Structural Interventions in Heart Failure: Mending a Broken Heart
Abstract
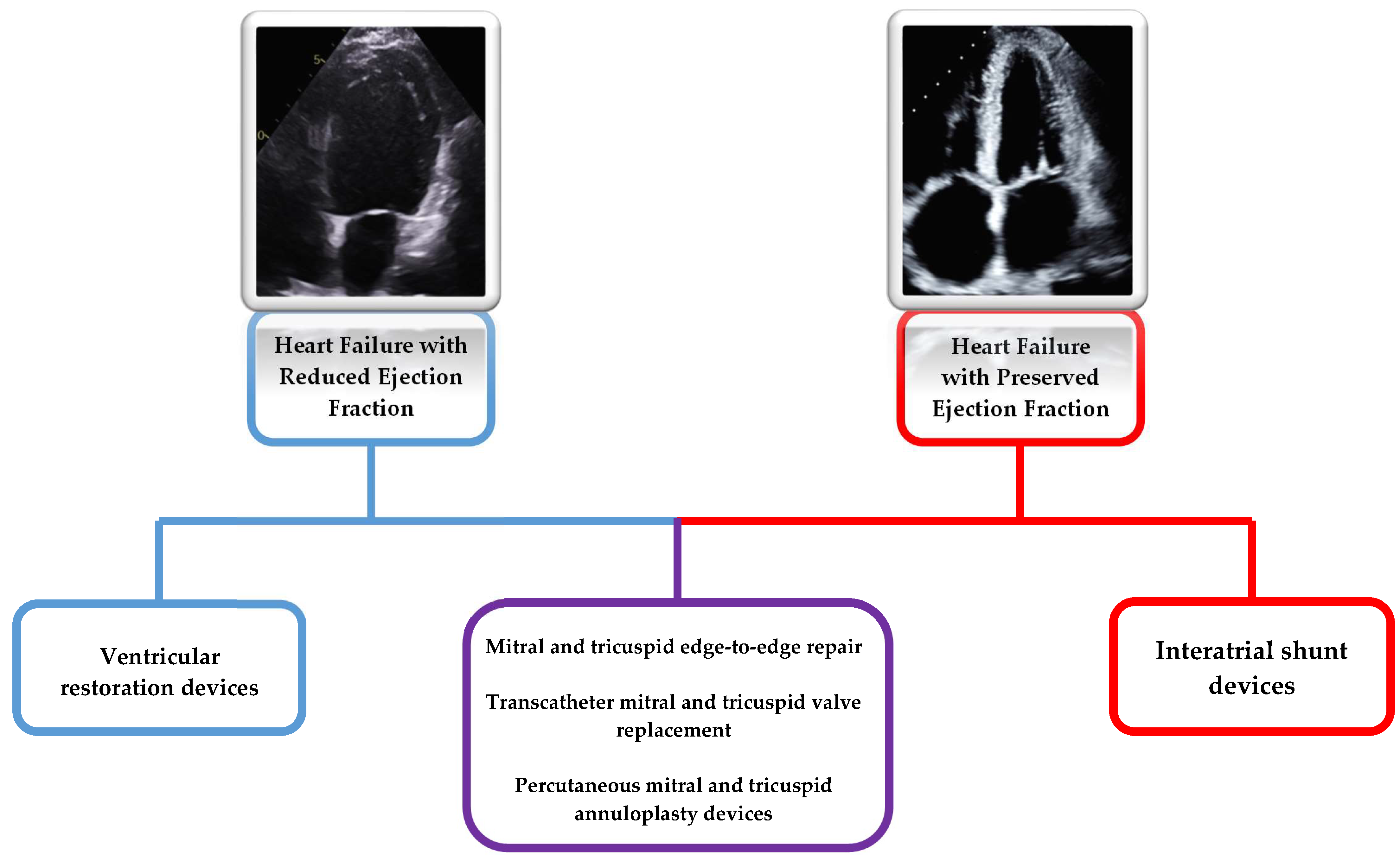
1. Introduction
2. Valvular Heart Disease
2.1. Mitral Regurgitation
2.2. Edge-to-Edge Mitral Valve Repair
2.3. MitraClip
2.4. PASCAL
2.5. Transcatheter Mitral Annuloplasty
2.6. Carillon Device
2.7. Cardioband Device
2.8. Mitralign
2.9. Transcatheter Mitral Valve Replacement (TMVR)
2.10. Tricuspid Valve
2.11. Tricuspid Annuloplasty Devices
2.12. Edge-to-Edge Tricuspid Valve Repair
2.13. Transcatheter Tricuspid Valve Replacement (TTVR)
3. Ventricular Restorative Devices
3.1. Parachute Device
3.2. AccuCinch
3.3. Revivent TC System
4. Interatrial Shunting Devices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated With Heart Failure With Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Gröger, M.; Scheffler, J.K.; Schösser, F.; Schneider, L.M.; Rottbauer, W.; Markovic, S.; Keßler, M. Percutaneous edge-to-edge mitral valve repair for mitral regurgitation improves heart failure symptoms in heart failure with preserved ejection fraction patients. ESC Heart Fail. 2021, 8, 5010–5021. [Google Scholar] [CrossRef]
- Szerlip, M.; Spargias, K.S.; Makkar, R.; Kar, S.; Kipperman, R.M.; O’Neill, W.W.; Ng, M.K.C.; Smith, R.L.; Fam, N.P.; Rinaldi, M.J.; et al. 2-Year Outcomes for Transcatheter Repair in Patients With Mitral Regurgitation From the CLASP Study. JACC Cardiovasc. Interv. 2021, 14, 1538–1548. [Google Scholar] [CrossRef]
- Siminiak, T.; Wu, J.C.; Haude, M.; Hoppe, U.C.; Sadowski, J.; Lipiecki, J.; Fajadet, J.; Shah, A.M.; Feldman, T.; Kaye, D.M.; et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: Results of the TITAN Trial. Eur. J. Heart Fail. 2012, 14, 931–938. [Google Scholar] [CrossRef][Green Version]
- Lipiecki, J.; Siminiak, T.; Sievert, H.; Müller-Ehmsen, J.; Degen, H.; Wu, J.C.; Schandrin, C.; Kalmucki, P.; Hofmann, I.; Reuter, D.; et al. Coronary sinus-based percutaneous annuloplasty as treatment for functional mitral regurgitation: The TITAN II trial. Open Heart 2016, 3, e000411. [Google Scholar] [CrossRef][Green Version]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Nickenig, G.; Latib, A.; Kuck, K.H.; Baldus, S.; Schueler, R.; La Canna, G.; Agricola, E.; Kreidel, F.; Huntgeburth, M.; et al. Transcatheter mitral valve repair for functional mitral regurgitation using the Cardioband system: 1 year outcomes. Eur. Heart J. 2019, 40, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.W.M.; Sorajja, P.; Duncan, A.; Bethea, B.; Dahle, G.; Grayburn, P.; Babaliaros, V.; Guerrero, M.; Thourani, V.H.; Bedogni, F.; et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J. Am. Coll. Cardiol. 2021, 78, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.G.; Kreidel, F.; Hell, M.M.; Praz, F.; Mach, M.; Adam, M.; Reineke, D.; Ruge, H.; Ludwig, S.; Conradi, L.; et al. Transapical mitral valve implantation for treatment of symptomatic mitral valve disease: A real-world multicentre experience. Eur. J. Heart Fail. 2022, 24, 899–907. [Google Scholar] [CrossRef]
