Non-Immersive Virtual Reality Telerehabilitation System Improves Postural Balance in People with Chronic Neurological Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- Between 25 and 70 years of age;
- Stage of disease: mild to moderate as documented by Hoehn and Yahr (H&Y score range between 2 and 3—PD group) or Expanded Disability Status Scale (EDSS score ≤ 6.5—MS group);
- Absence of cognitive impairment measured by the MoCA total score ≥ 18 [44] and sufficient cognitive and linguistic level to understand and comply with study procedures;
- Stabilized drug treatment for at least 3 months before starting this study;
- Absence of moderate and severe dyskinesia and freezing episodes as documented by MDS-UPDRS (PD group);
- No other neurologic conditions different from MS or PD;
- No psychiatric complications or personality disorders, as indicated in the medical documentation;
- Absence of severe primary sensory deficits such as blurring or low vision, severe hearing loss and speech disorder
2.2. Rehabilitation Procedures
2.3. Intervention Group (IG)
2.4. Control Group (CG)
2.5. Outcome Measures
- The mini-Balance Evaluation Systems Test (mini-BESTest) is a shortened version of the Balance Evaluation Systems Test. It is composed of a 14-item scale that evaluates balance with a total score of 28. Items are grouped into the following four subcomponents: anticipatory postural control (max score = 6), reactive postural control (max score = 6), somatosensory orientation (max score = 6), and dynamic walking (max score = 10). A summary of the subcomponents and the items of the mini-BESTest is depicted in Table 1. The mini-BESTest has been shown to have good psychometric properties in both PD and MS [45,46].
- The Timed Up-and-Go (TUG) test which involves rising from a seated position, walking to a pre-determined location, turning, and returning to a seated position, is a common test used to assess functional mobility, dynamic balance, and walking ability. The score is the time required to perform the following tasks: standing up from a chair; walking 3 m: turning around, walking back to the chair and sitting down. The validity and reliability of the TUG in people with PD and MS have been published [47,48]. TUG performance has been associated with mobility status and fall risk [49,50]. The TUG test used was the subtest included in the mini-BESTest.
- The Timed Up-and-Go-test Dual-task (TUG-D) is a dual-task measure of functional mobility that evaluates balance with a simultaneous cognitive task. The TUG-D score is the time required to perform the TUG when the following cognitive task is added: while walking, the participant counts backward in threes from a randomly chosen start number between 60 and 100 to avoid a learning effect. The TUG-D performance on the TUG-D represents a significant predictor of future falls in people with PD and MS [51,52]. The TUG-D test used was the subtest included in the mini-BESTest.
- The Montreal Cognitive Assessment (MoCA) is a rapid screening instrument for mild cognitive dysfunction. It assesses different cognitive domains: attention and concentration, executive functions, memory, language, visuo-constructional skills, conceptual thinking, calculations, and orientation. The total MoCA score is 30 points. The MoCA has been recognized as a valid and sensitive instrument to identify cognitive impairment in people with PD and MS [53,54].
- The MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) is a multimodal scale assessing impairment and disability consisting of four parts. Part I assessed non-motor experiences of daily living. Part II assessed motor experiences of daily living. Part III assessed the motor signs of PD. Part IV assessed motor fluctuations and dyskinesias. MDS-UPDRS Total Score equals the sum of Parts I, II, and III (Range 0–236). A higher score indicated more severe symptoms of PD [55].
- The Parkinson’s Disease Questionaire-8 (PDQ-8) is a short-form version of the Parkinson’s Disease Questionaire-39. It is a self-administered questionnaire, used to measure the quality of life in people with PD [56]. The total PDQ-8 score is 32 points.
- The Multiple Sclerosis Quality of Life 54 (MSQoL-54) is a structured, self-report questionnaire for measuring health-related quality of life in MS [57] consisting of four parts. The MSQoL-54 consists of 12 subscales and two single items. Each subscale is scored from 0 to 100, with higher scores indicating a better QoL. Subscale scores can be weighted and summed to generate Physical Health Composite Score (MSQOL-54_PHCS) and Mental Health Composite Score (MSQOL-54_MHCS).
