Effect of Sodium Hypochlorite 0.05% on MMP-9 Extracellular Release in Chronic Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Zymography
2.3. Magnetic Multiplex Immunoassay
2.4. Scratch Wound Healing Assay
2.5. Statistical Analysis
3. Results
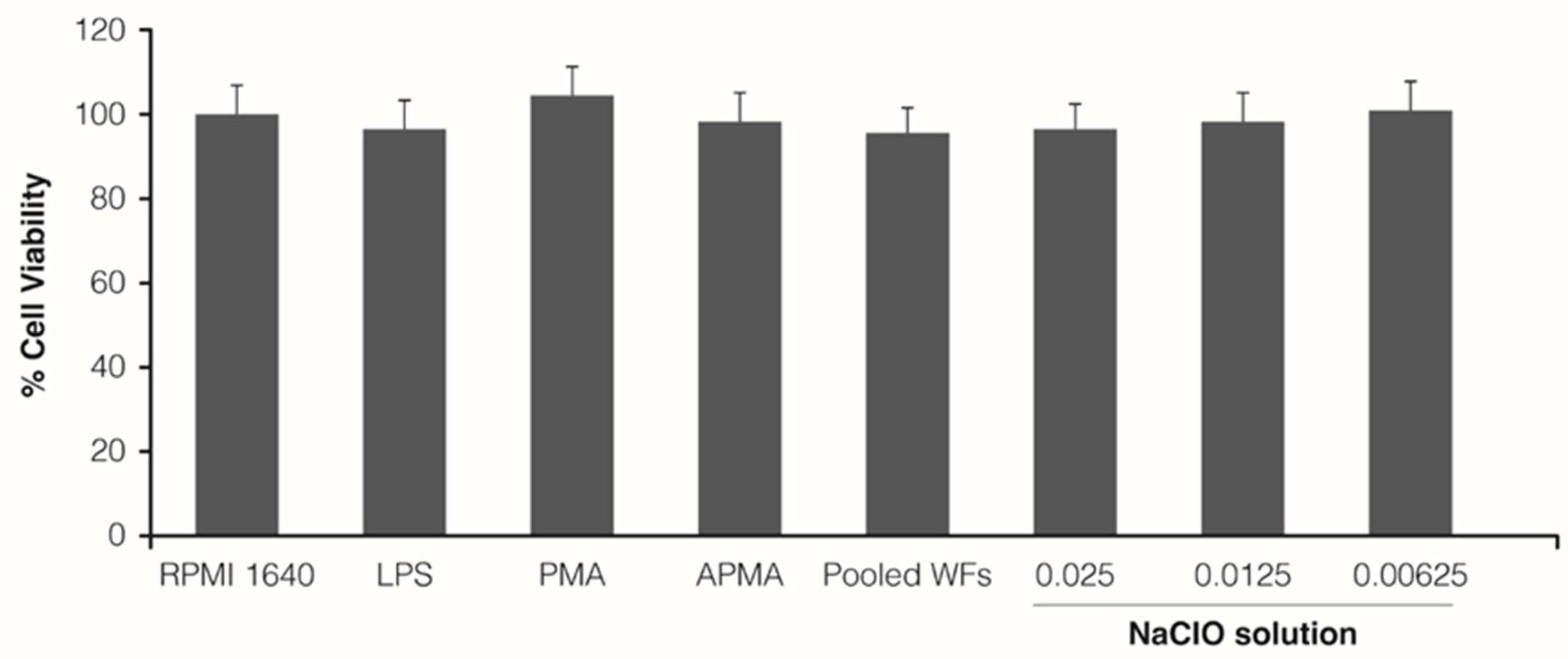
3.1. Cytotoxicity Assay
3.2. Zymography
3.2.1. Differentiated Macrophages
3.2.2. Peripheral Blood Serum
3.3. Quantitative Assay of MMPs Release
3.4. Exploratory Scratch Wound Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar] [PubMed]
- Cerofolini, L.; Fragai, M.; Luchinat, C. Mechanism and inhibition of matrix metalloproteinases. Curr. Med. Chem. 2019, 26, 2609–2633. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Mosti, G.; Santi, M.; Ligi, D.; Mannello, F. Matrix metalloproteinase profiles in chronic venous ulcer wound fluid of inflammatory and granulating venous leg ulcers. J. Vasc. Surg. Venous Lymphat. Disord. 2015, 3, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Rayment, E.A.; Upton, Z. Finding the culprit: A review of the influences of proteases on the chronic wound environment. Int. J. Low. Extrem. Wounds 2009, 8, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; De Franciscis, S. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013, 21, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Grzela, T.; Niderla-Bielinska, J.; Litwiniuk, M.; White, R. The direct inhibition of MMP-2 and MMP-9 by an enzyme alginogel: A possible mechanism of healing support for venous leg ulcers. J. Wound Care 2014, 23, 278–285. [Google Scholar] [CrossRef]
- Ren, Y.; Gu, G.; Yao, M.; Driver, V.R. Role of matrix metalloproteinases in chronic wound healing: Diagnostic and therapeutic implications. Chin. Med. J. 2014, 127, 1572–1581. [Google Scholar] [PubMed]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on wound antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef] [PubMed]
- Scalise, A. The new formulation of the 0,05% sodium hypochlorite electrolytic solution for cutaneous use: Reasons and advantages. AboutOpen 2021, 8, 14–22. [Google Scholar] [CrossRef]
- Santo, G.; D’Atanasio, N.; Capezzone de Joannon, A.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Valutazione della attività e tossicità di una soluzione di ipoclorito di sodio elettrolitico allo 0.05% nel processo di riparazione tissutale. Gazz. Med. Ital. Arch. Sci. Med. 2012, 171, 795–805. [Google Scholar]