- Guerrero, M.; Wang, D.D.; Pursnani, A.; Salinger, M.; Russell, H.M.; Eleid, M.; Chakravarty, T.; Ng, M.H.; Kodali, S.K.; Meduri, C.U.; et al. Prospective Evaluation of TMVR for Failed Surgical Annuloplasty Rings: MITRAL Trial Valve-in-Ring Arm 1-Year Outcomes. JACC Cardiovasc. Interv. 2021, 14, 846–858. [Google Scholar] [CrossRef]
- Guerrero, M.; Pursnani, A.; Narang, A.; Salinger, M.; Wang, D.D.; Eleid, M.; Kodali, S.K.; George, I.; Satler, L.; Waksman, R.; et al. Prospective Evaluation of Transseptal TMVR for Failed Surgical Bioprostheses: MITRAL Trial Valve-in-Valve Arm 1-Year Outcomes. JACC Cardiovasc. Interv. 2021, 14, 859–872. [Google Scholar] [CrossRef]
- Gray, W.A.; Abramson, S.V.; Lim, S.; Fowler, D.; Smith, R.L.; Grayburn, P.A.; Kodali, S.K.; Hahn, R.T.; Kipperman, R.M.; Koulogiannis, K.P.; et al. 1-Year Outcomes of Cardioband Tricuspid Valve Reconstruction System Early Feasibility Study. JACC Cardiovasc. Interv. 2022, 15, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Meduri, C.U.; Davidson, C.J.; Lim, S.; Nazif, T.M.; Ricciardi, M.J.; Rajagopal, V.; Ailawadi, G.; Vannan, M.A.; Thomas, J.D.; et al. Early Feasibility Study of a Transcatheter Tricuspid Valve Annuloplasty: SCOUT Trial 30-Day Results. J. Am. Coll. Cardiol. 2017, 69, 1795–1806. [Google Scholar] [CrossRef]
- Lurz, P.; Stephan von Bardeleben, R.; Weber, M.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.N.; Nabauer, M.; Tang, G.H.L.; et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2021, 77, 229–239. [Google Scholar] [CrossRef]
- Mehr, M.; Taramasso, M.; Besler, C.; Ruf, T.; Connelly, K.A.; Weber, M.; Yzeiraj, E.; Schiavi, D.; Mangieri, A.; Vaskelyte, L.; et al. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovasc. Interv. 2019, 12, 1451–1461. [Google Scholar] [CrossRef]
- Estévez-Loureiro, R.; Sánchez-Recalde, A.; Amat-Santos, I.J.; Cruz-González, I.; Baz, J.A.; Pascual, I.; Mascherbauer, J.; Abdul-Jawad Altisent, O.; Nombela-Franco, L.; Pan, M.; et al. 6-Month Outcomes of the TricValve System in Patients With Tricuspid Regurgitation: The TRICUS EURO Study. JACC Cardiovasc. Interv. 2022, 15, 1366–1377. [Google Scholar] [CrossRef]