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Zahra, W.; Rai, S.N.; Birla, H.; Singh, S.S.; Dilnashin, H.; Rathore, A.S.; Singh, S.P. The global economic impact of neurodegenerative diseases: Opportunities and challenges. In Bioeconomy for Sustainable Development; Springer Nature: Singapore, 2020; pp. 333–345. [Google Scholar]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Temel, Y.; Kessels, A.; Tan, S.; Topdag, A.; Boon, P.; Visser-Vandewalle, V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: A systematic review. Park. Relat. Disord. 2006, 12, 265–272. [Google Scholar] [CrossRef]
- Rosti-Otajärvi, E.; Hämäläinen, P. Behavioural symptoms and impairments in multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. J. 2013, 19, 31–45. [Google Scholar] [CrossRef]
- Jankovic, J.; Tolosa, E. (Eds.) Parkinson’s Disease and Movement Disorders; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Tranchant, C.; Bhatia, K.P.; Marsden, C.D. Movement disorders in multiple sclerosis. Mov. Disord. Off. J. Mov. Disord. Soc. 1995, 10, 418–423. [Google Scholar] [CrossRef]
- Cattaneo, D.; Carpinella, I.; Aprile, I.; Prosperini, L.; Montesano, A.; Jonsdottir, J. Comparison of upright balance in stroke, Parkinson and multiple sclerosis. Acta Neurol. Scand. 2016, 133, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Gervasoni, E.; Pupillo, E.; Bianchi, E.; Montesano, A.; Aprile, I.; Agostini, M.; Rovaris, M.; Cattaneo, D.; Iacobone, G.; et al. Prediction of falls in subjects suffering from Parkinson disease, multiple sclerosis, and stroke. Arch. Phys. Med. Rehabil. 2018, 99, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Nilsagård, Y.; Gunn, H.; Freeman, J.; Hoang, P.; Lord, S.; Mazumder, R.; Cameron, M. Falls in people with MS—An individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult. Scler. J. 2015, 21, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, S.S.; Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.; Fung, V.S. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov. Disord. 2013, 28, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise pre-scription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, D.; Gervasoni, E.; Pupillo, E.; Bianchi, E.; Aprile, I.; Imbimbo, I.; Russo, R.; Cruciani, A.; Turolla, A.; Jonsdottir, J.; et al. Educational and exercise intervention to prevent falls and im-prove participation in subjects with neurological conditions: The NEUROFALL random-ized controlled trial. Front. Neurol. 2019, 10, 865. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.H.; Chen, H.C.; Liou, T.H.; Li, W.; Chen, S.C. Exercise interventions for individuals with neurological disorders: A systematic review of systematic reviews. Am. J. Phys. Med. Rehabil. 2019, 98, 921–930. [Google Scholar] [CrossRef]
- Hayes, S.; Galvin, R.; Kennedy, C.; Finlayson, M.; McGuigan, C.; Walsh, C.D.; Coote, S. Interventions for preventing falls in people with multiple sclerosis. Cochrane Database Syst. Rev. 2019, 2019, CD012475. [Google Scholar] [CrossRef]
- Shen, X.; Wong-Yu, I.S.; Mak, M.K. Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: A meta-analysis. Neurorehabilit. Neural Repair 2016, 30, 512–527. [Google Scholar] [CrossRef] [Green Version]
- Salari, N.; Hayati, A.; Kazeminia, M.; Rahmani, A.; Mohammadi, M.; Fatahian, R.; Shohaimi, S. The effect of exercise on balance in patients with stroke, Parkinson, and multiple sclerosis: A systematic review and meta-analysis of clinical trials. Neurol. Sci. 2021, 43, 167–185. [Google Scholar] [CrossRef]
