Myopia Control Efficacy and Long-Term Safety of a Novel Orthokeratology Lens (MESOK Study)—A Randomized Controlled Clinical Trial Combining Clinical and Tear Proteomics Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization and Masking
2.4. Intervention
2.5. Sample Size and Power
2.6. Study Procedures
2.7. Clinical Outcome Measures
2.8. Tear Samples Collection
2.9. Tear Protein Extraction and Liquid Chromatography-Mass Spectrometry (LC-MS)/Mass Spectrometry (MS)
2.10. Ion Library Generation and SWATH-MS Analysis
- (1)
- Offline analysis with PeakView (v2.2, Sciex, Framingham, MA, USA). The peptide spectral library was generated using the identified peptides from the peptide fragments’ peak extracted by SWATH Acquisition MicroApp 2.0 in Peakview. Up to 10 peptides per protein, 6 transitions per peptide, 90% peptide confidence threshold, 1% FDR, with a 10 min extracted ion chromatogram (XIC) extraction window and 75 ppm width settings, were selected for processing. Processed data were normalized using the most likely ratio (MLR) method and exported using MarkerView (v1.3, Sceix, Framingham, MA, USA) for protein fold change calculation. Proteins that passed the 1% FDR and with more than 1 quantifiable peptide were used for quantification. Volcano plots were drawn using VolcaNoseR using the R program [21].
- (2)
- Cloud-based analysis with OneOmics. All the raw files (IDA and SWATH-MS) were uploaded to the OneOmics cloud-based analysis platform for protein library generation and spectral matching using PeakView. Up to 10 peptides per protein, 6 transitions per peptide, 90% peptide confidence threshold, 1% FDR, with a 10 min XIC extraction window and 75 ppm width settings, were selected for processing. Shared proteins and peptides with modifications were excluded from quantification, and the processed data were normalized using the MLR method. Proteins that passed the 1% FDR and with more than 1 quantifiable peptide were used for quantification.
2.11. Protein Confirmation Using MRMHR-MS
2.12. Statistical Analysis
3. Results
3.1. Changes in AL
3.2. Long-Term Clinical Safety
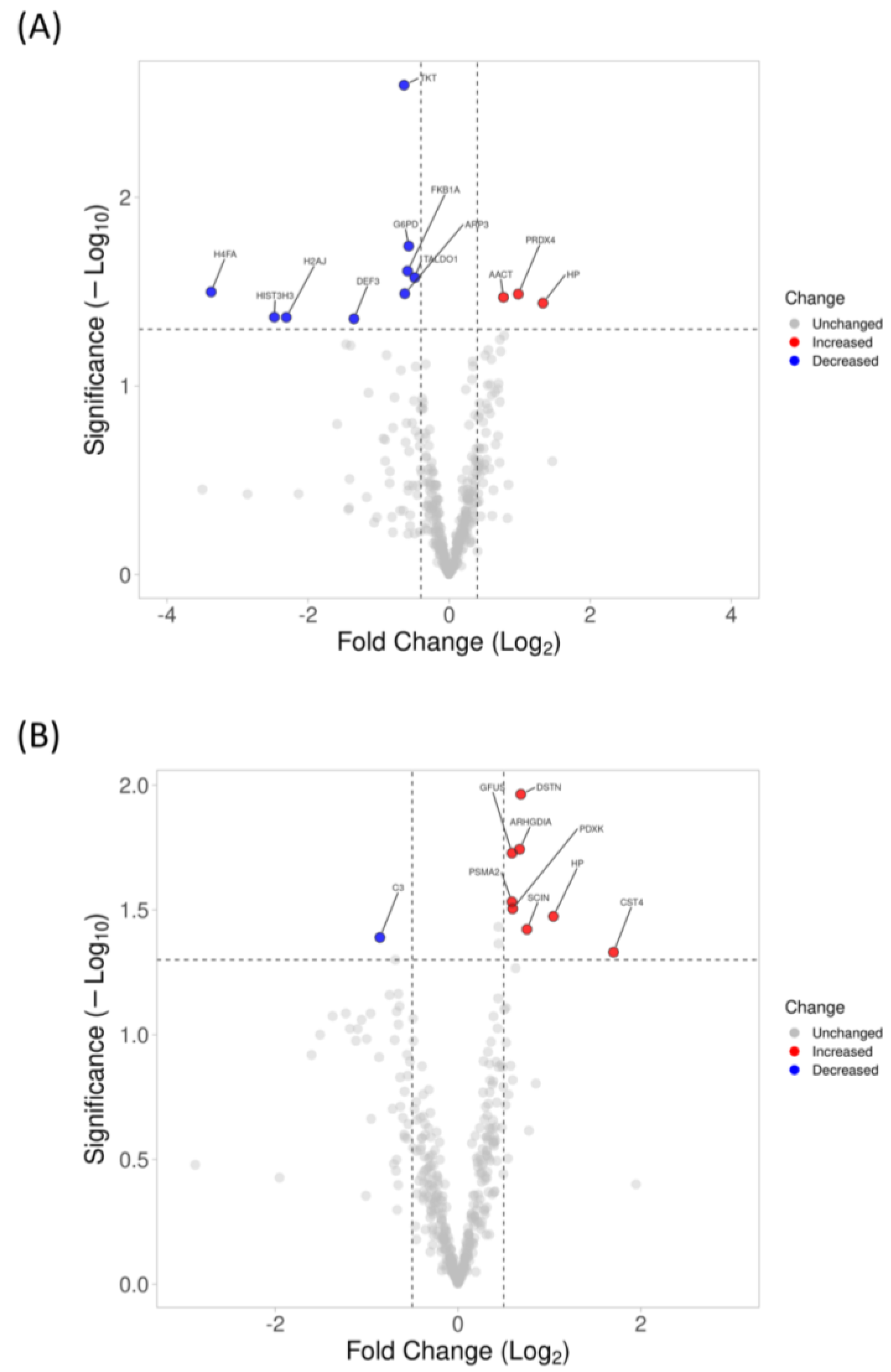
3.3. Changes in Proteomics Profiling of Tear with 6-Month and 1-Year Lens Wear Using SWATH-MS
