Does Forward Head Posture Influence Somatosensory Evoked Potentials and Somatosensory Processing in Asymptomatic Young Adults?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Techniques
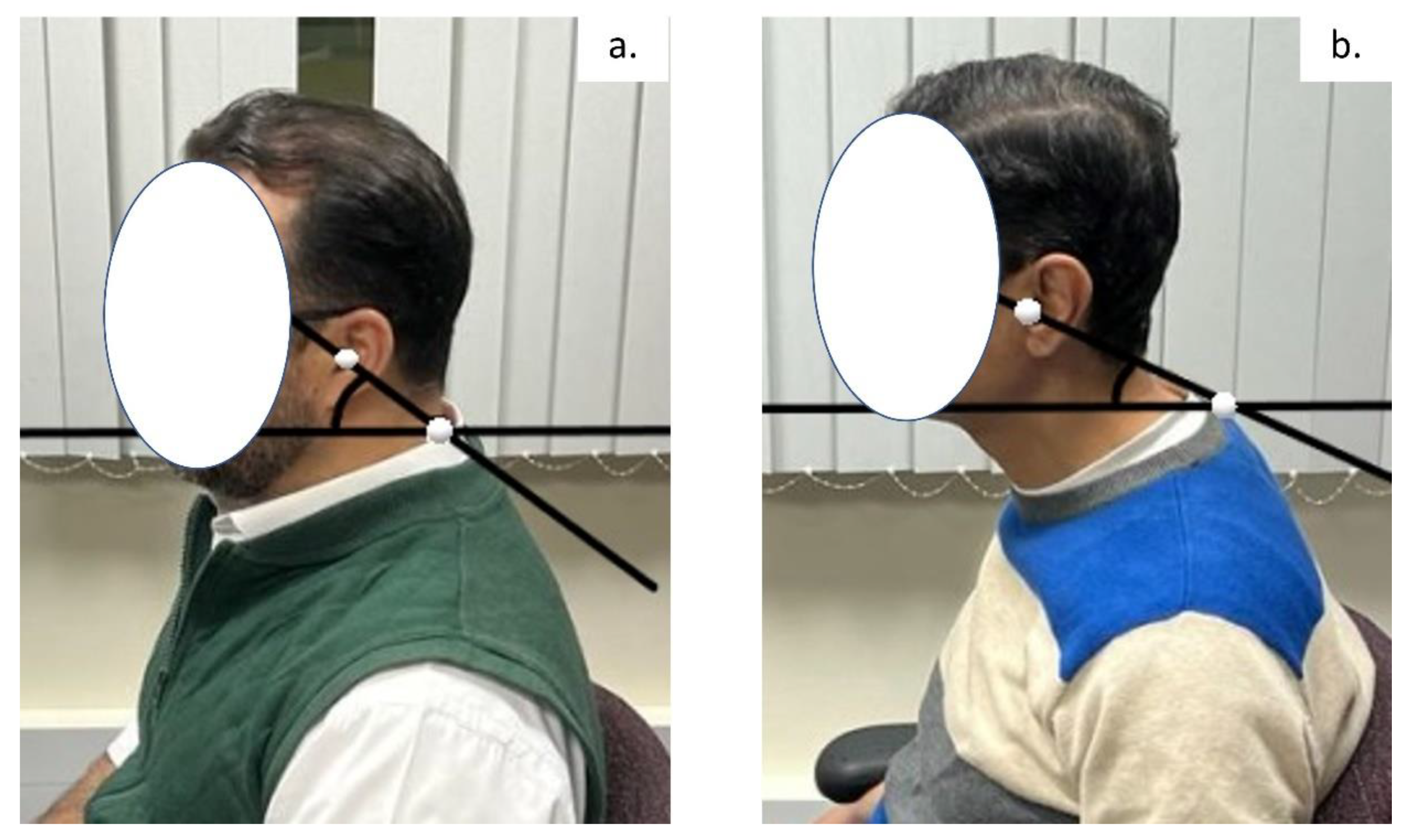
2.2.1. Craniovertebral Angle (CVA)
2.2.2. Evaluation of Sensorimotor Integration and Somatosensory Processing
2.3. Sample Size Determination
2.4. Data Analysis
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Between Group Analysis
3.3. Correlation of Findings between Groups
3.4. Logistic Regession Modelling
4. Discussion
4.1. Cortical, Subcortical, and Spinal Neural Changes
4.2. Central Somatosensory Conduction Time
4.3. Clinical Implications
4.4. Limitations
4.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machado, S.; Cunha, M.; Velasques, B.; Minc, D.; Teixeira, S.; Domingues, C.A.; Silva, J.G.; Bastos, V.H.; Budde, H.; Cagy, M.; et al. Sensorimotor integration: Basic concepts, abnormalities related to movement disorders and sensorimotor training-induced cortical reorganization. Rev. Neurol. 2010, 51, 427–436. [Google Scholar] [PubMed]
- Machado, D.; Bastos, V.H.; Cunha, M.; Velasques, B.; Machado, S.; Basile, L.; Cagy, M.; Piedade, R.; Ribeiro, P. Efectos del bromacepam en el desarrollo de una actividad sensoriomotora: Un estudio electroencefalográfico. Rev. Neurol. 2009, 49, 295–299. [Google Scholar] [PubMed]
- Krakauer, J.W.; Mazzoni, P.; Ghazizadeh, A.; Ravindran, R.; Shadmehr, R. Generalization of Motor Learning Depends on the History of Prior Action. PLoS Biol. 2006, 4, e316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, M.J.; Hwang, R.; Mroz, T.; Benzel, E.; Steinmetz, M.P. Relationship between degree of focal kyphosis correction and neurological outcomes for patients undergoing cervical deformity correction surgery. J. Neurosurg. Spine 2013, 18, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.S.; Lafage, V.; Ryan, D.J.; Shaffrey, C.I.; Schwab, F.J.; Patel, A.A.; Brodke, D.S.; Arnold, P.M.; Riew, K.D.; Traynelis, V.C.; et al. Association of Myelopathy Scores With Cervical Sagittal Balance and Normalized Spinal Cord Volume. Spine 2013, 38 (Suppl. 1), S161–S170. [Google Scholar] [CrossRef]
- Flor, H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl. Psychophysiol. Biofeedback 2002, 27, 215–227. [Google Scholar] [CrossRef]
- Mercier, C.; Léonard, G. Interactions between Pain and the Motor Cortex: Insights from Research on Phantom Limb Pain and Complex Regional Pain Syndrome. Physiother. Can. 2011, 63, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Bank, P.J.M.; Peper, C.E.; Marinus, J.; Beek, P.J.; van Hilten, J.J. Motor consequences of experimentally induced limb pain: A systematic review. Eur. J. Pain. 2013, 17, 145–157. [Google Scholar] [CrossRef]