- O’Malley, N.; Clifford, A.M.; Conneely, M.; Casey, B.; Coote, S. Effectiveness of interventions to prevent falls for people with multiple sclerosis, Parkinson’s disease and stroke: An umbrella review. BMC Neurol. 2021, 21, 378. [Google Scholar] [CrossRef]
- Calafiore, D.; Invernizzi, M.; Ammendolia, A.; Marotta, N.; Fortunato, F.; Paolucci, T.; Ferraro, F.; Curci, C.; Ćwirlej-Sozańska, A.B.; de Sire, A. Efficacy of virtual reality and exergaming in improving balance in patients with multiple sclerosis: A systematic review and meta-analysis. Front. Neurol. 2021, 12, 773459. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef] [Green Version]
- Topol, E. The topol review. In Preparing the Healthcare Workforce to Deliver the Digital Future; NHS: London, UK, 2019; pp. 1–48. [Google Scholar]
- De Angelis, M.; Lavorgna, L.; Carotenuto, A.; Petruzzo, M.; Lanzillo, R.; Brescia Morra, V.; Moccia, M. Digital technology in clinical trials for multiple sclerosis: Systematic review. J. Clin. Med. 2021, 10, 2328. [Google Scholar] [CrossRef]
- Prvu Bettger, J.; Resnik, L.J. Telerehabilitation in the age of COVID-19: An opportunity for learning health system research. Phys. Ther. 2020, 100, 1913–1916. [Google Scholar] [CrossRef]
- Nuara, A.; Fabbri-Destro, M.; Scalona, E.; Lenzi, S.E.; Rizzolatti, G.; Avanzini, P. Telerehabilitation in response to constrained physical distance: An opportunity to rethink neurorehabilitative routines. J. Neurol. 2021, 269, 627–638. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Bottiroli, S.; Realdon, O.; Riva, G.; Galvagni, L.; Platz, T.; Sandrini, G.; De Icco, R.; Tassorelli, C. Telemedicine and Virtual Reality at Time of COVID-19 Pandemic: An Overview for Future Perspectives in Neurorehabilitation. Front. Neurol. 2021, 12, 227. [Google Scholar] [CrossRef]
- Langer, A.; Gassner, L.; Flotz, A.; Hasenauer, S.; Gruber, J.; Wizany, L.; Pokan, R.; Maetzler, W.; Zach, H. How COVID-19 will boost remote exercise-based treatment in Parkinson’s disease: A narrative review. NPJ Park. Dis. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Vellata, C.; Belli, S.; Balsamo, F.; Giordano, A.; Colombo, R.; Maggioni, G. Effectiveness of Telerehabilitation on Motor Impairments, Non-motor Symptoms and Com-pliance in Patients with Parkinson’s Disease: A Systematic Review. Front. Neurol. 2021, 12, 627999. [Google Scholar] [CrossRef]
- Agostini, M.; Moja, L.; Banzi, R.; Pistotti, V.; Tonin, P.; Venneri, A.; Turolla, A. Telerehabilitation and recovery of motor function: A systematic review and meta-analysis. J. Telemed. Telecare 2015, 21, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Maresca, G.; Maggio, M.G.; De Luca, R.; Manuli, A.; Tonin, P.; Pignolo, L.; Calabrò, R.S. Tele-neuro-rehabilitation in Italy: State of the art and future perspectives. Front. Neurol. 2020, 11, 563375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Guan, B.S.; Li, Z.K.; Yang, Q.H.; Xu, T.J.; Li, H.B.; Wu, Q.Y. Application of telehealth intervention in Parkinson’s disease: A systematic review and meta-analysis. J. Telemed. Telecare 2020, 26, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: A multicenter, single-blind, randomized, controlled trial. BioMed Res. Int. 2017, 2017, 7962826. [Google Scholar] [CrossRef] [Green Version]