3.4. Protein Confirmation Using High-Resolution Multiple-Reaction Monitoring (MRMHR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. The Impact of Myopia and High Myopia; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Fricke, T.R.; Holden, B.A.; Wilson, D.A.; Schlenther, G.; Naidoo, K.S.; Resnikoff, S.; Frick, K.D. Global cost of correcting vision impairment from uncorrected refractive error. Bull. World Health Organ. 2012, 90, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.S.; Fricke, T.R.; Frick, K.D.; Jong, M.; Naduvilath, T.J.; Resnikoff, S.; Sankaridurg, P. Potential lost productivity resulting from the global burden of myopia: Systematic review, meta-analysis, and modeling. Ophthalmology 2019, 126, 338–346. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.-H.; Wen, L.; Fong, A.; Goon, Y.Y.; Tan, D. Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. Am. J. Ophthalmol. 2014, 157, 451–457.e1. [Google Scholar] [CrossRef]
- Yam, J.C.; Zhang, X.J.; Zhang, Y.; Wang, Y.M.; Tang, S.M.; Li, F.F.; Kam, K.W.; Ko, S.T.; Yip, B.H.; Young, A.L. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression Study: Continued Versus Washout: Phase 3 Report. Ophthalmology 2021, 129, 308–321. [Google Scholar] [CrossRef]
- Chan, H.H.; Choi, K.Y.; Ng, A.L.; Choy, B.N.; Chan, J.C.H.; Chan, S.S.; Li, S.Z.; Yu, W.Y. Efficacy of 0.01% atropine for myopia control in a randomized, placebo-controlled trial depends on baseline electroretinal response. Sci. Rep. 2022, 12, 11588. [Google Scholar] [CrossRef]
- Lam, C.S.; Tang, W.C.; Lee, P.H.; Zhang, H.Y.; Qi, H.; Hasegawa, K.; To, C.H. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: Results of a 3-year follow-up study. Br. J. Ophthalmol. 2022, 106, 1110–1114. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chun, R.K.M.; Tang, W.C.; To, C.H.; Lam, C.S.-y.; Chan, H.H.-l. Evaluation of an optical defocus treatment for myopia progression among schoolchildren during the COVID-19 pandemic. JAMA Netw. Open 2022, 5, e2143781. [Google Scholar] [CrossRef]
- Walline, J.J.; Greiner, K.L.; McVey, M.E.; Jones-Jordan, L.A. Multifocal contact lens myopia control. Optom. Vis. Sci. 2013, 90, 1207–1214. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.; Tang, Y.Y.; To, C.H. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2014, 98, 40–45. [Google Scholar] [CrossRef]
- Cho, P.; Cheung, S.W. Retardation of myopia in orthokeratology (ROMIO) study: A 2-year randomized clinical trial. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7077–7085. [Google Scholar] [CrossRef]
- Lau, J.K.; Wan, K.; Cho, P. Orthokeratology lenses with increased compression factor (OKIC): A 2-year longitudinal clinical trial for myopia control. Cont. Lens Anterior Eye 2022, 46, 101745. [Google Scholar] [CrossRef]
