Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps—A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Research Questions and Eligibility Criteria
2.2. Search Strategy
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Search Results
3.2. NPDF Cytokines
4. Discussion
4.1. Chemokine C-C Motif Ligand 5 (CCL5)
4.2. Eotaxins
4.3. Interleukin-4 (IL-4)
4.4. Interleukin-6 (IL-6) and (IL-8)
4.5. Interleukin-13 (IL-13)
4.6. Interleukin-17 (IL-17)
4.7. Interleukin-32 (IL-32)
4.8. CXC Motif Chemokine Ligand 10 (CXCL10)
4.9. Chemokine C-C Motif Ligand 2 (CCL2)
4.10. Chemokine C-C Motif Ligand 17 (CCL17)
4.11. Chemokine C-C Motif Ligand 20 (CCL20)
4.12. Thymic Stromal Lymphopoietin (TSLP)
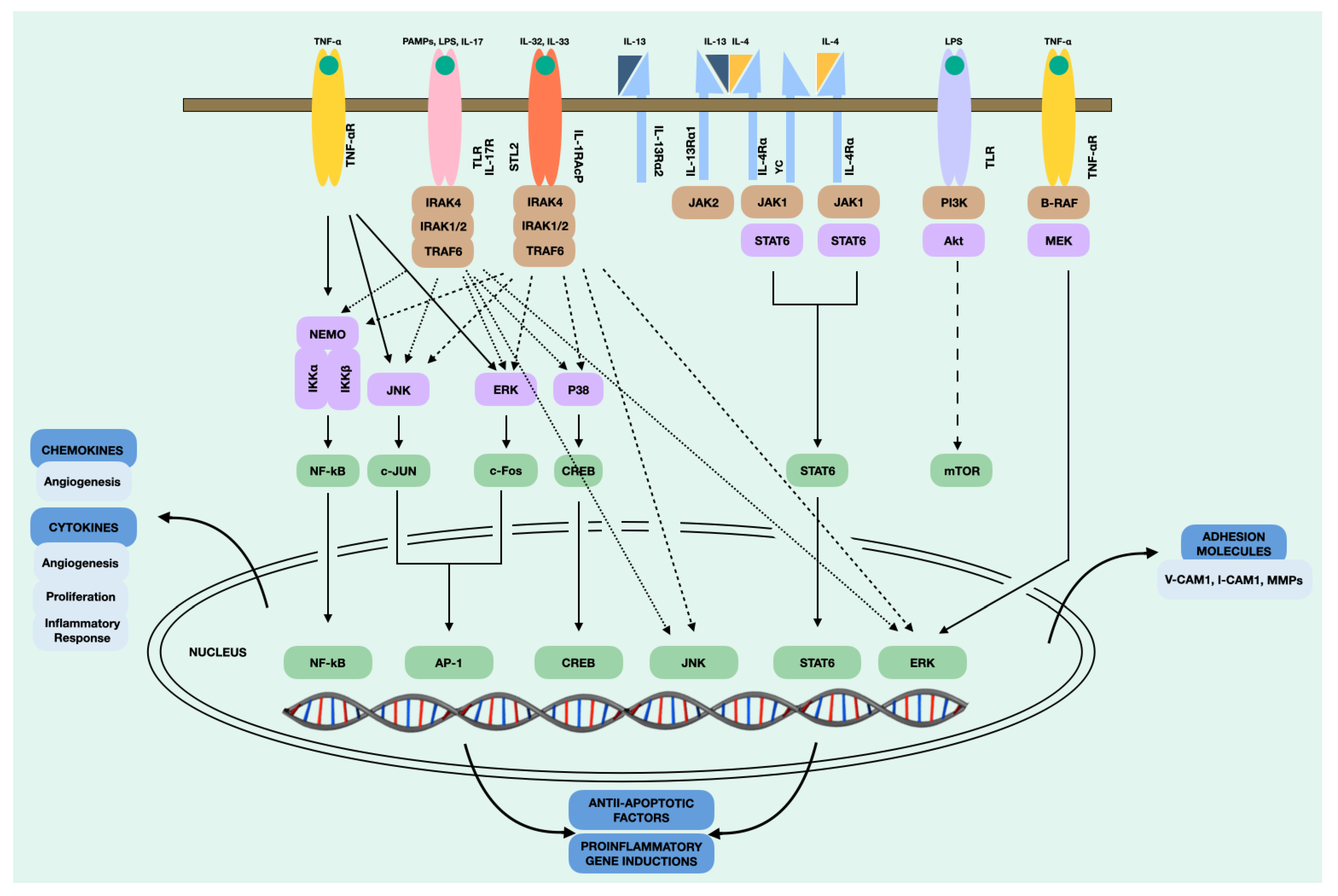
4.13. Overall Effects of Cytokines Produced by NPDF in CRSwNP
5. Limitations
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.R.; Ah-See, K.W. A review of nasal polyposis. Ther. Clin. Risk Manag. 2008, 4, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.K.; Durham, S.R.; Mirakian, R.; Jones, N.S.; Drake-Lee, A.B.; Ryan, D.; Dixon, T.A.; Huber, P.A.J.; Nasser, S.M. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin. Exp. Allergy 2008, 38, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Tos, M.; Mogensen, C. Pathogenesis of nasal polyps. Rhinology 1977, 15, 87–95. [Google Scholar] [PubMed]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Kusama, K.; Pawankar, R.; Yagi, T. Nasal polyp fibroblasts produce MIP-3alpha in response to toll-like receptor ligands and cytokine stimulation. Rhinology 2010, 48, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Pawankar, R.; Fukumoto, A.; Ogihara, N.; Sakanushi, A.; Yagi, T. Distinct role for nasal fibroblasts in initiation of the eosinophilic inflammatory response. Clin. Exp. Allergy Rev. 2005, 5, 77–80. [Google Scholar] [CrossRef]
- Bernstein, J.M.; Gorfien, J.; Noble, B.; Yankaskas, J.R. Nasal polyposis: Immunohistochemistry and bioelectrical findings (a hypothesis for the development of nasal polyps). J. Allergy Clin. Immunol. 1997, 99, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Shiozawa, A.; Ono, N.; Kusunoki, T.; Hirotsu, M.; Homma, H.; Saitoh, T.; Murata, J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013, 123, E1–E9. [Google Scholar] [CrossRef]
- Ho, J.; Bailey, M.; Zaunders, J.; Mrad, N.; Sacks, R.; Sewell, W.; Harvey, R.J. Cellular comparison of sinus mucosa vs polyp tissue from a single sinus cavity in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Klingler, A.I.; Stevens, W.W.; Tan, B.K.; Peters, A.T.; Poposki, J.A.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; Kern, R.C.; et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Cavagnero, K.J.; Gallo, R.L. Essential immune functions of fibroblasts in innate host defense. Front. Immunol. 2022, 13, 1058862. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, L.; Roth, M.; Tian, J.; He, Q.; Zhong, B.; Bao, R.; Lan, X.; Jiang, C.; Sun, J.; et al. PRMT1 Upregulated by Epithelial Proinflammatory Cytokines Participates in COX2 Expression in Fibroblasts and Chronic Antigen-Induced Pulmonary Inflammation. J. Immunol. 2015, 195, 298–306. [Google Scholar] [CrossRef]
- Park, S.-K.; Jin, Y.-D.; Park, Y.-K.; Yeon, S.-H.; Xu, J.; Han, R.-N.; Rha, K.-S.; Kim, Y.-M. IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis. PLoS ONE 2017, 3, e0181806. [Google Scholar] [CrossRef]