- Di Tella, S.; Pagliari, C.; Blasi, V.; Mendozzi, L.; Rovaris, M.; Baglio, F. Inte-grated telerehabilitation approach in multiple sclerosis: A systematic review and meta-analysis. J. Telemed. Telecare 2020, 26, 385–399. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Kesselring, J.; Galea, M. Telerehabilitation for persons with multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, CD010508. [Google Scholar] [CrossRef]
- Pagliari, C.; Di Tella, S.; Jonsdottir, J.; Mendozzi, L.; Rovaris, M.; De Icco, R.; Milanesi, T.; Federico, S.; Agostini, M.; Goffredo, M.; et al. Effects of home-based virtual reality telerehabilitation system in people with multiple sclerosis: A randomized controlled trial. J. Telemed. Telecare 2021, 1, 12. [Google Scholar] [CrossRef]
- Herdman, S.J.; Schubert, M.C.; Tusa, R.J. Strategies for balance rehabilitation: Fall risk and treatment. Ann. N. Y. Acad. Sci. 2001, 942, 394–412. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [Green Version]
- King, L.A.; Priest, K.C.; Salarian, A.; Pierce, D.; Horak, F.B. Comparing the Mini-BESTest with the Berg Balance Scale to evaluate balance disorders in Parkinson’s disease. Park. Dis. 2012, 2012, 375419. [Google Scholar] [CrossRef] [Green Version]
- Ross, E.; Purtill, H.; Uszynski, M.; Hayes, S.; Casey, B.; Browne, C.; Coote, S. Cohort study comparing the Berg Balance Scale and the Mini-BESTest in people who have multiple sclerosis and are ambulatory. Phys. Ther. 2016, 96, 1448–1455. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.; Morris, M.E.; Iansek, R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys. Ther. 2001, 81, 810–818. [Google Scholar]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go Test as a Measure of Functional Mobility in Persons with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J.; National Parkinson Foundation Quality Improvement Initiative Investigators. Using the Timed Up & Go test in a clinical setting to predict falling in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar]
- Quinn, G.; Comber, L.; McGuigan, C.; Galvin, R.; Coote, S. Discriminative ability and clinical utility of the Timed Up and Go (TUG) in identifying falls risk in people with multiple sclerosis: A prospective cohort study. Clin. Rehabil. 2019, 33, 317–326. [Google Scholar] [CrossRef]
- Vance, R.C.; Healy, D.G.; Galvin, R.; French, H.P. Dual tasking with the timed “up & go” test improves detection of risk of falls in people with Parkinson disease. Phys. Ther. 2015, 95, 95–102. [Google Scholar]
- Nilsagård, Y.; Lundholm, C.; Denison, E.; Gunnarsson, L.G. Predicting accidental falls in people with multiple sclerosis—A longitudinal study. Clin. Rehabil. 2009, 23, 259–269. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; MacAskill, M.R.; Nakas, C.T.; Livingston, L.; Graham, C.; Crucian, G.P.; Melzer, T.R.; Kirwan, J.; Keenan, R.; Wells, S.; et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010, 75, 1717–1725. [Google Scholar] [CrossRef]
- Rosca, E.C.; Simu, M. Montreal cognitive assessment for evaluating cognitive impairment in multiple sclerosis: A systematic review. Acta Neurol. Belg. 2020, 120, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The PDQ-8: Development and validation of a short-form Parkinson’s disease questionnaire. Psychol. Health 1997, 12, 805–814. [Google Scholar] [CrossRef]