- Xu, S.; Li, Z.; Zhao, W.; Zheng, B.; Jiang, J.; Ye, G.; Feng, Z.; Long, W.; He, L.; He, M. Effect of atropine, orthokeratology and combined treatments for myopia control: A 2-year stratified randomised clinical trial. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Wang, X.; Yang, B.; Liu, L.; Cho, P. Analysis of parental decisions to use orthokeratology for myopia control in successful wearers. Ophthalmic Physiol. Opt. 2021, 41, 3–12. [Google Scholar] [CrossRef]
- Bullimore, M.A.; Sinnott, L.T.; Jones-Jordan, L.A. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom. Vis. Sci. 2013, 90, 937–944. [Google Scholar] [CrossRef]
- Cheung, S.-W.; Lam, C.; Cho, P. Parents’ knowledge and perspective of optical methods for myopia control in children. Optom. Vis. Sci. 2014, 91, 634–641. [Google Scholar] [CrossRef]
- Tse, J.; Cheung, J.K.; Wong, G.T.; Lam, T.C.; Choi, K.Y.; So, K.H.; Lam, C.D.; Sze, A.Y.; Wong, A.C.; Yee, G.M. Integrating clinical data and tear proteomics to assess efficacy, ocular surface status, and biomarker response after orthokeratology lens wear. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Cheung, J.K.-W.; Bian, J.; Sze, Y.-H.; So, Y.-K.; Chow, W.-Y.; Woo, C.; Wong, M.T.-K.; Li, K.-K.; Lam, T.C. Human tear proteome dataset in response to daily wear of water gradient contact lens using SWATH-MS approach. Data Brief 2021, 36, 107120. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Yau, H.T.; Cheung, S.W.; Cho, P. Corneal thickness changes in myopic children during and after short-term orthokeratology lens wear. Ophthalmic Physiol. Opt. 2021, 41, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wei, S.; Li, S.-M.; Yang, X.; Cao, K.; Hu, J.; Fan, S.; Zhang, L.; Wang, N. Progression of myopia in a natural cohort of Chinese children during COVID-19 pandemic. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2813–2820. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Musch, D.C.; Wei, N.; Qi, X.; Ding, G.; Li, X.; Li, J.; Song, L.; Zhang, Y. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol. 2021, 139, 293–300. [Google Scholar] [CrossRef]
- Wong, C.W.; Tsai, A.; Jonas, J.B.; Ohno-Matsui, K.; Chen, J.; Ang, M.; Ting, D.S.W. Digital screen time during the COVID-19 pandemic: Risk for a further myopia boom? Am. J. Ophthalmol. 2021, 223, 333–337. [Google Scholar] [CrossRef]
- Erdinest, N.; London, N.; Levinger, N.; Lavy, I.; Pras, E.; Morad, Y. Decreased effectiveness of 0.01% atropine treatment for myopia control during prolonged COVID-19 lockdowns. Cont. Lens Anterior Eye 2021, 45, 101475. [Google Scholar] [CrossRef]
