Reversing Neuromuscular Blockade without Nerve Stimulator Guidance in a Postsurgical ICU—An Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. Baseline Characteristics and Follow-Up
2.3. Intraoperative and Perioperative Anesthesia
2.4. Neostigmine Administration
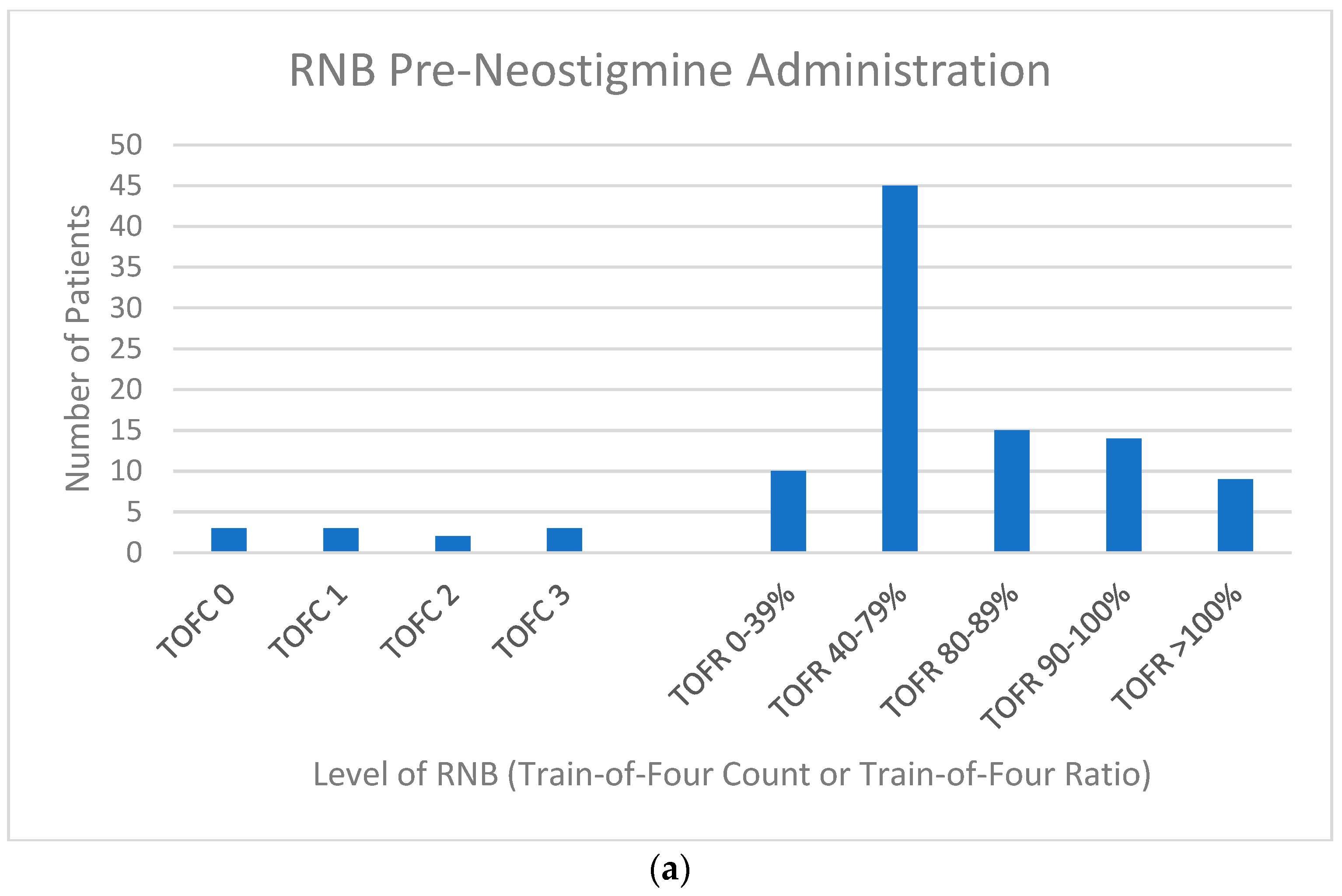
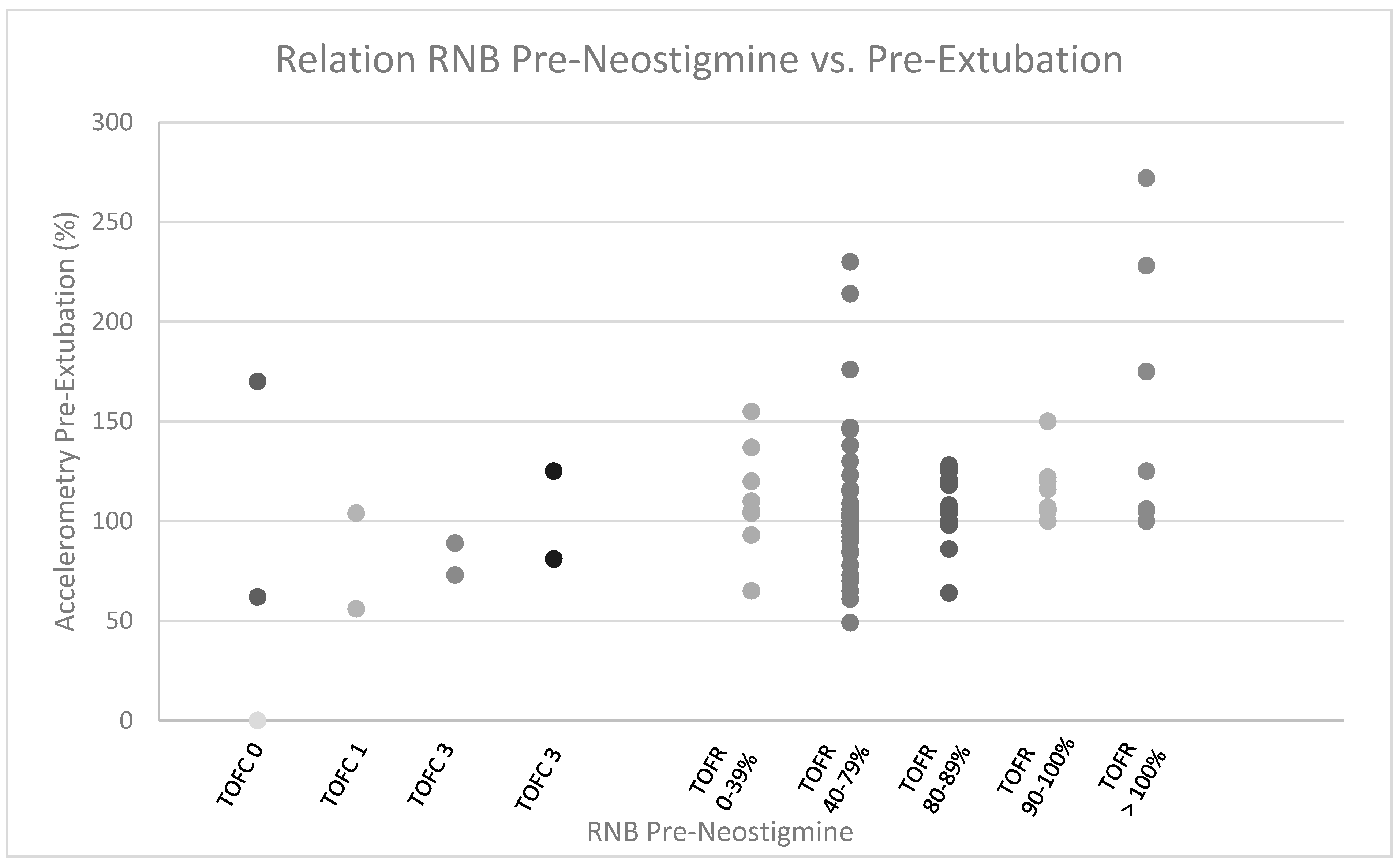
2.5. Residual Neuromuscular Blockade Assessment
2.6. Extubation Timing
3. Results
3.1. Patients Receiving Unplanned Postextubation Pulmonary Adjunct Support
3.2. Patients Receiving Planned Postextubation Pulmonary Adjunct Support
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| TOFC | Train-of-Four Count |
| TOFR | Train-of-Four Ratio |
| RV | Right Ventricle |
| HFNC | High-Flow Nasal Canula |
| CPAP | Continuous Positive Airway Pressure |
| BiPAP | Bilevel Positive Airway Pressure |
| OSA | Obstructive Sleep Apnea |
| CAB | Coronary Artery Bypass |
| MV | Mitral Valve |
| TV | Tricuspid Valve |
| LVAD | Left Ventricular Assist Device |
| ASD | Atrial Septal Defect |
| AscAo | Ascending Aorta |
| AoRoot | Aortic Root |
| IABP | Intra-aortic Balloon Pump |
| OR | Operating Room |
| RSBI | Rapid Shallow Breathing Index |
| BMI | Body Mass Index |
| SD | Standard Deviation |
References
- Stoelting, R.K. Monitoring of Neuromuscular Blockade: What Would You Expect If You Were the Patient? APSF Newsl. 2016, 30, 45–47. [Google Scholar]
- Brull, S.J.; Kopman, A.F. Current Status of Neuromuscular Reversal and Monitoring: Challenges and Opportunities. Anesthesiology 2017, 126, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.S.; Szokol, J.W.; Marymont, J.H.; Greenberg, S.B.; Avram, M.J.; Vender, J.S. Residual neuromuscular blockade and critical respiratory events in the postanesthesia care unit. Anesth. Analg. 2008, 107, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Cormack, R.S.; Lehane, J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984, 39, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Gottschalk, A.; Whitman, G.; Lawton, J.S.; Dodd, O.J.; Barodka, V. Opioid-Sparing Cardiac Anesthesia: Secondary Analysis of an Enhanced Recovery Program for Cardiac Surgery. Anesth. Analg. 