- Solari, A.; Filippini, G.; Mendozzi, L.; Ghezzi, A.; Cifani, S.; Barbieri, E.; Baldini, S.; Salmaggi, A.; La Mantia, L.; Farinotti, M.; et al. Validation of Italian multiple sclerosis quality of life 54 questionnaire. J. Neurol. Neurosurg. Psychiatry 1999, 67, 158–162. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation System’s Test: The mini-BESTest. J. Rehabil. Med. Off. J. UEMS Eur. Board Phys. Rehabil. Med. 2010, 42, 323. [Google Scholar]
- Godi, M.; Franchignoni, F.; Caligari, M.; Giordano, A.; Turcato, A.M.; Nardone, A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys. Ther. 2013, 93, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Montedori, A.; Bonacini, M.I.; Casazza, G.; Luchetta, M.L.; Duca, P.; Cozzolino, F.; Abraha, I. Modified versus standard intention-to-treat reporting: Are there differences in methodological quality, sponsorship, and findings in randomized trials? A cross-sectional study. Trials 2011, 12, 58. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials–a practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, M. GAMLj: General Analyses for Linear Models. 2019. Available online: https://gamlj.github.io/ (accessed on 1 December 2022).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Seidler, K.J.; Duncan, R.P.; McNeely, M.E.; Hackney, M.E.; Earhart, G.M. Feasibility and preliminary efficacy of a telerehabilitation approach to group adapted tango instruction for people with Parkinson disease. J. Telemed. Telecare 2017, 23, 740–746. [Google Scholar] [CrossRef]
- Ortiz Gutierrez, R.; Galan del Rio, F.; Cano de la Cuerda, R.; Alguacil-Diego, I.M.; Arroyo González, R.; Miangolarra Page, J.C. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. Neurorehabilitation 2013, 33, 545–554. [Google Scholar] [CrossRef]
- Hoang, P.; Schoene, D.; Gandevia, S.; Smith, S.; Lord, S.R. Effects of a home-based step training programme on balance, stepping, cognition and functional perfor-mance in people with multiple sclerosis—A randomized controlled trial. Mult. Scler. J. 2016, 22, 94–103. [Google Scholar] [CrossRef]
- Finkelstein, J.; Lapshin, O.; Castro, H.; Cha, E.; Provance, P.G. Home-based physical telerehabilitation in patients with multiple sclerosis: A pilot study. J. Rehabil. Res. Dev. 2008, 45, 1361–1373. [Google Scholar] [CrossRef]
- dos Santos Mendes, F.A.; Pompeu, J.E.; Lobo, A.M.; da Silva, K.G.; de Paula Oliveira, T.; Zomignani, A.P.; Piemonte, M.E.P. Motor learning, retention and transfer af-ter virtual-reality-based training in Parkinson’s disease–effect of motor and cognitive demands of games: A longitudinal, controlled clinical study. Physiotherapy 2012, 98, 217–223. [Google Scholar] [CrossRef]
- Ferraris, C.; Nerino, R.; Chimienti, A.; Pettiti, G.; Cau, N.; Cimolin, V.; Azzaro, C.; Priano, L.; Mauro, A. Feasibility of home-based automated assessment of postural instability and lower limb impairments in Parkinson’s disease. Sensors 2019, 19, 1129. [Google Scholar] [CrossRef] [Green Version]
- Intzandt, B.; Beck, E.N.; Silveira, C.R. The effects of exercise on cognition and gait in Parkinson’s disease: A scoping review. Neurosci. Biobehav. Rev. 2018, 95, 136–169. [Google Scholar] [CrossRef]
- Fritz, N.E.; Kloos, A.D.; Kegelmeyer, D.A.; Kaur, P.; Nichols-Larsen, D.S. Supplementary motor area connectivity and dual-task walking variability in multiple sclerosis. J. Neurol. Sci. 2019, 396, 159–164. [Google Scholar] [CrossRef]
- Wajda, D.A.; Motl, R.W.; Sosnoff, J.J. Dual task cost of walking is related to fall risk in persons with multiple sclerosis. J. Neurol. Sci. 2013, 335, 160–163. [Google Scholar] [CrossRef]