- Yum, H.R.; Park, S.H.; Shin, S.Y. Influence of coronavirus disease 2019 on myopic progression in children treated with low-concentration atropine. PLoS ONE 2021, 16, e0257480. [Google Scholar] [CrossRef]
- Bian, J.; Sze, Y.-H.; Tse, D.Y.-Y.; To, C.-H.; McFadden, S.A.; Lam, C.S.-Y.; Li, K.-K.; Lam, T.C. SWATH based quantitative proteomics reveals significant lipid metabolism in early myopic guinea pig retina. Int. J. Mol. Sci. 2021, 22, 4721. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Lu, D.; To, C.-H.; Lam, T.C.; Lin, B. Retinal Proteomic Analysis in a Mouse Model of Endotoxin-Induced Uveitis Using Data-Independent Acquisition-Based Mass Spectrometry. Int. J. Mol. Sci. 2022, 23, 6464. [Google Scholar] [CrossRef]
- Zhu, Y.; Bian, J.F.; Lu, D.Q.; To, C.H.; Lam, C.S.-Y.; Li, K.K.; Yu, F.J.; Gong, B.T.; Wang, Q.; Ji, X.W. Alteration of EIF2 signaling, glycolysis, and dopamine secretion in form-deprived myopia in response to 1% atropine treatment: Evidence from interactive iTRAQ-MS and SWATH-MS proteomics using a guinea pig model. Front. Pharmacol. 2022, 13, 78. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A double-edged sword in host immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Oppenheim, J.J.; Biragyn, A.; Kwak, L.W.; Yang, D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann. Rheum. Dis. 2003, 62, ii17. [Google Scholar] [CrossRef]
- Ganz, T.; Selsted, M.E.; Lehrer, R.I. Defensins. Eur. J. Haematol. 1990, 44, 1–8. [Google Scholar] [CrossRef]
- Lehrer, R.; Barton, A.; Daher, K.A.; Harwig, S.; Ganz, T.; Selsted, M.E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 1989, 84, 553–561. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Lu, W. α-Defensins in human innate immunity. Immunol. Rev. 2012, 245, 84–112. [Google Scholar] [CrossRef]
- de Leeuw, E.; Li, C.; Zeng, P.; Li, C.; Diepeveen-de Buin, M.; Lu, W.-Y.; Breukink, E.; Lu, W. Functional interaction of human neutrophil peptide-1 with the cell wall precursor lipid II. FEBS Lett. 2010, 584, 1543–1548. [Google Scholar] [CrossRef]
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.K.; Mygind, P.H.; Raventós, D.S. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 2010, 328, 1168–1172. [Google Scholar] [CrossRef]
- Zhou, L.; Beuerman, R.W.; Ang, L.P.; Chan, C.M.; Li, S.F.; Chew, F.T.; Tan, D.T. Elevation of human α-defensins and S100 calcium-binding proteins A8 and A9 in tear fluid of patients with pterygium. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2077–2086. [Google Scholar] [CrossRef]