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131, e149538. [Google Scholar] [CrossRef]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernández, L.A.; Gómez-Olivares, J.L.; Buentello-Volante, B.; Bautista-de Lucio, V.M. Fibroblasts: The unknown sentinels eliciting immune responses against microorganisms. Eur. J. Microbiol. Immunol. 2017, 7, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Porras-González, C.; Palacios-García, J.M.; Sánchez-Gómez, S.; Maza-Solano, J.M.; Alba, G.; Sánchez-Margalet, V.; Palorames, O.; Del Cuvillo, A.; Cordero-Varela, J.A.; Moreno-Luna, R.; et al. Transcriptional analysis of nasal polyps fibroblasts reveals a new source of pro-inflammatory signaling in CRSwNP. Rhinology 2023, 61, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Saji, F.; Nonaka, M.; Pawankar, R. Expression of RANTES by IL-1 beta and TNF-alpha stimulated nasal polyp fibroblasts. Auris Nasus Larynx 2000, 27, 247–252. [Google Scholar] [CrossRef]
- Yamada, T.; Fujieda, S.; Yanagi, S.; Yamamura, H.; Inatome, R.; Yamamoto, H.; Igawa, H.; Saito, H. IL-1 induced chemokine production through the association of Syk with TNF receptor-associated factor-6 in nasal fibroblast lines. J. Immunol. 2001, 167, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yoshifuku, K.; Matsune, S.; Ohori, J.; Sagara, Y.; Fukuiwa, T.; Kurono, Y. IL-4 and TNF-alpha increased the secretion of eotaxin from cultured fibroblasts of nasal polyps with eosinophil infiltration. Rhinology 2007, 45, 235–241. [Google Scholar] [PubMed]
- Bishara, N. The Use of Biomarkers for Detection of Early- and Late-Onset Neonatal Sepsis. In Hematology, Immunology and Infectious Disease: Neonatology Questions and Controversies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 303–315. [Google Scholar] [CrossRef]
- Meyer, J.E.; Bartels, J.; Görögh, T.; Sticherling, M.; Rudack, C.; Ross, D.A.; Maune, S. The role of RANTES in nasal polyposis. Am. J. Rhinol. 2005, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, J.; Bourdon, C.; Yao, Y.; Rousseau, S. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell. Signal. 2009, 21, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Erin, E.M.; Williams, T.J.; Barnes, P.J.; Hansel, T.T. Eotaxin receptor (CCR3) antagonism in asthma and allergic disease. Curr. Drug Targets Inflamm. Allergy 2002, 1, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Hamano, N.; Nomura, T.; Numata, T.; Hirai, K.; Nakajima, T.; Yamada, H.; Yoshie, O.; Ito, I.; Konno, A. Interleukin-13 and tumour necrosis factor-alpha synergistically induce eotaxin production in human nasal fibroblasts. Clin. Exp. Allergy 2000, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.; Meyer, J.; Pods, R.; Pethe, W.; Hedderich, J.; Schmidt, C.; Maune, S. Endothelial and epithelial expression of eotaxin-2 (CCL24) in nasal polyps. Int. Arch. Allergy Immunol. 2006, 140, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Pawankar, R.; Fukumoto, A.; Ogihara, N.; Sakanushi, A.; Yagi, T. Induction of eotaxin production by interleukin-4, interleukin-13 and lipopolysaccharide by nasal fibroblasts. Clin. Exp. Allergy 2004, 34, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Crouse, C.D.; Bradley, D.; Hise, K.; Lynch, K.; Kountakis, S.E.; Borish, L. Characterization of Interleukin-4-Stimulated Nasal Polyp Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2004, 30, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Tojima, I.; Takezawa, K.; Matsumoto, K.; Kouzaki, H.; Shimizu, T. Thrombin and Activated coagulation factor x stimulate the release of cytokines and fibronectin from Nasal polyp fibroblasts via proteaseactivated receptors. Am. J. Rhinol. Allergy 2017, 31, e13–e18. [Google Scholar] [CrossRef] [PubMed]
- Imoto, Y.; Ueki, S.; Kato, Y.; Yoshida, K.; Morikawa, T.; Kimura, Y.; Kidoguchi, M.; Tsutsumiuchi, T.; Koyama, K.; Adachi, N.; et al. Elevated Serum Leptin Levels in Patients with Eosinophilic Chronic Rhinosinusitis. Front. Pharmacol. 2022, 12, 793607. [Google Scholar] [CrossRef]