- Costa, M.A.; Mazzaferri, E.L.; Sievert, H.; Abraham, W.T. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: Three-year outcomes of the PARACHUTE first-in-human study. Circ. Heart Fail. 2014, 7, 752–758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, P.; Anker, S.D.; Wechsler, A.; Skalsky, I.; Neuzil, P.; Annest, L.S.; Bifi, M.; McDonagh, T.; Frerker, C.; Schmidt, T.; et al. Less invasive ventricular reconstruction for ischaemic heart failure. Eur. J. Heart Fail. 2019, 21, 1638–1650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, S.J.; Borlaug, B.A.; Chung, E.S.; Cutlip, D.E.; Debonnaire, P.; Fail, P.S.; Gao, Q.; Hasenfuß, G.; Kahwash, R.; Kaye, D.M.; et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): A randomised, multicentre, blinded, sham-controlled trial. Lancet 2022, 399, 1130–1140. [Google Scholar] [CrossRef]
- Shah, S.J.; Feldman, T.; Ricciardi, M.J.; Kahwash, R.; Lilly, S.; Litwin, S.; Nielsen, C.D.; van der Harst, P.; Hoendermis, E.; Penicka, M.; et al. One-Year Safety and Clinical Outcomes of a Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure with Preserved Ejection Fraction in the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP-HF I) Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 968–977. [Google Scholar] [CrossRef][Green Version]
- Asgar, A.W.; Mack, M.J.; Stone, G.W. Secondary mitral regurgitation in heart failure: Pathophysiology, prognosis, and therapeutic considerations. J. Am. Coll. Cardiol. 2015, 65, 1231–1248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartko, P.E.; Heitzinger, G.; Pavo, N.; Heitzinger, M.; Spinka, G.; Prausmüller, S.; Arfsten, H.; Andreas, M.; Gabler, C.; Strunk, G.; et al. Burden, treatment use, and outcome of secondary mitral regurgitation across the spectrum of heart failure: Observational cohort study. BMJ 2021, 373, n1421. [Google Scholar] [CrossRef] [PubMed]
- Noly, P.-E.; Pagani, F.D.; Obadia, J.-F.; Bouchard, D.; Bolling, S.F.; Ailawadi, G.; Tang, P.C. The role of surgery for secondary mitral regurgitation and heart failure in the era of transcatheter mitral valve therapies. Rev. Cardiovasc. Med. 2022, 23, 87. [Google Scholar] [CrossRef]
- Rossi, A.; Dini, F.L.; Faggiano, P.; Agricola, E.; Cicoira, M.; Frattini, S.; Simioniuc, A.; Gullace, M.; Ghio, S.; Enriquez-Sarano, M.; et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 1675–1680. [Google Scholar] [CrossRef]
- Chan, K.M.J.; Punjabi, P.P.; Flather, M.; Wage, R.; Symmonds, K.; Roussin, I.; Rahman-Haley, S.; Pennell, D.J.; Kilner, P.J.; Dreyfus, G.D.; et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: Final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation 2012, 126, 2502–2510. [Google Scholar] [CrossRef][Green Version]
- Percutaneous MitraClip Device or Surgical Mitral Valve REpair in PAtients with PrImaRy MItral Regurgitation Who Are Candidates for Surgery (REPAIR MR). Available online: https://clinicaltrials.gov/ct2/show/NCT04198870 (accessed on 22 April 2023).