- Gökçınar, N.B.; Karabulut, A.A.; Onaran, Z.; Yumuşak, E.; Budak Yıldıran, F.A. Elevated Tear Human Neutrophil Peptides 1-3, Human Beta Defensin-2 Levels and Conjunctival Cathelicidin LL-37 Gene Expression in Ocular Rosacea. Ocul. Immunol. Inflamm. 2019, 27, 1174–1183. [Google Scholar] [CrossRef]
- Lo, L.-H.; Wu, P.-C.; Wu, Y.-C.; Shiea, J. Characterization of human neutrophil peptides (α-Defensins) in the tears of dry eye patients. Anal. Methods 2010, 2, 1934–1940. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Fan, X.; Tang, D. Release and activity of histone in diseases. Cell Death Dis. 2014, 5, e1370. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.; Papareddy, P.; Dahlgren, M.W.; Chakrakodi, B.; Norrby-Teglund, A.; Smeds, E.; Linder, A.; Mörgelin, M.; Johansson-Lindbom, B.; Egesten, A. Extracellular histones induce chemokine production in whole blood ex vivo and leukocyte recruitment in vivo. PLoS Pathog. 2015, 11, e1005319. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kadiyala, U.; Weerappuli, P.; Valdez, J.J.; Yalavarthi, S.; Louttit, C.; Knight, J.S.; Moon, J.J.; Weiss, D.S.; VanEpps, J.S. Antimicrobial Microwebs of DNA–Histone Inspired from Neutrophil Extracellular Traps. Adv. Mater. 2019, 31, 1807436. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Aziz, M.; Wang, P. The vitals of NETs. J. Leukoc. Biol. 2021, 110, 797–808. [Google Scholar] [CrossRef]

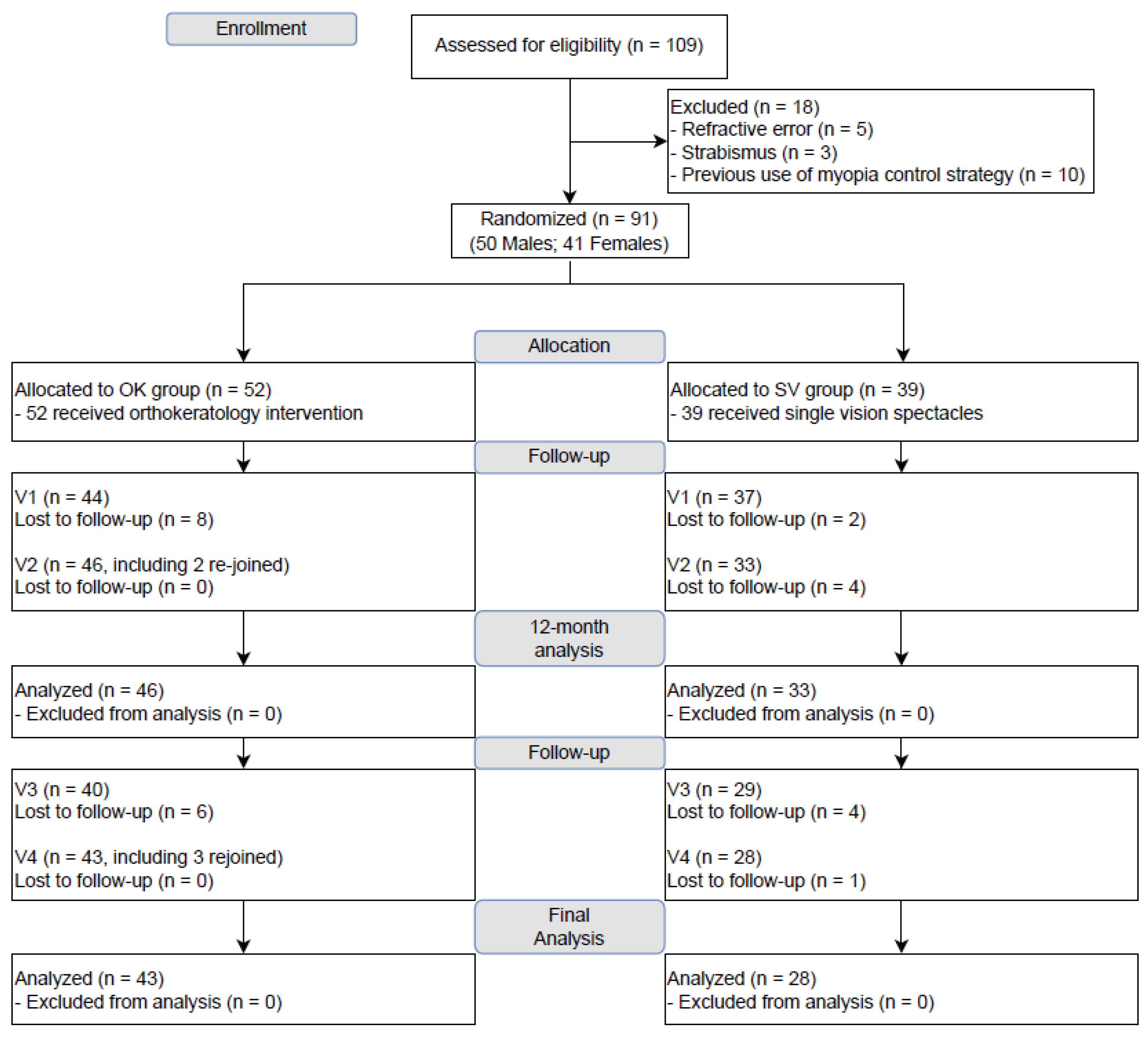

| All | OK | SV | ||
|---|---|---|---|---|
| Subjects (n) | 52 | 39 | ||
| Age (years) | 9.8 ± 1.3 | 9.5 ± 1.4 | ||
| SER (D) | −2.67 ± 1.08 | −2.50 ± 1.02 | ||
| AL (mm) | 24.62 ± 0.94 | 24.42 ± 0.69 | ||
| Analyzed | Excluded | |||
| 12-month | OK | SV | OK | SV |
| Subjects (n) | 46 | 33 | 6 | 6 |
| Age (years) | 9.8 ± 1.3 | 9.3 ± 1.4 | 9.8 ± 1.3 | 10.3 ± 1.6 |
| SER (D) | −2.59 ± 1.10 | −2.50 ± 1.05 | −3.37 ± 0.67 | −2.47 ± 0.88 |
| AL (mm) | 24.57 ± 0.96 | 24.43 ± 0.73 | 25.08 ± 0.57 | 24.33 ± 0.49 |
| 24-month | OK | SV | OK | SV |
| Subjects (n) | 43 | 28 | 9 | 11 |
| Age (years) | 9.8 ± 1.3 | 9.5 ± 1.4 | 9.9 ± 1.4 | 9.5 ± 1.6 |
| SER (D) | −2.61 ± 1.07 | −2.51 ± 1.03 | −2.92 ± 1.18 | −2.47 ± 1.03 |
| AL (mm) | 24.53 ± 0.93 | 24.52 ± 0.73 | 25.04 ± 0.87 | 24.17 ± 0.52 |
| Baseline | 12 Months | Interaction [F(p)] | |||
|---|---|---|---|---|---|
| OK (n = 46) | SV (n = 33) | OK | SV | ||
| BCVA | −0.02 ± 0.05 | 0.00 ± 0.07 | 0.04 ± 0.09 | 0.01 ±0.05 | 2.41 (0.13) |
| CCT * | 550.3 ± 33.7 | 540.0 ± 34.0 | 531.2 ± 38.0 | 537.6 ± 34.7 | 15.84 (<0.001) |