- Wajda, D.A.; Sosnoff, J.J. Cognitive-motor interference in multiple sclerosis: A systematic review of evidence, correlates, and consequences. BioMed Res. Int. 2015, 2015, 720856. [Google Scholar] [CrossRef] [Green Version]
- Brustio, P.R.; Magistro, D.; Zecca, M.; Liubicich, M.E.; Rabaglietti, E. Fear of falling and activities of daily living function: Mediation effect of dual-task ability. Aging Ment. Health 2018, 22, 856–861. [Google Scholar] [CrossRef] [Green Version]
- Pedullà, L.; Tacchino, A.; Podda, J.; Bragadin, M.M.; Bonzano, L.; Battaglia, M.A.; Bove, M.; Brichetto, G.; Ponzio, M. The patients’ perspective on the perceived difficulties of dual-tasking: Development and validation of the Dual-task Impact on Daily-living Activities Questionnaire (DIDA-Q). Mult. Scler. Relat. Disord. 2020, 46, 102601. [Google Scholar] [CrossRef]
- Charvet, L.E.; Yang, J.; Shaw, M.T.; Sherman, K.; Haider, L.; Xu, J.; Krupp, L.B. Cognitive function in multiple sclerosis improves with telerehabilitation: Results from a randomized controlled trial. PLoS ONE 2017, 12, e0177177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacciante, L.; Pieta, C.D.; Rutkowski, S.; Cieślik, B.; Szczepańska-Gieracha, J.; Agostini, M.; Kiper, P. Cognitive telerehabilitation in neurological patients: Systematic review and meta-analysis. Neurol. Sci. 2022, 43, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Morelli, N.; Morelli, H. Dual task training effects on gait and balance out-comes in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2021, 49, 102794. [Google Scholar] [CrossRef] [PubMed]
- Martino Cinnera, A.; Bisirri, A.; Leone, E.; Morone, G.; Gaeta, A. Effect of dual-task training on balance in patients with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2021, 35, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, T.; Liu, H.; Jiang, Y.; Wang, Z.; Zhuang, J. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: A systematic re-view and meta-analysis. Clin. Rehabil. 2020, 34, 1355–1367. [Google Scholar] [CrossRef]
- De Freitas, T.B.; Leite, P.H.W.; Doná, F. The effects of dual task gait and balance training in Parkinson’s disease: A systematic review. Physiother. Theory Pract. 2018, 36, 1088–1096. [Google Scholar] [CrossRef]
- Prosperini, L.; Piattella, M.C.; Giannì, C.; Pantano, P. Functional and structural brain plasticity enhanced by motor and cognitive rehabilitation in multiple sclerosis. Neural Plast. 2015, 2015, 481574. [Google Scholar] [CrossRef] [Green Version]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Centonze, D.; Leocani, L.; Feys, P. Advances in physical rehabilitation of multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 255–261. [Google Scholar] [CrossRef]
- Sehm, B.; Taubert, M.; Conde, V.; Weise, D.; Classen, J.; Dukart, J.; Draganski, B.; Villringer, A.; Ragert, P. Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol. Aging 2014, 35, 232–239. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; García-Muñoz, C.; Martín-Valero, R.; Lucena-Anton, D.; Moral-Munoz, J.A.; Cortés-Vega, M.D. Dropout rate in randomised controlled trials of balance and gait rehabilitation in multiple sclerosis: Is it expected to be different for virtual reality-based interventions? A systematic review with meta-analysis and meta-regression. Virtual Real. 2022, 1–17. [Google Scholar] [CrossRef]