- Ikenaga, H.; Higashihara, T.; Itakura, K.; Utsunomiya, H.; Fukuda, Y.; Takasaki, T.; Takahashi, S.; Nakano, Y. Successful MitraClip Therapy for Atrial Functional Mitral Regurgitation With Severe Mitral Annular Calcification. JACC Cardiovasc. Interv. 2021, 14, 101–102. [Google Scholar] [CrossRef]
- Lavall, D.; Hagendorff, A.; Schirmer, S.H.; Böhm, M.; Borger, M.A.; Laufs, U. Mitral valve interventions in heart failure. ESC Heart Fail. 2018, 5, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Maisano, F.; Torracca, L.; Oppizzi, M.; Stefano, P.L.; D’Addario, G.; La Canna, G.; Zogno, M.; Alfieri, O. The edge-to-edge technique: A simplified method to correct mitral insufficiency. Eur. J. Cardiothorac. Surg. 1998, 13, 240–245; discussion 245–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, D.S.; Smith, R.L.; Gillam, L.D.; Zahr, F.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. Randomized Comparison of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation in Prohibitive Surgical Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Hinohara, T.T.; Reardon, M.J.; Goel, S.S. Latest Advances in Transcatheter Mitral Valve Replacement. Heart Int. 2021, 15, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Starling, R.C.; Khan, M.S.; Friede, T.; Filippatos, G.; Lindenfeld, J.; Stephan von Bardeleben, R.; Coats, A.J.S.; Butler, J. Percutaneous Mitral Valve Annuloplasty in Patients With Secondary Mitral Regurgitation and Severe Left Ventricular Enlargement. JACC Heart Fail. 2021, 9, 453–462. [Google Scholar] [CrossRef]
- Nickenig, G.; Schueler, R.; Dager, A.; Martinez Clark, P.; Abizaid, A.; Siminiak, T.; Buszman, P.; Demkow, M.; Ebner, A.; Asch, F.M.; et al. Treatment of Chronic Functional Mitral Valve Regurgitation With a Percutaneous Annuloplasty System. J. Am. Coll. Cardiol. 2016, 67, 2927–2936. [Google Scholar] [CrossRef]
- Acker, M.A.; Parides, M.K.; Perrault, L.P.; Moskowitz, A.J.; Gelijns, A.C.; Voisine, P.; Smith, P.K.; Hung, J.W.; Blackstone, E.H.; Puskas, J.D.; et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N. Engl. J. Med. 2014, 370, 23–32. [Google Scholar] [CrossRef][Green Version]
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar] [CrossRef][Green Version]
- Nagaraja, V.; Kapadia, S.R.; Krishnaswamy, A. Current and Future Application of Transcatheter Mitral Valve Replacement. Cardiol. Clin. 2021, 39, 221–232. [Google Scholar] [CrossRef]
- Taramasso, M.; Vanermen, H.; Maisano, F.; Guidotti, A.; La Canna, G.; Alfieri, O. The growing clinical importance of secondary tricuspid regurgitation. J. Am. Coll. Cardiol. 2012, 59, 703–710. [Google Scholar] [CrossRef][Green Version]
- Koelling, T.M.; Aaronson, K.D.; Cody, R.J.; Bach, D.S.; Armstrong, W.F. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am. Heart J. 2002, 144, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.N.; Menon, V.; Sammour, Y.M.; Gajulapalli, R.D.; Meenakshisundaram, C.; Nusairat, L.; Mohananey, D.; Hernandez, A.V.; Navia, J.; Krishnaswamy, A.; et al. Outcomes of patients with severe tricuspid regurgitation and congestive heart failure. Heart 2019, 105, 1813–1817. [Google Scholar] [CrossRef]