- Izuhara, K.; Arima, K.; Yasunaga, S. IL-4 and IL-13: Their pathological roles in allergic diseases and their potential in developing new therapies. Curr. Drug Targets Inflamm. Allergy 2002, 1, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, D.K. Effect of Airborne Particulate Matter on the Immunologic Characteristics of Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2022, 23, 1018. [Google Scholar] [CrossRef]
- Leland, E.M.; Zhang, Z.; Kelly, K.M.; Ramanathan, M. Role of Environmental Air Pollution in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2021, 21, 42. [Google Scholar] [CrossRef]
- Park, M.; Lee, J.S.; Park, M.K. The Effects of Air Pollutants on the Prevalence of Common Ear, Nose, and Throat Diseases in South Korea: A National Population-Based Study. Clin. Exp. Otorhinolaryngol. 2019, 12, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Fukumoto, A.; Ogihara, N.; Pawankar, R.; Sakanushi, A.; Yagi, T. Expression of MCP-4 by TLR ligand-stimulated nasal polyp fibroblasts. Acta Oto-Laryngol. 2007, 127, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Bamba, S.; Andoh, A.; Yasui, H.; Makino, J.; Kim, S.; Fujiyama, Y. Regulation of IL-11 expression in intestinal myofibroblasts: Role of c-Jun AP-1- and MAPK-dependent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G529–G538. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, R.; Chen, H.; Jia, P.; Bao, L.; Tang, H. Bornyl acetate has an anti-inflammatory effect in human chondrocytes via induction of IL-11. IUBMB Life 2014, 66, 854–859. [Google Scholar] [CrossRef]
- Allen, J.S.; Eisma, R.; Leonard, G.; Lafreniere, D.; Kreutzer, D. Interleukin-8 expression in human nasal polyps. Otolaryngol. Head. Neck Surg. 1997, 117, 535–541. [Google Scholar] [PubMed]
- Peters, A.T.; Kato, A.; Zhang, N.; Conley, D.B.; Suh, L.; Tancowny, B.; Carter, D.; Carr, T.; Radtke, M.; Hulse, K.E.; et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2010, 125, 397–403.e10. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sun, S.; Zhang, Y.; Tang, P.; Lv, C.; Ma, H.; Yu, Y.; Xu, S.; Deng, Z. Serum IL-8 and VEGFA are Two Promising Diagnostic Biomarkers of Asthma-COPD Overlap Syndrome. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Bequignon, E.; Mangin, D.; Bécaud, J.; Pasquier, J.; Angely, C.; Bottier, M.; Escudier, E.; Isabey, D.; Filoche, M.; Louis, B.; et al. Pathogenesis of chronic rhinosinusitis with nasal polyps: Role of IL-6 in airway epithelial cell dysfunction. J. Transl. Med. 2020, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hong, C.Y.; Shun, C.T.; Hsiao, T.Y.; Wang, C.C.; Wang, J.S.; Hsiao, M.; Lin, S.K. Inducible cyclooxygenase and interleukin 6 gene expressions in nasal polyp fibroblasts: Possible implication in the pathogenesis of nasal polyposis. Arch. Otolaryngol.-Head Neck Surg. 2002, 128, 945–951. [Google Scholar] [CrossRef]
- Cho, J.S.; Han, I.H.; Lee, H.R.; Lee, H.M. Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-kappa B Pathways in Nasal Polyp-Derived Fibroblasts. Allergy Asthma Immunol. Res. 2014, 6, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kang, J.H.; Um, J.Y.; Han, I.H.; Park, I.H.; Lee, H.M. Lipopolysaccharide induces pro-inflammatory cytokines and MMP production via TLR4 in nasal polyp-derived fibroblast and organ culture. PLoS ONE 2014, 9, e90683. [Google Scholar] [CrossRef]