- Parra, A.G.; Gonzalez-Medina, G.; Perez-Cabezas, V.; Casuso-Holgado, M.J.; Vinolo-Gil, M.J.; García-Muñoz, C. Dropout Rate of Participants in Randomized Clinical Trials That Use Virtual Reality to Train Balance and Gait in Parkinson’s Disease. A Systematic Review with Meta-analysis and Meta-regression. J. Med. Syst. 2023, 47, 46. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Chen, H. The impact of COVID-19 on the clinical trial. PLoS ONE 2021, 16, e0251410. [Google Scholar] [CrossRef]

| Mini-BESTest (Max 28 Points) | |
|---|---|
Anticipatory postural control (max 6 points)
| Somatosensory orientation (max 6 points)
|
Reactive postural control (max 6 points)
| Dynamic walking (max 10 points)
|
| Variables | IG | CG | Group Comparison [p-Value] | |
|---|---|---|---|---|
| Full sample (N = 132) | N = 65 | N = 67 | ||
| Age years, [M, (SD)] | 58.12 (12.43) | 61.12 (11.06) | 0.146 § | |
| Education, N (%) | ||||
| Primary | 0 (0%) | 3 (4.5%) | 0.288 ^ | |
| Secondary | 13 (20.0%) | 17 (25.4%) | ||
| High School | 36 (55.4%) | 33 (49.3%) | ||
| College | 16 (24.6%) | 14 (20.9%) | ||
| Sex (Male/Female), N% | 29 (44.6%)/36 (55.4%) | 30 (44.8%)/37 (55.2%) | 0.985 ^ | |
| Mini-BESTest, [M, (SD)]—primary outcome | 19.43 (5.75) | 19.70 (5.98) | 0.791 § | |
| Mini-BESTest Anticipatory postural control, [M, (SD)] | 3.94 (1.55) | 3.93 (1.54) | 0.961 § | |
| Mini-BESTest Reactive postural control, [M, (SD)] | 4.22 (1.84) | 4.21 (1.90) | 0.984 § | |
| Mini-BESTest Somatosensory orientation, [M, (SD)] | 4.55 (1.48) | 4.58 (1.43) | 0.911 § | |
| Mini-BESTest Dynamic walking, [M, (SD)] | 6.74 (2.05) | 6.99 (2.27) | 0.514 § | |
| TUG [ln], [M, (SD)] | 2.29 (0.54) | 2.33 (0.49) | 0.668 § | |
| TUG-D [ln], [M, (SD)] | 2.50 (0.57) | 2.50 (0.52) | 0.942 § | |
| MoCA, [M, (SD)] | 25.88 (2.67) | 25.19 (3.22) | 0.341 * | |
| people with PD (N = 72) | N = 35 | N = 37 | ||
| Age years, [M, (SD)] | 66.51 (7.37) | 68.32 (5.89) | 0.252 § | |
| Education, N (%) | ||||
| Primary | 0 (0%) | 3 (8.1%) | 0.166 ^ | |
| Secondary | 9 (25.7%) | 12 (32.4%) | ||
| High School | 16 (45.7%) | 17 (45.9%) | ||
| College | 10 (28.6%) | 5 (13.5%) | ||
| Sex (Male/Female), N% | 17 (48.6%)/18 (51.4%) | 18 (48.6%)/19(51.4%) | 0.995 ^ | |
| H&Y, [median, (25th–75th)] | 2.00 (2.00–2.00) | 2.00 (1.50–2.50) | 0.340 ° | |
| Disease Duration years [M, (SD)] | 5.84 (4.57) | 4.70 (3.76) | 0.331 § | |
| Mini-BESTest, [M, (SD)]—primary outcome | 19.86 (5.60) | 21.00 (5.52) | 0.386 § | |
| Mini-BESTest Anticipatory postural control, [M, (SD)] | 4.20 (1.57) | 4.05 (1.51) | 0.689 § | |
| Mini-BESTest Reactive postural control, [M, (SD)] | 4.37 (1.63) | 4.54 (1.64) | 0.662 § | |
| Mini-BESTest Somatosensory orientation, [M, (SD)] | 4.71 (1.38) | 5.00 (1.27) | 0.364 § | |
| Mini-BESTest Dynamic walking, [M, (SD)] | 6.60 (1.99) | 7.41 (1.95) | 0.087 § | |
| TUG [ln], [M, (SD)] | 2.15 (0.54) | 2.17 (0.50) | 0.856 § | |
| TUG-D [ln], [M, (SD)] | 2.39 (0.58) | 2.38 (0.55) | 0.932 § | |
| MoCA, [M, (SD)] | 25.51 (2.66) | 24.76 (3.09) | 0.405 * | |
| MDS-UPDRS part III, [median, (25th–75th)] | 27.00 (18.50–44.00) | 33.00 (22.00–44.00) | 0.388 ° | |
| PDQ-8, [M, (SD)] | 28.75 (18.82) | 27.96 (15.62) | 0.845 § | |
| people with MS (N = 60) | N = 30 | N = 30 | ||
| Age years, [M, (SD)] | 48.33 (9.66) | 52.23 (9.34) | 0.117 § | |
| Education, N (%) | ||||
| Primary | 0 (0%) | 0 (0%) | 0.561 ^ | |
| Secondary | 4 (13.3%) | 5 (16.7%) | ||
| High School | 20 (66.7%) | 16 (53.3% | ||
| College | 6 (20.0%) | 9 (30.0%) | ||