- Bowering, K.J.; O’Connell, N.; Tabor, A.; Catley, M.; Leake, H.B.; Moseley, L.; Stanton, T. The Effects of Graded Motor Imagery and Its Components on Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain. 2013, 14, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Moseley, L.G. Graded motor imagery is effective for long-standing complex regional pain syndrome: A randomised controlled trial. Pain 2004, 108, 192–198. [Google Scholar] [CrossRef]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in Cortical and Cerebellar Motor Processing in Subclinical Neck Pain Patients Following Spinal Manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B. Altered Sensorimotor Integration With Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2008, 31, 115–126. [Google Scholar] [CrossRef]
- Hishinuma, M.; Yamaguchi, T. Axonal projection of descending pathways responsible for eliciting forelimb stepping into the cat cervical spinal cord. Exp. Brain Res. 1990, 82, 597–605. [Google Scholar] [CrossRef]
- Knight, R.T.; Staines, W.R.; Swick, D.; Chao, L.L. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. 1999, 101, 159–178. [Google Scholar] [CrossRef]
- Fernandez-de-las-Penas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Pareja, J.A. Forward head posture and neck mobility in chronic tension-type headache: A blinded, controlled study. Cephalgia 2006, 26, 314–319. [Google Scholar] [CrossRef]
- Patwardhan AGKhayatzadeh, S.; Havey, R.M.; Voronov, L.I.; Smith, Z.A.; Kalmanson, O.; Ghanayem, A.J.; Sears, W. Cervical sagittal balance: A biomechanical perspecive can help clinical practice. Eur. Spine J. 2018, 27 (Suppl. 1), 25–28. [Google Scholar] [CrossRef]
- Khayatzadey, S.; Kalmanson, O.A.; Schuit, D.; Havey, R.M.; Voronov, L.I.; Ghanayem, A.J.; Patwardhan, A.G. Cervical Spine Muscle-Tendon Unit Length Differences between Neutral and Forward Head Postures: Biomechanical Study Using Human Cadaveric Specimens. Phys. Ther. 2017, 97, 756–766. [Google Scholar] [CrossRef] [Green Version]
- Mousavi-Khatir, R.; Talebian, S.; Toosizadeh, N.; Olyaei, G.R.; Maroufi, N. Disturbance of neck proprioception and feed-forward motor control following static neck flexion in healthy young adults. J. Electromyogr. Kinesiol. 2018, 41, 160–167. [Google Scholar] [CrossRef]
- Nese, G.Y.; Yasemin, E. Diagnostic value of the F-wave in loss of the cervical lordosis. Neurophysiology 2020, 52, 192–197. [Google Scholar] [CrossRef]
- Moustafa, I.; Kim, M.; Harrison, D.E. Comparison of Sensorimotor Integration and Skill-Related Physical Fitness Components Between College Athletes with and Without Forward Head Posture. J. Sport. Rehabil. 