2020, 131, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Whitman, G.; Lawton, J.S.; Dodd, O.J.; Barodka, V.; Johns Hopkins Enhanced Recovery Program for the Cardiac Surgery Working Group. Results from an enhanced recovery program for cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 1393–1402.e1397. [Google Scholar] [CrossRef]
- Yang, K.L.; Tobin, M.J. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. New Engl. J. Med. 1991, 324, 1445–1450. [Google Scholar] [CrossRef]
- Klein, A.A.; Meek, T.; Allcock, E.; Cook, T.M.; Mincher, N.; Morris, C.; Nimmo, A.F.; Pandit, J.J.; Pawa, A.; Rodney, G.; et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: Guideline from the Association of Anaesthetists. Anaesthesia 2021, 76, 1212–1223. [Google Scholar] [CrossRef]
- Naguib, M.; Brull, S.J.; Kopman, A.F.; Hunter, J.M.; Fulesdi, B.; Arkes, H.R.; Elstein, A.; Todd, M.M.; Johnson, K.B. Consensus Statement on Perioperative Use of Neuromuscular Monitoring. Anesth. Analg. 2018, 127, 71–80. [Google Scholar] [CrossRef]
- Plaud, B.; Baillard, C.; Bourgain, J.L.; Bouroche, G.; Desplanque, L.; Devys, J.M.; Fletcher, D.; Fuchs-Buder, T.; Lebuffe, G.; Meistelman, C.; et al. Guidelines on muscle relaxants and reversal in anaesthesia. Anaesth. Crit. Care Pain Med. 2020, 39, 125–142. [Google Scholar] [CrossRef]
- Thilen, S.R.; Weigel, W.A.; Todd, M.M.; Dutton, R.P.; Lien, C.A.; Grant, S.A.; Szokol, J.W.; Eriksson, L.I.; Yaster, M.; Grant, M.D.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Monitoring and Antagonism of Neuromuscular Blockade: A Report by the American Society of Anesthesiologists Task Force on Neuromuscular Blockade. Anesthesiology 2023, 138, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Heier, T.; Caldwell, J.E.; Sessler, D.I.; Miller, R.D. Mild intraoperative hypothermia increases duration of action and spontaneous recovery of vecuronium blockade during nitrous oxide-isoflurane anesthesia in humans. Anesthesiology 1991, 74, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.M.; Kim, H.J.; Choi, J.M.; Kim, Y.B.; Song, J.S.; Yang, H.S. Effects of hyperthermia on the effective concentration of rocuronium and sugammadex-mediated reversal in isolated phrenic nerve hemidiaphragm preparations of rats. BMC Anesthesiol. 