- Shin, S.H.; Ye, M.K.; Kim, Y.H.; Kim, J.K. Role of TLRs in the production of chemical mediators in nasal polyp fibroblasts by fungi. Auris Nasus Larynx 2016, 43, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Olsson, S.; Cagnoni, F.; Dignetti, P.; Melioli, G.; Canonica, G.W. Low concentrations of cytokines produced by allergen-stimulated peripheral blood mononuclear cells have potent effects on nasal polyp-derived fibroblasts. Clin. Exp. Immunol. 2003, 132, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Shun, C.T.; Lin, S.K.; Hong, C.Y.; Huang, H.M.; Liu, C.M. Hypoxia induces cysteine-rich 61, vascular endothelial growth factor, and interleukin-8 expressions in human nasal polyp fibroblasts: An implication of neutrophils in the pathogenesis of nasal polyposis. Am. J. Rhinol. Allergy 2011, 25, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Laury, A.M.; Katz, E.H.; Den Beste, K.A.; Parkos, C.A.; Nusrat, A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int. Forum Allergy Rhinol. 2014, 4, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vatrella, A.; Fabozzi, I.; Calabrese, C.; Maselli, R.; Pelaia, G. Dupilumab: A novel treatment for asthma. J. Asthma Allergy 2014, 7, 123–130. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nakajima, T.; Tsukidate, T.; Matsumoto, K.; Iida, M.; Otori, N.; Haruna, S.-I.; Moriyama, H.; Saito, H. TNF-alpha and IL-4 regulate expression of IL-13 receptor alpha2 on human fibroblasts. Biochem. Biophys. Res. Commun. 2003, 312, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Sharma, P.; Harbor, P.C.; Aman, M.J.; Vogelbaum, M.A.; Haque, S.J. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002, 62, 1103–1109. [Google Scholar]
- Jiang, H.; Harris, M.B.; Rothman, P. IL-4/IL-13 signaling beyond JAK/STAT. J. Allergy Clin. Immunol. 2000, 105 Pt 1, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Taguchi, J.; Puri, R.K.; Mohri, H. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int. J. Hematol. 1999, 69, 13–20. [Google Scholar] [PubMed]
- Fuchs, A.; Colonna, M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr. Opin. Gastroenterol. 2013, 29, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases: IL-17 cytokine family. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.J.; Zhou, M.L.; Liu, Y.C.; Zhou, S.H. Roles Played by the PI3K/Akt/HIF-1 alpha Pathway and IL-17A in the Chinese Subtype of Chronic Sinusitis with Nasal Polyps. Mediat. Inflamm. 2022, 2022, 8609590. [Google Scholar] [CrossRef]
- Niu, Y.Z.; Gong, G.Q.; Chen, S.; Chen, J.J.; Kong, W.J.; Wang, Y.J. Effects of IL-17 on expression of GRO-alpha and IL-8 in fibroblasts from nasal polyps. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2014, 34, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Rudack, C.; Sachse, F.; Alberty, J. Primary role of growth-related oncogene-alpha and granulocyte chemotactic protein-2 as neutrophil chemoattractants in chronic rhinosinusitis. Clin. Exp. Allergy 2006, 36, 748–759. [Google Scholar] [CrossRef]
- Homma, H.; Kamiya, K.; Kusunoki, T.; Ikeda, K. Multiplex analyses of cytokine and chemokine release from the cultured fibroblast of nasal polyps: The effect of IL-17A. Acta Oto-Laryngol. 2013, 133, 1065–1072. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.-D.; Kunz, S.; Asadullah, K.; Volk, H.-D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Hao, D.; Wu, Y.; Li, P.; Li, C.; Jiang, T.; Zhang, Q.; Liu, S.; Shi, L. An Integrated Analysis of Inflammatory Endotypes and Clinical Characteristics in Chronic Rhinosinusitis with Nasal Polyps. JIR 2022, 15, 5557–5565. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Kim, S.H. IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis. 2006, 65 (Suppl. S3), iii61–iii64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.Y.; Azam, T.; Yoon, D.Y.; Dinarello, C.A. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity 2005, 22, 131–142. [Google Scholar] [PubMed]