- Alperi, A.; Almendárez, M.; Álvarez, R.; Moris, C.; Leon, V.; Silva, I.; Hernández-Vaquero, D.; Pascual, I.; Avanzas, P. Transcatheter tricuspid valve interventions: Current status and future perspectives. Front. Cardiovasc. Med. 2022, 9, 994502. [Google Scholar] [CrossRef]
- Calen, C.; Taramasso, M.; Guidotti, A.; Kuwata, S.; Nietlispach, F.; Zuber, M.; Maisano, F. Successful TriCinch-in-TriCinch Transcatheter Tricuspid Valve Repair. JACC Cardiovasc. Interv. 2017, 10, e75–e77. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Nietlispach, F.; Zuber, M.; Maisano, F. Transcatheter repair of persistent tricuspid regurgitation after MitraClip with the TriCinch system: Interventional valve treatment toward the surgical standard. Eur. Heart J. 2017, 38, 1259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nickenig, G.; Weber, M.; Schueler, R.; Hausleiter, J.; Näbauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Kuck, K.H.; et al. 6-Month Outcomes of Tricuspid Valve Reconstruction for Patients With Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 1905–1915. [Google Scholar] [CrossRef]
- Romeo, J.D.; Bashline, M.J.; Fowler, J.A.; Kliner, D.E.; Toma, C.; Smith, A.C.; Sultan, I.; Sanon, S. Current Status of Transcatheter Tricuspid Valve Therapies. Heart Int. 2022, 16, 49–58. [Google Scholar] [CrossRef]
- Sorajja, P.; Whisenant, B.; Hamid, N.; Naik, H.; Makkar, R.; Tadros, P.; Price, M.J.; Singh, G.; Fam, N.; Kar, S.; et al. Transcatheter Repair for Patients with Tricuspid Regurgitation. N. Engl. J. Med. 2023. [Google Scholar] [CrossRef]
- Wild, M.G.; Löw, K.; Rosch, S.; Gerçek, M.; Higuchi, S.; Massberg, S.; Näbauer, M.; Rudolph, V.; Markovic, S.; Boekstegers, P.; et al. Multicenter Experience With the Transcatheter Leaflet Repair System for Symptomatic Tricuspid Regurgitation. JACC Cardiovasc. Interv. 2022, 15, 1352–1363. [Google Scholar] [CrossRef]
- Ponna, P.K.; Patin, S.; Turaga, N.S.S.; Zoltowska, D.M.; Devarkonda, V.; Botta, R.K.; Agrawal, Y.; Dhar, G. Transcatheter interventions for severe tricuspid regurgitation: A literature review. J. Geriatr. Cardiol. 2022, 19, 539–550. [Google Scholar] [CrossRef]
- Lauten, A.; Dreger, H.; Laule, M.; Stangl, K.; Figulla, H.R.; Eng, M.H. Caval Valve Implantation. Interv. Cardiol. Clin. 2022, 11, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kir, D.; Munagala, M. Restructuring the Heart From Failure to Success: Role of Structural Interventions in the Realm of Heart Failure. Front. Cardiovasc. Med. 2022, 9, 839483. [Google Scholar] [CrossRef] [PubMed]
- Ige, M.; Al-Kindi, S.G.; Attizzani, G.; Costa, M.; Oliveira, G.H. Percutaneous left ventricular restoration. Heart Fail. Clin. 2015, 11, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.P.; Oliveira, G.H. Percutaneous Ventricular Restoration with a Partitioning Device for Ischemic Heart Failure Treatment. Curr. Heart Fail. Rep. 2017, 14, 87–99. [Google Scholar] [CrossRef]
- Ancora Heart the AccuCinch Ventricular Restoration System. Available online: https://www.ancoraheart.com/physicians/ (accessed on 23 November 2022).