| Sex (Male/Female), N% | 12 (40.0%)/18 (60.0%) | 12 (40.0%)/18 (60.0%) | 1.000 ^ | |
| EDSS, [median, (25th–75th)] | 5.00 (3.63–6.00) | 4.50 (3.50–5.88) | 0.634 ° | |
| MS Phenotype (RR/SP), N% | RR (13, 43%)/SP (17; 57%) | RR (16; 53%)/SP (14; 47%) | 0.438 ^ | |
| Disease Duration years [M, (SD)] | 15.36 (7.17) | 12.68 (6.72) | 0.618 § | |
| Mini-BESTest, [M, (SD)]—primary outcome | 18.93 (5.98) | 18.10 (6.23) | 0.599 § | |
| Mini-BESTest Anticipatory postural control, [M, (SD)] | 3.63 (1.50) | 3.77 (1.59) | 0.739 § | |
| Mini-BESTest Reactive postural control, [M, (SD)] | 4.03 (2.08) | 3.80 (2.12) | 0.669 § | |
| Mini-BESTest Somatosensory orientation, [M, (SD)] | 4.37 (1.59) | 4.07 (1.46) | 0.449 § | |
| Mini-BESTest Dynamic walking, [M, (SD)] | 6.90 (2.14) | 6.47 (2.56) | 0.479 § | |
| TUG [ln], [M, (SD)] | 2.45 (0.50) | 2.51 (0.41) | 0.570 § | |
| TUG-D [ln], [M, (SD)] | 2.64 (0.55) | 2.64 (0.44) | 0.961 § | |
| MoCA, [M, (SD)] | 26.30 (2.67) | 25.73 (3.34) | 0.510 § | |
| MSQOL-54_PHCS, [M, (SD)] | 55.58 (16.47) | 53.09 (19.13) | 0.590 § | |
| MSQOL-54_MHCS, [M, (SD)] | 67.94 (16.59) | 58.58 (22.10) | 0.069 § |
| Variables | IG (N = 65) | CG (N = 67) | Time [p-Value] | Group [p-Value] | Time✻Group [p-Value] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EMM T0 | SE T0 | EMM T1 | SE T1 | EMM T0 | SE T0 | EMM T1 | SE T1 | ||||
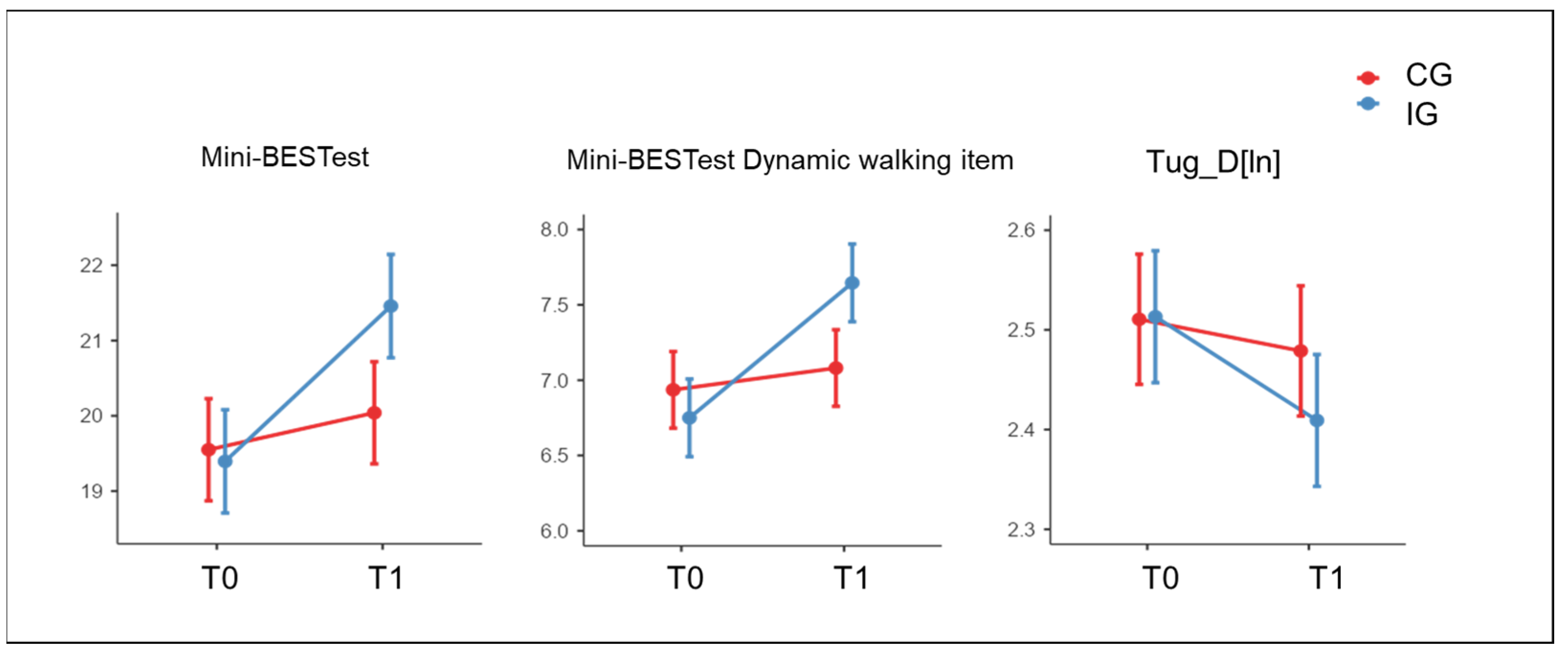
| Mini-BESTest—primary outcome | 19.40 | 0.69 | 21.46 | 0.69 | 19.55 | 0.68 | 20.04 | 0.68 | <0.001 | 0.487 | 0.020 |
| Mini-BESTest Anticipatory postural control | 3.92 | 0.18 | 4.51 | 0.18 | 3.91 | 0.18 | 4.12 | 0.18 | <0.001 | 0.394 | 0.082 |
| Mini-BESTest Reactive postural control | 4.20 | 0.22 | 4.46 | 0.22 | 4.17 | 0.22 | 4.26 | 0.22 | 0.207 | 0.688 | 0.554 |
| Mini-BESTest Somatosensory orientation | 4.54 | 0.17 | 4.85 | 0.17 | 4.53 | 0.16 | 4.57 | 0.16 | 0.055 | 0.513 | 0.137 |
| Mini-BESTest Dynamic walking | 6.75 | 0.26 | 7.65 | 0.26 | 6.94 | 0.25 | 7.08 | 0.25 | <0.001 | 0.568 | 0.011 |
| TUG [ln] | 2.30 | 0.06 | 2.23 | 0.06 | 2.34 | 0.06 | 2.31 | 0.06 | 0.002 | 0.469 | 0.250 |
| TUG-D [ln] | 2.51 | 0.07 | 2.41 | 0.07 | 2.51 | 0.07 | 2.48 | 0.07 | <0.001 | 0.714 | 0.048 |
| MoCA | 25.85 | 0.35 | 26.62 | 0.35 | 25.29 | 0.34 | 25.85 | 0.34 | 0.003 | 0.125 | 0.616 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goffredo, M.; Pagliari, C.; Turolla, A.; Tassorelli, C.; Di Tella, S.; Federico, S.; Pournajaf, S.; Jonsdottir, J.; De Icco, R.; Pellicciari, L.; et al. Non-Immersive Virtual Reality Telerehabilitation System Improves Postural Balance in People with Chronic Neurological Diseases. J. Clin. Med. 2023, 12, 3178. https://doi.org/10.3390/jcm12093178
Goffredo M, Pagliari C, Turolla A, Tassorelli C, Di Tella S, Federico S, Pournajaf S, Jonsdottir J, De Icco R, Pellicciari L, et al. Non-Immersive Virtual Reality Telerehabilitation System Improves Postural Balance in People with Chronic Neurological Diseases. Journal of Clinical Medicine. 2023; 12(9):3178. https://doi.org/10.3390/jcm12093178
Chicago/Turabian StyleGoffredo, Michela, Chiara Pagliari, Andrea Turolla, Cristina Tassorelli, Sonia Di Tella, Sara Federico, Sanaz Pournajaf, Johanna Jonsdottir, Roberto De Icco, Leonardo Pellicciari, and et al. 2023. "Non-Immersive Virtual Reality Telerehabilitation System Improves Postural Balance in People with Chronic Neurological Diseases" Journal of Clinical Medicine 12, no. 9: 3178. https://doi.org/10.3390/jcm12093178