- Shoda, H.; Fujio, K.; Yamaguchi, Y.; Okamoto, A.; Sawada, T.; Kochi, Y.; Yamamoto, K. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res. Ther. 2006, 8, R166. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bae, S.; Kang, Y.; Yoon, D.; Bai, X.; Chan, E.D.; Azam, T.; Dinarello, C.A.; Lee, S.; Her, E.; et al. Suppressing IL-32 in monocytes impairs the induction of the proinflammatory cytokines TNFalpha and IL-1beta. Cytokine 2010, 49, 171–176. [Google Scholar] [CrossRef]
- Keswani, A.; Chustz, R.T.; Suh, L.; Carter, R.; Peters, A.T.; Tan, B.K.; Chandra, R.; Kim, S.H.; Azam, T.; Dinarello, C.A. Differential expression of interleukin-32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012, 67, 25–32. [Google Scholar] [CrossRef]
- Soyka, M.B.; Treis, A.; Eiwegger, T.; Menz, G.; Zhang, S.; Holzmann, D.; Akdis, C.A.; Meyer, N. Regulation and expression of IL-32 in chronic rhinosinusitis. Allergy 2012, 67, 790–798. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, J.A.; Park, J.H.; Park, I.H.; Han, I.H.; Lee, H.M. Toll-like receptor 4-mediated expression of interleukin-32 via the c-Jun N-terminal kinase/protein kinase B/cyclic adenosine monophosphate response element binding protein pathway in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2016, 6, 1020–1028. [Google Scholar] [CrossRef]
- Mirchandani, A.S.; Salmond, R.J.; Liew, F.Y. Interleukin-33 and the function of innate lymphoid cells. Trends Immunol. 2012, 33, 389–396. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-γ-Inducible Protein 10 (IP-10; CXCL10)-Deficient Mice Reveal a Role for IP-10 in Effector T Cell Generation and Trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Wada, K.; Yoshimura, T.; Asaka, D.; Okada, N.; Matsumoto, K.; Moriyama, H. Increased CXCL10 expression in nasal fibroblasts from patients with refractory chronic rhinosinusitis and asthma. Allergol. Int. 2013, 62, 495–502. [Google Scholar] [CrossRef]
- Li, Z.; Levast, B.; Madrenas, J. Staphylococcus aureus Downregulates IP-10 Production and Prevents Th1 Cell Recruitment. J. Immunol. 2017, 198, 1865–1874. [Google Scholar] [CrossRef]
- Nam, Y.R.; Lee, K.J.; Lee, H.; Joo, C.H. CXCL10 production induced by high levels of IKKε in nasal airway epithelial cells in the setting of chronic inflammation. Biochem. Biophys. Res. Commun. 2019, 514, 607–612. [Google Scholar] [CrossRef]
- Evers, T.M.J.; Sheikhhassani, V.; Haks, M.C.; Storm, C.; Ottenhoff, T.H.M.; Mashaghi, A. Single-cell analysis reveals chemokine-mediated differential regulation of monocyte mechanics. iScience 2022, 25, 103555. [Google Scholar] [CrossRef]
- Shun, C.T.; Lin, S.K.; Hong, C.Y.; Kok, S.H.; Juan, Y.H.; Wang, C.C.; Hsu, M.C.; Liu, C.M. CC chemokine ligand 2 gene expression in nasal polyp fibroblasts: Possible implication in the pathogenesis of nasal polyposis. Ann. Otol. Rhinol. Laryngol. 2005, 114, 879–885. [Google Scholar] [CrossRef]
- Lin, S.-K.; Kok, S.-H.; Shun, C.-T.; Hong, C.-Y.; Wang, C.-C.; Hsu, M.-C.; Liu, C.-M. Tumor necrosis factor-alpha stimulates the expression of C-C chemokine ligand 2 gene in fibroblasts from the human nasal polyp through the pathways of mitogen-activated protein kinase. Am. J. Rhinol. 2007, 21, 251–255. [Google Scholar] [CrossRef]
- Pujols, L.; Mullol, J.; Alobid, I.; Roca-Ferrer, J.; Xaubet, A.; Picado, C. Dynamics of COX-2 in nasal mucosa and nasal polyps from aspirin-tolerant and aspirin-intolerant patients with asthma. J. Allergy Clin. Immunol. 2004, 114, 814–819. [Google Scholar] [CrossRef]