- Dorfs, S.; Zeh, W.; Hochholzer, W.; Jander, N.; Kienzle, R.P.; Pieske, B.; Neumann, F.J. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3103–3112. [Google Scholar] [CrossRef][Green Version]
- Ahlgrim, C.; Kocher, S.; Minners, J.; Jander, N.; Savarese, G.; Neumann, F.J.; Arentz, T.; Jadidi, A.; Mueller-Edenborn, B. Pulmonary Capillary Wedge Pressure during Exercise Is Prognostic for Long-Term Survival in Patients with Symptomatic Heart Failure. J. Clin. Med. 2022, 11, 5901. [Google Scholar] [CrossRef]
- Riccardi, M.; Tomasoni, D.; Vizzardi, E.; Metra, M.; Adamo, M. Device-based percutaneous treatments to decompress the left atrium in heart failure with preserved ejection fraction. Heart Fail. Rev. 2022, 28, 315–330. [Google Scholar] [CrossRef]
- Emani, S.; Burkhoff, D.; Lilly, S.M. Interatrial shunt devices for the treatment of heart failure. Trends Cardiovasc. Med. 2021, 31, 427–432. [Google Scholar] [CrossRef]
- Yum, B.K.W.; Frishman, W.H. Interatrial Shunting, a Novel Device-Based Therapy for Patients With Heart Failure. Cardiol. Rev. 2018, 26, 267–273. [Google Scholar] [CrossRef]
| Device | Implantation Approach | Population | Results | References | |
|---|---|---|---|---|---|
| Mitral Valve | |||||
| Edge-to-Edge Repair | |||||
| MitraClip (Abbott Medical, USA) | Femoral vein | HFrEF (LVEF 20–50%) Moderate-to-severe or severe functional MR | ↓ HF hospitalization at 24 months (annualized rate, HR 0.53, p < 0.001) ↓ MR severity in device group Improvement in NYHA functional class, KCCQ score at 12 months Improvement in LVEDV at 12 months ↓ All-cause mortality at 24 months (HR 0.62, p < 0.001) | [5] | |
| MitraClip (Abbott Medical, USA) | Femoral vein | HFpEF (LVEF > 50%) Degenerative and functional MR | ↓ MR severity at 12 months Improvement in NYHA functional class at 12 months ↓ HF hospitalization at 12 months | [6] | |
| PASCAL (Edwards Lifesciences LLC, USA) | Femoral vein | LVEF ≥ 20% MR grade ≥ 3+ NYHA class II–III Degenerative and functional MR | ↓ MR severity at 24 months in both DMR and FMR ↓ LVEDV at 24 months in both DMR and FMR Improvement in NYHA functional class at 24 months in both DMR and FMR ↓ Annualized rate of HF hospitalization in both DMR and FMR | [7] | |
| Annuloplasty Devices | |||||
| Carillon device (Cardiac Dimension Inc, USA) | Right internal jugular vein | LVEF < 40%, functional MR (TITAN I) LVEF < 40%, functional MR (TITAN II) LVEF < 50%, functional MR (REDUCE FMR) | ↓ Regurgitant volume (TITAN I, TITAN II, REDUCE FMR) ↓ MR Echocardiographic quantitative parameters (TITAN I, TITAN II) ↓ LVEDV (TITAN I, REDUCE FMR) ↓ LVESV (TITAN I, REDUCE FMR) ↑ 6MWD (TITAN I) ↑ KCCQ (TITAN I) ↓ Mitral annular diameter (TITAN II) | [8,9,10] | |
| Cardioband device (Edwards Lifesciences, USA) | Femoral vein | LVEF ≥ 25% Moderate or severe functional MR | ↓ MR severity at 12 months Improvement in NYHA functional class at 12 months ↑ 6MWD at 12 months | [11] | |
| Transcatheter Mitral-Valve Replacement (TMVR) | |||||
| Tendyne mitral-valve replacement system (Abbott, USA) | Transapical | Symptomatic moderate-to-severe MR | ↓ MR severity at 24 months ↓ RV systolic pressure at 24 months ↓ NYHA functional class ↓ Annualized rate of HF hospitalization | [12,13] | |
| Sapien 3 mitral-valve replacement system (Edwards Lifesciences, USA) | Femoral vein | Valve-in-MAC, valve-in-ring, valve-in-valve LVEF ≥20% NYHA functional class II or greater symptoms Severe mitral stenosis Moderate-to-severe MR | ↓ NYHA functional class at 12 months | [14,15] | |