| CD | 3067 ± 232 | 3096 ± 159 | 3042 ± 224 | 2992 ± 200 | 2.26 (0.14) |
| CV | 24.17 ± 3.10 | 23.61 ± 3.60 | 24.67 ± 3.85 | 25.89 ± 3.45 | 2.65 (0.11) |
| CH | 68.13 ± 5.34 | 69.5 ± 5.15 | 68.53 ± 3.82 | 68.04 ± 5.00 | 2.08 (0.16) |
| OSDI | 5.87 ± 4.71 | 8.41 ± 6.82 | 4.28 ± 4.28 | 5.56 ± 4.81 | 0.77 (0.39) |
| Baseline | Final (24 months) | Interaction [F(p)] | |||
| OK (n = 43) | SV (n = 28) | OK | SV | ||
| BCVA | −0.01 ± 0.04 | −0.01 ± 0.07 | 0.03 ± 0.10 | 0.00 ± 0.03 | 2.38 (0.13) |
| CCT * | 547.5 ± 35.2 | 540.8 ± 36.4 | 538.5 ± 41.0 | 545.0 ± 36.0 | 8.56 (0.01) |
| CD | 3051 ± 239 | 3100 ± 168 | 2973 ± 253 | 3005 ± 200 | 0.13 (0.72) |
| CV | 24.10 ± 3.00 | 23.71 ± 3.88 | 24.48 ± 4.36 | 23.88 ± 2.72 | 0.05 (0.82) |
| CH | 68.03 ± 5.27 | 69.50 ± 5.44 | 69.90 ± 5.21 | 68.48 ± 4.41 | 2.85 (0.10) |
| OSDI | 5.80 ± 4.74 | 7.29 ± 5.01 | 4.74 ± 4.81 | 5.57 ± 5.76 | 0.21 (0.65) |
| Protein Accession | Protein Name | Gene Name | Peptide Sequence Used for MRMHR | Transitions Used for MRMHR | MRMHR (5 OK; 5 SV) | SWATH-MS (16 OK; 12 SV) | ||
|---|---|---|---|---|---|---|---|---|
| OK/SV FC | p | OK/SV FC | p | |||||
| P59666 | Neutrophil defensin 3 | DEFA3, DEF3 | IPAC[CAM]IAGER YGTC[CAM]IYQGR | +2y6, +2y7, +2y8 +2y4, +2y5, +2y6 | 0.11 | 0.019 | 0.39 | 0.044 |
| P62805 | Histone H4 | H4C1, H4/A, H4FA, HIST1H4A | VFLENVIR | +2y6 | 0.06 | 0.051 | 0.10 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.Y.; Cheung, J.K.W.; Wong, G.T.K.; Li, P.H.; Chan, S.S.H.; Lam, T.C.; Chan, H.H.L. Myopia Control Efficacy and Long-Term Safety of a Novel Orthokeratology Lens (MESOK Study)—A Randomized Controlled Clinical Trial Combining Clinical and Tear Proteomics Data. J. Clin. Med. 2023, 12, 3210. https://doi.org/10.3390/jcm12093210
Choi KY, Cheung JKW, Wong GTK, Li PH, Chan SSH, Lam TC, Chan HHL. Myopia Control Efficacy and Long-Term Safety of a Novel Orthokeratology Lens (MESOK Study)—A Randomized Controlled Clinical Trial Combining Clinical and Tear Proteomics Data. Journal of Clinical Medicine. 2023; 12(9):3210. https://doi.org/10.3390/jcm12093210
Chicago/Turabian StyleChoi, Kai Yip, Jimmy K. W. Cheung, Gigi T. K. Wong, Peter H. Li, Sonia S. H. Chan, Thomas C. Lam, and Henry H. L. Chan. 2023. "Myopia Control Efficacy and Long-Term Safety of a Novel Orthokeratology Lens (MESOK Study)—A Randomized Controlled Clinical Trial Combining Clinical and Tear Proteomics Data" Journal of Clinical Medicine 12, no. 9: 3210. https://doi.org/10.3390/jcm12093210