- Tanaka, S.; Tatsuguchi, A.; Futagami, S.; Gudis, K.; Wada, K.; Seo, T.; Mitsui, K.; Yonezawa, M.; Nagata, K.; Fujimori, S.; et al. Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut 2006, 55, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Yoshie, O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 1997, 272, 15036–15042. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, A.; Nonaka, M.; Ogihara, N.; Pawankar, R. Induction of TARC production by lipopolysaccharide and interleukin-4 in nasal fibroblasts. Int. Arch. Allergy Immunol. 2008, 145, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Pawankar, R.; Yagi, T. Toll-like receptor 2, 3, 4, 5 ligands and interleukin-4 synergistically induce TARC production in nasal polyp fibroblasts. Auris Nasus Larynx 2008, 35, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Kusama, K.; Pawankar, R.; Yagi, T. Combined stimulation with Poly(I:C), TNF-alpha and Th2 cytokines induces TARC production by human fibroblasts from the nose, bronchioles and lungs. Int. Arch. Allergy Immunol. 2010, 152, 327–341. [Google Scholar] [CrossRef]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Pawankar, R.; Yagi, T. Combined stimulation of nasal polyp fibroblasts with poly IC, interleukin 4, and tumor necrosis factor α potently induces production of thymus- and activation-regulated chemokine. Arch. Otolaryngol.-Head. Neck Surg. 2008, 134, 630–635. [Google Scholar] [CrossRef]
- Hieshima, K.; Imai, T.; Opdenakker, G.; Van Damme, J.; Kusuda, J.; Tei, H.; Sakaki, Y.; Takatsuki, K.; Miura, R.; Yoshie, O.; et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997, 272, 5846–5853. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- van Tongeren, J.; Reinartz, S.M.; Fokkens, W.J.; de Jong, E.C.; van Drunen, C.M. Interactions between epithelial cells and dendritic cells in airway immune responses: Lessons from allergic airway disease. Allergy 2008, 63, 1124–1135. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Menten, P.; Lenaerts, J.-P.; Conings, R.; Put, W.; Wuyts, A.; Proost, P.; Van Damme, J. Regulated production and molecular diversity of human liver and activation-regulated chemokine/macrophage inflammatory protein-3 alpha from normal and transformed cells. J. Immunol. 2000, 165, 4470–4477. [Google Scholar] [CrossRef]
- Nonaka, M.; Ogihara, N.; Fukumoto, A.; Sakanushi, A.; Kusama, K.; Pawankar, R.; Yagi, T. Synergistic Induction of Macrophage Inflammatory Protein-3 alpha/CCL20 Production by Interleukin-17A and Tumor Necrosis Factor-alpha in Nasal Polyp Fibroblasts. World Allergy Organ. J. 2009, 2, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.N.; Kohanski, M.A.; Maina, I.W.; Workman, A.D.; Herbert, D.R.; Cohen, N.A. Sentinels at the wall: Epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation: Epithelial cytokines in upper airway. Int. Forum Allergy Rhinol. 2019, 9, 93–99. [Google Scholar] [CrossRef]
- Nonaka, M.; Fukumoto, A.; Ogihara, N.; Sakanushi, A.; Pawankar, R.; Yagi, T. Synergistic induction of thymic stromal lymphopoietin by tumor necrosis factor alpha and Th2 cytokine in nasal polyp fibroblasts. Am. J. Rhinol. Allergy 2010, 24, e14–e18. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Wang, J.; Wang, Q.; Xia, D.; Sun, W.; Zhang, L.; Yu, H.; Liu, Y.; Cao, X. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol. Lett. 2004, 94, 141–151. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, Y.H.; Jin, H.S.; Kang, S.H. Alternaria Induces Production of Thymic Stromal Lymphopoietin in Nasal Fibroblasts Through Toll-like Receptor 2. Allergy Asthma Immunol. Res. 2016, 8, 63–68. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol. Rev. 2018, 286, 37–52. [Google Scholar] [CrossRef]
- Rostkowska-Nadolska, B.; Latocha, M.; Gawron, W.; Kutner, A.; Bochnia, M. The influence of calcitriol and tacalcitol on proliferation of fibroblasts cultured from nasal polyps. Adv. Clin. Exp. Med. 2007, 16, 213–219. [Google Scholar]
- Carsuzaa, F.; Béquignon, É.; Bainaud, M.; Jégou, J.F.; Dufour, X.; Lecron, J.C.; Favot, L. Oncostatin M Counteracts the Fibrotic Effects of TGF-β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps? Int. J. Mol. Sci. 2022, 23, 6308. [Google Scholar] [CrossRef]