2022, 32, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, I.M.; Youssef, A.; Ahbouch, A.; Tamim, M.; Harrison, D.E. Is forward head posture relevant to autonomic nervous system function and cervical sensorimotor control? Cross sectional study. Gait Posture 2020, 77, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.T.; Chiu, T.T.W.; Poon, A.T.K. The relationship between head posture and severity and disability of patients with neck pain. Man. Ther. 2008, 13, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartolini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle pain in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, S.M.; Louw, Q.; Vaughan, C.; Grimmer-Somers, K.; Schreve, K. Photographic measurement of upper-body sitting posture of high school students: A reliability and validity study. BMC Musculoskelet. Disord. 2008, 9, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falla, D.; Jull, G.; Russell, T.; Vicenzino, B.; Hodges, P. Effect of Neck Exercise on Sitting Posture in Patients With Chronic Neck Pain. Phys. Ther. 2007, 87, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Mauguière, F.; Allison, T.; Babiloni, C.; Buchner, H.; Eisen, A.A.; Goodin, D.S.; Jones, S.J.; Kakigi, R.; Matsuoka, S.; Nuwer, M.; et al. Somatosensory evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 79–90. [Google Scholar]
- Ulas, U.H.; Özdag, F.; Eroglu, E.; Odabasi, Z.; Kutukcu, Y.; Demirkaya, S.; Gökçil, Z.; Hamamcioglu, K.; Vural, O. Median Nerve Somatosensory Evoked Potentials Recorded with Cephalic and Noncephalic References in Central and Peripheral Nervous System Lesions. Clin. EEG Neurosci. 2001, 32, 191–196. [Google Scholar] [CrossRef]
- Tinazzi, M.; Priori, A.; Bertolasi, L.; Frasson, E.; Mauguière, F.; Fiaschi, A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 2000, 123, 42–50. [Google Scholar] [CrossRef]
- Tinazzi, M.; Fiaschi, A.; Rosso, T.; Faccioli, F.; Grosslercher, J.; Aglioti, S.M. Neuroplastic Changes Related to Pain Occur at Multiple Levels of the Human Somatosensory System: A Somatosensory-Evoked Potentials Study in Patients with Cervical Radicular Pain. J. Neurosci. 2000, 20, 9277–9283. [Google Scholar] [CrossRef] [Green Version]
- Desmedt, J.E.; Cheron, G. Prevertebral (oesophageal) recording of subcortical somatosensory evoked potentials in man: The spinal P13 component and the dual nature of the spinal generators. Electroencephalogr. Clin. Neurophysiol. 1981, 52, 257–275. [Google Scholar] [CrossRef]
- Allison, T.; McCarthy, G.; Wood, C.C.; Jones, S.J. Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 1991, 114, 2465–2503. [Google Scholar] [CrossRef]
- Mauguière, F.; Desmedt, J.E.; Courjon, J. Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning. Brain 1983, 106 Pt 2, 271–311. [Google Scholar] [CrossRef]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef]
- Cebolla, A.M.; Palmero-Soler, E.; Dan, B.; Cheron, G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. Neuroimage 2011, 54, 1297–1306. [Google Scholar] [CrossRef]