- Retana-Lobo, C.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Mendes de Souza, B.D.; Reyes-Carmona, J. Sodium hypochlorite and chlorhexidine downregulate mmp expression on radicular dentin. Med. Princ. Pract. 2021, 30, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.A.T.; Abu Hasna, A.; Carvalho, A.S.; Vilela, P.D.G.F.; Ramos, L.D.P.; Valera, M.C.; De Oliveira, L.D. Clinical study of sodium hypochlorite, polymyxin b and limewater effect on MMP-3,-8,-9 in apical periodontitis. Braz. Dent. J. 2020, 31, 116–121. [Google Scholar] [CrossRef]
- AIFA. Amukine Med 0.05% Spray Cutaneo, Soluzione. 2021. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000219_032192_FI.pdf&retry=0&sys=m0b1l3 (accessed on 12 December 2022).
- Ligi, D.; Mosti, G.; Croce, L.; Raffetto, J.D.; Mannello, F. Chronic venous disease–Part II: Proteolytic biomarkers in wound healing. Biochim. Biophys. Acta 2016, 1862, 1900–1908. [Google Scholar] [CrossRef]
- Malara, A.; Ligi, D.; Di Buduo, C.A.; Mannello, F.; Balduini, A. Sub-cellular localization of metalloproteinases in megakaryocytes. Cells 2018, 7, 80. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Saraiva-Romanholo, B.M.; de Genaro, I.S.; de Almeida, F.M.; Felix, S.N.; Lopes, M.R.C.; Amorim, T.S.; Vieira, R.P.; Arantes-Costa, F.M.; Martins, M.A.; Tibério, I.D.F.L.C.; et al. Exposure to sodium hypochlorite or cigarette smoke induces lung injury and mechanical impairment in Wistar rats. Inflammation 2022, 45, 1464–1483. [Google Scholar] [CrossRef] [PubMed]
- Alfakry, H.; Malle, E.; Koyani, C.N.; Pussinen, P.J.; Sorsa, T. Neutrophil proteolytic activation cascades: A possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun. 2016, 22, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J. Biol. Chem. 2001, 276, 41279–41287. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Kassim, S.Y.; Parks, W.C.; Heinecke, J.W. Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): An oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 2003, 278, 28403–28409. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniscalco, R.; Mangano, G.; de Joannon, A.C.; Vergassola, M.; Zucchi, S.; Mannello, F.; Ragni, L. Effect of Sodium Hypochlorite 0.05% on MMP-9 Extracellular Release in Chronic Wounds. J. Clin. Med. 2023, 12, 3189. https://doi.org/10.3390/jcm12093189
Maniscalco R, Mangano G, de Joannon AC, Vergassola M, Zucchi S, Mannello F, Ragni L. Effect of Sodium Hypochlorite 0.05% on MMP-9 Extracellular Release in Chronic Wounds. Journal of Clinical Medicine. 2023; 12(9):3189. https://doi.org/10.3390/jcm12093189
Chicago/Turabian StyleManiscalco, Rosanna, Giorgina Mangano, Alessandra Capezzone de Joannon, Matteo Vergassola, Sara Zucchi, Ferdinando Mannello, and Lorella Ragni. 2023. "Effect of Sodium Hypochlorite 0.05% on MMP-9 Extracellular Release in Chronic Wounds" Journal of Clinical Medicine 12, no. 9: 3189. https://doi.org/10.3390/jcm12093189