- Carsuzaa, F.; Béquignon, É.; Dufour, X.; de Bonnecaze, G.; Lecron, J.C.; Favot, L. Cytokine Signature and Involvement in Chronic Rhinosinusitis with Nasal Polyps. IJMS 2021, 23, 417. [Google Scholar] [CrossRef]
- Park, S.A.; Park, I.H.; Cho, J.S.; Moon, Y.M.; Lee, S.H.; Kim, T.H.; Lee, S.H.; Lee, H.M. Effect of [6]-Gingerol on Myofibroblast Differentiation in Transforming Growth Factor Beta 1–Induced Nasal polyp–Derived Fibroblasts. Am. J. Rhinol. Allergy 2012, 26, 97–103. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yan, D.; Wang, H.W.; Bowman, R.L.; Joyce, J.A. STAT3 and STAT6 Signaling Pathways Synergize to Promote Cathepsin Secretion from Macrophages via IRE1α Activation. Cell Rep. 2016, 16, 2914–2927. [Google Scholar] [CrossRef]
- McCain, J. The MAPK (ERK) Pathway: Investigational Combinations for the Treatment Of BRAF-Mutated Metastatic Melanoma. Pharm. Ther. 2013, 38, 96–108. [Google Scholar]
- Nemec, C.M.; Singh, A.K.; Ali, A.; Tseng, S.C.; Syal, K.; Ringelberg, K.J.; Ho, Y.H.; Hintermair, C.; Ahmad, M.F.; Kar, R.K.; et al. Noncanonical CTD kinases regulate RNA polymerase II in a gene-class-specific manner. Nat. Chem. Biol. 2019, 15, 123–131. [Google Scholar] [CrossRef]
- Hong, C.J.; Tsang, A.C.; Quinn, J.G.; Bonaparte, J.P.; Stevens, A.; Kilty, S.J. Anti-IgE monoclonal antibody therapy for the treatment of chronic rhinosinusitis: A systematic review. Syst. Rev. 2015, 4, 166. [Google Scholar] [CrossRef]
- Franzese, C.B. The Role of Biologics in the Treatment of Nasal Polyps. Immunol. Allergy Clin. 2020, 40, 295–302. [Google Scholar] [CrossRef]
- Patel, G.B.; Peters, A.T. The Role of Biologics in Chronic Rhinosinusitis with Nasal Polyps. Ear Nose Throat J. 2021, 100, 44–47. [Google Scholar] [CrossRef]
- Moreno-Luna, R.; González-García, J.; Palacios-García, J.; Maza-Solano, J.M.; del Cuvillo Bernal, A.; Sánchez-Gómez, S. Usefulness of endonasal mucoplasty in the surgical treatment of chronic rhinosinusitis with nasal polyps. Prospective study [Utilidad de la mucoplastia endonasal en el tratamiento quirúrgico de la rinosinusitis crónica con pólipos nasales. Estudio prospectivo]. Acta Otorrinolaringol. Esp. 2021, 72, 256–261. [Google Scholar]
- Moreno-Luna, R.; Martin-Jimenez, D.I.; Callejon-Leblic, M.A.; Gonzalez-Garcia, J.; Maza-Solano, J.M.; Porras-Gonzalez, C.; Del Cuvillo-Bernal, A.; Sanchez-Gomez, S. Usefulness of bilateral mucoplasty plus reboot surgery in severe type-2 chronic rhinosinusitis with nasal polyps. Rhinology 2022, 60, 368–376. [Google Scholar] [CrossRef]
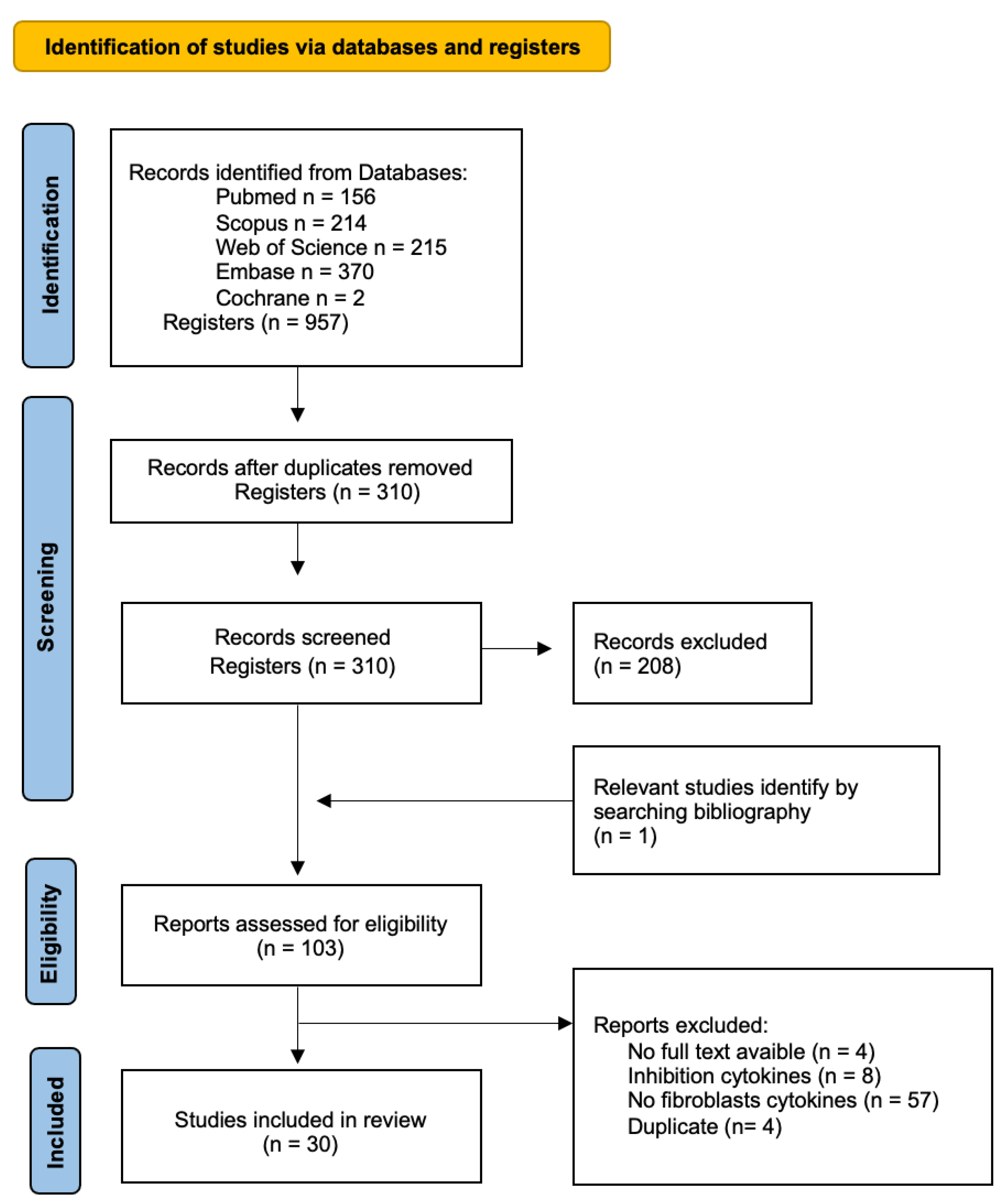


| Criteria | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) | All other diseases |
| Intervention/Comparators | Stimulation of nasal-polyp-derived fibroblasts (NPDF) |
|
| Outcomes | Cytokines and/or chemokines |
|
| Study Design |
|
|
| Language | Articles in English a | All non-English articles |
| Author (Year) | Aim | Features of Patients | Previous Treatment | Control Group Fibroblasts | Stimulation by | ARN Detection | Immunoassay | Cytokine Produced | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Saji (2000) | To assess the production of RANTES cytokine by NPDF | No other associated disease | No comment | No | TNF-⍺ IL-1β | RT-PCR | ELISA | CCL5 (RANTES) | RANTES from NPDF was expressed after stimulation with TNF-⍺ and IL-1β |
| Yamada (2001) | To assess if Syk is involved in the RANTES production | No comment | No comment | Nasal polyps | TNF-⍺ IL-1β | No | Western Blot | CCL5 (RANTES) | TNF-⍺ induced RANTES production in an independent manner at the Syk-expression level |