- Zabihhosseinian, M.; Yielder, P.; Wise, R.; Holmes, M.; Murphy, B. Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials. Brain Sci. 2021, 11, 1481. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yagi, K.; Tsuruta, K.; Taniguchi, A.; Ishii, N.; Shiomi, K.; Nakazato, M. Prolonged central sensory conduction time in patients with chronic arsenic exposure. J. Neurol. Sci. 2016, 361, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Bouwes, A.; Doesborg, P.G.G.; Laman, D.M.; Koelman, J.H.T.M.; Imanse, J.G.; Tromp, S.C.; van Geel, B.M.; van der Kooi, E.L.; Zandbergen, E.G.J.; Horn, J. Hypothermia after CPR prolongs conduction times of somatosensory evoked potentials. Neurocrit Care 2013, 19, 25–30. [Google Scholar] [CrossRef]
- Cohen, J. Some statistical issues in psychological research. In Handbook of Clinical Psychology; Wolman, B.B., Ed.; McGraw-Hill: New York, NY, USA, 1965; pp. 95–121. [Google Scholar]
- Parker, J.L.; Dostrovsky, J.O. Cortical involvement in the induction, but not expression, of thalamic plasticity. J. Neurosci. 1999, 19, 8623–8629. [Google Scholar] [CrossRef] [Green Version]
- Florence, S.L.; Hackett, T.A.; Strata, F. Thalamic and cortical contributions to neural plasticity after limb amputation. J. Neurophysiol. 2000, 83, 3154–3159. [Google Scholar] [CrossRef]
- Taylor, H.H.; Murphy, B. Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Jones, E.W.; Janik, T.J.; Harrison, D.D. Evaluation of axial and flexural stresses in the vertebral body cortex and trabecular bone in lordosis and two sagittal cervical translation configurations with an elliptical shell model. J. Manip. Physiol. Ther. 2002, 25, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Thoomes, E.J.; Scholten-Peeters, W.; Koes, B.; Falla, D.; Verhagen, A.P. The effectiveness of conservative treatment for patients with cervical radiculopathy: A systematic review. Clin. J. Pain. 2013, 29, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Yeo, H.G.; Kim, T.U.; Hyun, J.K.; Kim, J.Y. Radiologic assessment of forward head posture and its relation to myofascial pain syndrome. Ann. Rehabil. Med. 2014, 38, 821–826. [Google Scholar] [CrossRef]
- Harrison, D.D.E.; Cailliet, R.; Harrison, D.D.E.; Troyanovich, S.J.; Harrison, S.O. A review of biomechanics of the central nervous system–part II: Spinal cord strains from postural loads. J. Manip. Physiol. Ther. 1999, 22, 322–332. [Google Scholar] [CrossRef]
- Breig, A. Adverse Mechanical Tension in the Central Nervous System: An Analysis of Cause and Effect: Relief by Functional Neurosurgery; Wiley: New York, NY, USA, 1978; 130p. [Google Scholar]
- Moustafa, I.M.; Diab, A.A. The effect of adding forward head posture corrective exercises in the management of lumbosacral radiculopathy: A randomized controlled study. J. Manip. Physiol. Ther. 2015, 38, 167–178. [Google Scholar] [CrossRef]