| Tricuspid Valve | |||||
| Annuloplasty Devices | |||||
| TriAlign (Mitralign Inc, USA) TriCinch (4Tech Cardio Ltd., Ireland) Cardioband (Edwards Lifesciences, USA) Millipede device (Boston Scientific, USA) | Internal jugular vein (TriAlign) Femoral vein (Cardioband) Femoral vein (TriCinch) | NYHA functional class ≥ II and moderate or greater functional TR (TriAlign) ≥Moderate TR (Cardioband) | ↓ Tricuspid dimensions at 30 days (TriAlign) and 12 months (Cardioband) ↓ EROA at 30 days (TriAlign) and 12 months (Cardioband) ↑ LV stroke volume at 30 days (TriAlign) Improvement in NYHA functional class at 30 days (TriAlign) and 12 months (Cardioband) ↑ 6MWD at 30 days (TriAlign) and 6 months (Cardioband) | [16,17] | |
| Edge-to-Edge Repair | |||||
| TriClip (Abbott, USA) PASCAL (Edwards Lifesciences, USA) | Femoral vein | ↓ Tricuspid regurgitation at 12 months (TriClip) Improvement in NYHA functional class at 12 months (TriClip) ↑ 6MWD at 12 months (TriClip) ↑ KCCQ at 12 months (TriClip) | [18,19] | ||
| Tricuspid Valve Replacement | |||||
| Caval Techniques | TricValve system (P&F, Germany) | Femoral vein | ≥3+ tricuspid regurgitation NYHA functional class III–IV | Improvement in NYHA functional class at 6 months (TricValve) ↑ KCCQ at 6 months (TricValve) ↓ Hepatic vein backflow at 6 months (TricValve) | [20] |
| Ventricular Restoration Devices | |||||
| Parachute device (CardioKinetix, USA) AccuCinch (Ancora Heart, USA) Revivent TC system (BioVentrix, USA) | Femoral artery (Parachute device) Femoral artery (AccuCinch) Median sternotomy or hybrid mini thoracotomy + right internal jugular vein (Revivent) | NYHA functional class II–IV symptoms (PARACHUTE) LVEF 15–40% (PARACHUTE) Dilated, akinetic, or dyskinetic anterior apex (PARACHUTE) NYHA functional class II–IV symptoms (Revivent) LV dilation (Revivent) Transmural scar in anteroseptal, anterolateral, or apical segments (Revivent) | ↓ End diastolic volume index at 36 months (PARACHUTE) ↑ LVEF at 12 months (Revivent) ↓ LVESVi at 12 months (Revivent) ↓ LVEDVi at 12 months (Revivent) Improvement in NYHA functional class at 12 months (Revivent) ↑ 6MWD at 12 months (Revivent) | [21,22] | |
| Interatrial shunt devices | |||||
| Interatrial shunt device (IASD) (Corvia Medical, USA) V-wave shunt device (V-wave Ltd., Israel) | Femoral vein | NYHA functional class III or IVa (IASD) LVEF ≥ 40% (IASD) Exercise PCWP ≥ 25 mmHg (IASD) PCWP—RAP ≥ 5 mmHg (IASD) | ↓ Annual rate of HF hospitalization at 12 months (REDUCE LAP I) ↑ Right ventricular dimensions at 6 months (REDUCE LAP I) No reduction in CV death or non-fatal ischemic stroke at 12 months, HF events at 24 months, or KCCQ at 12 months (REDUCE LAP II) | [23,24] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzianer, D.; Albert, C. Structural Interventions in Heart Failure: Mending a Broken Heart. J. Clin. Med. 2023, 12, 3243. https://doi.org/10.3390/jcm12093243
Katzianer D, Albert C. Structural Interventions in Heart Failure: Mending a Broken Heart. Journal of Clinical Medicine. 2023; 12(9):3243. https://doi.org/10.3390/jcm12093243
Chicago/Turabian StyleKatzianer, David, and Chonyang Albert. 2023. "Structural Interventions in Heart Failure: Mending a Broken Heart" Journal of Clinical Medicine 12, no. 9: 3243. https://doi.org/10.3390/jcm12093243
APA StyleKatzianer, D., & Albert, C. (2023). Structural Interventions in Heart Failure: Mending a Broken Heart. Journal of Clinical Medicine, 12(9), 3243. https://doi.org/10.3390/jcm12093243