2020, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Beaufort, A.M.; Wierda, J.M.; Belopavlovic, M.; Nederveen, P.J.; Kleef, U.W.; Agoston, S. The influence of hypothermia (surface cooling) on the time-course of action and on the pharmacokinetics of rocuronium in humans. Eur. J. Anaesthesiol. Suppl. 1995, 11, 95–106. [Google Scholar]
- Caldwell, J.E.; Heier, T.; Wright, P.M.; Lin, S.; McCarthy, G.; Szenohradszky, J.; Sharma, M.L.; Hing, J.P.; Schroeder, M.; Sessler, D.I. Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology 2000, 92, 84–93. [Google Scholar] [CrossRef]
- Frutos-Vivar, F.; Ferguson, N.D.; Esteban, A.; Epstein, S.K.; Arabi, Y.; Apezteguia, C.; Gonzalez, M.; Hill, N.S.; Nava, S.; D’Empaire, G.; et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 2006, 130, 1664–1671. [Google Scholar] [CrossRef]
- Berg, H.; Roed, J.; Viby-Mogensen, J.; Mortensen, C.R.; Engbaek, J.; Skovgaard, L.T.; Krintel, J.J. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol. Scand. 1997, 41, 1095–1103. [Google Scholar] [CrossRef]
- Bissinger, U.; Schimek, F.; Lenz, G. Postoperative residual paralysis and respiratory status: A comparative study of pancuronium and vecuronium. Physiol. Res. 2000, 49, 455–462. [Google Scholar]
- Morin, J.F.; Mistry, B.; Langlois, Y.; Ma, F.; Chamoun, P.; Holcroft, C. Fluid Overload after Coronary Artery Bypass Grafting Surgery Increases the Incidence of Post-Operative Complications. World J. Cardiovasc. Surg. 2011, 1, 18–23. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Pasquina, P.; Tramer, M.R.; Walder, B. Prophylactic respiratory physiotherapy after cardiac surgery: Systematic review. BMJ 2003, 327, 1379. [Google Scholar] [CrossRef]
- Jonsson, M.; Wyon, N.; Lindahl, S.G.; Fredholm, B.B.; Eriksson, L.I. Neuromuscular blocking agents block carotid body neuronal nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2004, 497, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.I.; Lennmarken, C.; Wyon, N.; Johnson, A. Attenuated ventilatory response to hypoxaemia at vecuronium-induced partial neuromuscular block. Acta Anaesthesiol. Scand. 