- Diab, A.A.; Moustafa, I.M. The efficacy of forward head correction on nerve root function and pain in cervical spondylotic radiculopathy: A randomized trial. Clin. Rehab 2012, 26, 351–361. [Google Scholar] [CrossRef]
- Baker, P.F.; Ladds, M.; Rubinson, K.A. Measurement of the flow properties of isolated axoplasm in a defined chemical environment. J. Physiol. 1977, 269, 10P–11P. [Google Scholar]
- Kim, M.S.; Cha, Y.J.; Choi, J.D. Correlation between forward head posture, respiratory functions, and respiratory accessory muscles in young adults. J. Back. Musculoskelet. Rehabil. 2017, 30, 711–715. [Google Scholar] [CrossRef]
- Zafar, H.; Albarrati, A.; Alghadir, A.H.; Iqbal, Z.A. Effect of different head-nec postures on the respiratory function in healthy males. Biomed. Res. Int. 2018, 2018, 4518269. [Google Scholar] [CrossRef] [Green Version]
- McCormick, P.C.; Stein, B.M. Functional anatomy of the spinal cord and related structures. Neurosurg. Clin. N. Am. 1990, 1, 469–489. [Google Scholar] [CrossRef]
- Bulut, M.D.; Alpayci, M.; Şenköy, E.; Bora, A.; Yazmalar, L.; Yavuz, A.; Gülşen, I. Decreased Vertebral Artery Hemodynamics in Patients with Loss of Cervical Lordosis. Med. Sci. Monit. 2016, 22, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Katz, E.; Katz, S.; Fedorchuk, C.; Lightstone, D.; Banach, C.; Podoll, J. Increase in cerebral blood flow indicated by increased cerebral arterial area and pixel intensity on brain magnetic resonance angiogram following correction of cervical lordosis. Brain Circ. 2019, 5, 19. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Diab, A.A.; Hegazy, F.; Harrison, D.E. Demonstration of central conduction time and neuroplastic changes after cervical lordosis rehabilitation in asymptomatic subjects: A randomized, placebo-controlled trial. Sci. Rep. 2021, 11, 15379. [Google Scholar] [CrossRef]
- Moustafa, I.; Youssef, A.S.A.; Ahbouch, A.; Harrison, D. Demonstration of Autonomic Nervous Function and Cervical Sensorimotor Control After Cervical Lordosis Rehabilitation: A Randomized Controlled Trial. J. Athl. Train. 2021, 56, 427–436. [Google Scholar] [CrossRef]
- Singla, D.; Veqar, Z.; Hussain, M.E. Photogrammetric Assessment of Upper Body Posture Using Postural Angles: A Literature Review. J. Chiropr. Med. 2017, 16, 131–138. [Google Scholar] [CrossRef] [Green Version]





| Variable | FHP (n = 60) | CG (n = 60) | p-Value |
|---|---|---|---|
| Age (years) | 23.5 ± 2 | 25.9 ± 2 | 0.07 |
| Weight (kg) | 67.2 ± 3 | 69.2 ± 5 | 0.11 |
| Gender (%) | |||
| Male | 35 (58%) | 33 (55%) | 0.3 |
| Female | 25 (42%) | 27 (45%) | |
| Smoking | |||
| Light smoker | 18 | 16 | 0.2 |
| Heavy smoker | 0 | 0 | |
| No Smoker | 42 | 44 | |
| Educational level | |||
| Bachelor or Master | 43 | 36 | <0.005 |
| High school or less | 17 | 24 | |
| Marital status | |||
