.60 | 8.43 ± 13.55 * | 0.661 |
| FMS | 11.03 ± 11.52 | 17.79 ± 22.33 | 6.76 ± 12.69 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudonienė, V.; Kirklytė, I.; Žlibinaitė, L.; Jerez-Roig, J.; Rutkauskaitė, R. Pelvic Floor Muscle Training versus Functional Magnetic Stimulation for Stress Urinary Incontinence in Women: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3157. https://doi.org/10.3390/jcm12093157
Dudonienė V, Kirklytė I, Žlibinaitė L, Jerez-Roig J, Rutkauskaitė R. Pelvic Floor Muscle Training versus Functional Magnetic Stimulation for Stress Urinary Incontinence in Women: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(9):3157. https://doi.org/10.3390/jcm12093157
Chicago/Turabian StyleDudonienė, Vilma, Indrė Kirklytė, Laura Žlibinaitė, Javier Jerez-Roig, and Renata Rutkauskaitė. 2023. "Pelvic Floor Muscle Training versus Functional Magnetic Stimulation for Stress Urinary Incontinence in Women: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 9: 3157. https://doi.org/10.3390/jcm12093157