1992, 36, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Broens, S.J.L.; Boon, M.; Martini, C.H.; Niesters, M.; van Velzen, M.; Aarts, L.; Dahan, A. Reversal of Partial Neuromuscular Block and the Ventilatory Response to Hypoxia: A Randomized Controlled Trial in Healthy Volunteers. Anesthesiology 2019, 131, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Claudius, C.; Skovgaard, L.T.; Viby-Mogensen, J. Is the performance of acceleromyography improved with preload and normalization? A comparison with mechanomyography. Anesthesiology 2009, 110, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Baillard, C.; Bourdiau, S.; Le Toumelin, P.; Ait Kaci, F.; Riou, B.; Cupa, M.; Samama, C.M. Assessing residual neuromuscular blockade using acceleromyography can be deceptive in postoperative awake patients. Anesth. Analg. 2004, 98, 854–857, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Samet, A.; Capron, F.; Alla, F.; Meistelman, C.; Fuchs-Buder, T. Single acceleromyographic train-of-four, 100-Hertz tetanus or double-burst stimulation: Which test performs better to detect residual paralysis? Anesthesiology 2005, 102, 51–56. [Google Scholar] [CrossRef]
- Bowdle, A.; Bussey, L.; Michaelsen, K.; Jelacic, S.; Nair, B.; Togashi, K.; Hulvershorn, J. A comparison of a prototype electromyograph vs. a mechanomyograph and an acceleromyograph for assessment of neuromuscular blockade. Anaesthesia 2020, 75, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Capron, F.; Alla, F.; Hottier, C.; Meistelman, C.; Fuchs-Buder, T. Can acceleromyography detect low levels of residual paralysis? A probability approach to detect a mechanomyographic train-of-four ratio of 0.9. Anesthesiology 2004, 100, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Eikermann, M.; Groeben, H.; Husing, J.; Peters, J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology 2003, 98, 1333–1337. [Google Scholar] [CrossRef]