| Married | 32 | 24 | <0.005 |
| Not married | 28 | 36 | |
| BMI | |||
| Normal | 45 | 26 | <0.005 |
| Obese | 15 | 34 | |
| Working hours | |||
| Full-time | 22 | 42 | <0.005 |
| Part-time | 38 | 18 | |
| Neurophysiological Outcome Measure | FHP Group | Control Group | Mean Difference between the Two Groups | (95% CI)/ Cohen’s d | p Value | p Value (A) |
|---|---|---|---|---|---|---|
| N9 | 1.8 ± 0.2 | 1.7 ± 0.34 | 0.1 | [0.07, 0.21]/0.1 | =0.07 | 0.6 |
| P14 | 1.67 ± 0.6 | 1.3 ± 0.63 | 0.37 | [0.25, 0.49]/0.77 | <0.005 | 0.02 |
| N20 | 2.61 ± 0.61 | 2.1 ± 0.52 | 0.51 | [0.33, 0.6]/0.9 | <0.005 | <0.005 |
| P27 | 3.2 ± 0.7 | 2.7 ± 0.5 | 0.5 | [0.41, 0.69]/0.8 | <0.005 | 0.04 |
| N30 | 2.91 ± 0.64 | 2.4 ± 0.58 | 0.51 | [0.359, 0.69]/2.45 | <0.005 | 0.003 |
| N13 | 2 ± 0.5 | 1.6 ± 0.45 | 0.4 | [0.11, 0.35]/0.8 | <0.005 | 0.004 |
| N13–N20 | 1.77 ± 0.46 | 1.5 ± 0.51 | 0.27 | [0.07, 0.51]/0.56 | =0.004 | <0.005 |
| Correlation | CVA FHP r (p-Value) | CVA CG r (p-Value) |
|---|---|---|
| N9 | −0.44 <0.001 | −0.5 <0.001 |
| N13 | −0.67 <0.001 | −0.54 <0.001 |
| P14 | −0.58 <0.001 | −0.57 <0.001 |
| N20 | −0.49 <0.001 | −0.51 <0.001 |
| P27 | −0.58 <0.001 | −0.6 <0.001 |
| N30 | −0.64 <0.001 | −0.61 <0.001 |
| N13–N20 | −0.61 <0.001 | −0.56 <0.001 |
| P14 | N20 | P27 | N30 | N13 | N13–N20 | |
|---|---|---|---|---|---|---|
| Predictors | Odds ratios (p-value) | Odds ratios (p-value) | Odds ratios (p-value) | Odds ratios (p-value) | Odds ratios (p-value) | Odds ratios (p-value) |
| BMI (Obesity) | 0.4 0.06 | 0.23 0.06 | 0.13 0.3 | 0.16 0.34 | 0.2 0.06 | 0.2 0.06 |
| Educational level (Bachelor or Master) | 1.2 0.4 | 3.2 0.08 | 2.3 0.3 | 1.2 0.4 | 2.4 0.32 | 1.5 0.42 |
| Marital status (Not married) | 1.54 0.2 | 1.54 0.2 | 1.3 0.3 | 1.3 0.3 | 1.5 0.2 | 1.8 0.09 |
| Weekly working hours (Full-time) | 13.1 <0.005 | 12.4 <0.005 | 19.5 <0.005 | 25.9 <0.005 | 28 <0.005 | 19.4 <0.005 |
| CVA | 0.41 <0.005 | 0.3 <0.005 | 0.3 <0.005 | 0.57 <0.005 | 0.23 <0.005 | 0.34 <0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa, I.M.; Diab, A.A.M.; Harrison, D.E. Does Forward Head Posture Influence Somatosensory Evoked Potentials and Somatosensory Processing in Asymptomatic Young Adults? J. Clin. Med. 2023, 12, 3217. https://doi.org/10.3390/jcm12093217
Moustafa IM, Diab AAM, Harrison DE. Does Forward Head Posture Influence Somatosensory Evoked Potentials and Somatosensory Processing in Asymptomatic Young Adults? Journal of Clinical Medicine. 2023; 12(9):3217. https://doi.org/10.3390/jcm12093217
Chicago/Turabian StyleMoustafa, Ibrahim M., Aliaa Attiah Mohamed Diab, and Deed E. Harrison. 2023. "Does Forward Head Posture Influence Somatosensory Evoked Potentials and Somatosensory Processing in Asymptomatic Young Adults?" Journal of Clinical Medicine 12, no. 9: 3217. https://doi.org/10.3390/jcm12093217