- Kirmeier, E.; Eriksson, L.I.; Lewald, H.; Jonsson Fagerlund, M.; Hoeft, A.; Hollmann, M.; Meistelman, C.; Hunter, J.M.; Ulm, K.; Blobner, M.; et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): A multicentre, prospective observational study. Lancet Respir. Med. 2019, 7, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; He, Q.; Liu, Y. Residual neuromuscular block: Beware of long-term adverse respiratory outcomes after departure from postanesthesia care unit (PACU). Asian J. Surg. 2022. [Google Scholar] [CrossRef] [PubMed]

| CAB | CAB/Valve | AoV | MV | TV | AscAo/AoRoot | Arch | Other |
|---|---|---|---|---|---|---|---|
| 58 | 3 | 13 | 13 | 3 | 6 | 2 | 6 * |
| Rocuronium (mg/kg) | Final NMBA Dose-1st TOF Interval (h) | Neostimine (mg/kg) | Final RNB Evaluation—Extubation Interval (h) | |
|---|---|---|---|---|
| 1.6 | 5.1 | 0.045 | 1.5 | |
| IQR | 0.6–1.9 | 3.25–6.75 | 0.037–0.051 | 0.12–1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calef, A.; Castelgrande, R.; Crawley, K.; Dorris, S.; Durham, J.; Lee, K.; Paras, J.; Piazza, K.; Race, A.; Rider, L.; et al. Reversing Neuromuscular Blockade without Nerve Stimulator Guidance in a Postsurgical ICU—An Observational Study. J. Clin. Med. 2023, 12, 3253. https://doi.org/10.3390/jcm12093253
Calef A, Castelgrande R, Crawley K, Dorris S, Durham J, Lee K, Paras J, Piazza K, Race A, Rider L, et al. Reversing Neuromuscular Blockade without Nerve Stimulator Guidance in a Postsurgical ICU—An Observational Study. Journal of Clinical Medicine. 2023; 12(9):3253. https://doi.org/10.3390/jcm12093253
Chicago/Turabian StyleCalef, Andrea, Rashel Castelgrande, Kristin Crawley, Sara Dorris, Joanna Durham, Kaitlin Lee, Jen Paras, Kristen Piazza, Abigail Race, Laura Rider, and et al. 2023. "Reversing Neuromuscular Blockade without Nerve Stimulator Guidance in a Postsurgical ICU—An Observational Study" Journal of Clinical Medicine 12, no. 9: 3253. https://doi.org/10.3390/jcm12093253
APA StyleCalef, A., Castelgrande, R., Crawley, K., Dorris, S., Durham, J., Lee, K., Paras, J., Piazza, K., Race, A., Rider, L., Shelley, M., Stewart, E., Tamok, M., Tate, J., & Dodd-o, J. M. (2023). Reversing Neuromuscular Blockade without Nerve Stimulator Guidance in a Postsurgical ICU—An Observational Study. Journal of Clinical Medicine, 12(9), 3253. https://doi.org/10.3390/jcm12093253