| Liu (2002) | To evaluate the production of IL-6 and COX-2 by NPDF | No history of nasal allergy, asthma, or aspirin sensitivity | Not regular topical or oral medication within 3 weeks | Inferior turbinate mucosa | IL-1⍺ TNF-⍺ | Northern Blot in situ hybridization | No | IL-6 | IL-6 and COX-2 were expressed with a stimulatory effect of IL-1⍺ and TNF-⍺ |
| Olsson (2003) | Proinflammatory functions of NPDF after PBMC mediators | No comment | Not anti-inflammatory treatment 2 weeks before | No | Dp-stimulated PBMC supernatants | No | Flow cytometry ELISA | Il-6 IL-8 | IL-6 and IL-8 were significantly higher in NPDFs cultured with Dp-stimulated PBMC supernatants compared to nonstimulated supernatants |
| Yoshikawa (2003) | To assess the expression IL-13Rs | No comment | No comment | Lung and skin | TNF-⍺ IL-4 | RT-PCR | Flow cytometry ELISA | IL-13R⍺2 | TNF-⍺ and IL-4 upregulated the cell surface expression of IL-13R⍺2 in NPDF |
| Steinke (2004) | To assess the effect of IL-4 and IL-13 on CysLT receptor expression on NPDF | No comment | No oral steroids or leukotriene modifier within 4 weeks | No | IL-4 IL-13 | RT-PCR | Flow cytometry | IL-6 CCL11 (eotaxin-1) TFG-β1 TFG-β2 | IL-4 induced changes in the mRNA and protein expression of fibrotic (TFG-β1, TFG-β2) and inflammatory cytokines and chemokines (IL-6 and CCL11) by NPDF |
| Nonaka (2004) | To assess eotaxin production by NPDF | No comment | No comment | Uncinate process mucosa, lung and skin | IL-4 IL-13 TGF-β1 LPS | RT-PCR | ELISA | Eotaxin | LPS was necessary for IL-4 to strongly induce eotaxin in NPDF |
| Shun (2005) | To assess CCL2 expression on NPDF | No history of nasal allergy, asthma, or aspirin sensitivity | Not regular topical or oral medication within 3 weeks | Inferior turbinate mucosa | TNF-⍺ | Northern Blot | Inmunohistochemistry | CCL2 (MCP-1) | CCL2 synthesis by NPDF may promote the macrophage recruitment |
| Yoshifuku (2007) | To assess the expression of RANTES and eotaxin in NPDF |
| No treatment administered at least 2 weeks prior to surgery | Unstimulated cells of both groups | TNF-⍺ IL-4 | RT-PCR | ELISA | CCL5 (RANTES) eotaxin | Eotaxin had an important role in the selective recruitment of eosinophils in the eosinophilic group. |
| Nonaka (2007) | To assess the production of MCP-4 by NPDF | No associated airway disease | No comment | No | IL-4 TLRs | RT-PCR | ELISA | CCL13 (MCP-4) | The induction of CCL13 by TLRs and IL-4 suggested an important role for NPDF in regulating eosinophilic infiltration |
| Lin (2007) | To assess the CCL2 expression on NPDF | No history of nasal allergy, asthma, or aspirin sensitivity | No regular topical or oral medication within 3 weeks | Inferior turbinate mucosa | TNF-⍺ | Northern Blot | Western Blot | CCL2 (MCP-1) | TNF-α contributed to NP through the induction of CCL2 expression in NPDF |
| Fukumoto (2008) | To assess if NPDF produced TARC | No comment | No comment | Uncinate process mucosa and lung | IL-4 LPS | RT-PCR | ELISA | CCL17 (TARC) | A combined stimulation with LPS and IL-4 induced CCL17 in NPDF |
| Nonaka (2008) | To assess if NPDF produced TARC | Allergic chronic sinusitis but no associated lower airway disease | No comment | No | IL-4 TLRs | RT-PCR | ELISA | CCL17 (TARC) | A combined stimulation with TLR ligands and IL-4 induced TARC in NPDF |
| Nonaka (2008) | To assess the expression of TARC by NPDF | Allergic chronic sinusitis | No comment | No | TNF-⍺ IL-4 poly I:C | RT-PCR | ELISA | CCL17 (TARC) | A combined stimulation with poly I:C, IL-4, and TNF-⍺ resulted in substantial amounts of TARC in NPDF |
| Nonaka (2009) | To assess the expression of CCL20 by NPDF | Two atopic patients | No comment | No | TNF-α IL-4 IL-17 | RT-PCR | ELISA | CCL20 (MIP-3α) | IL-17A and IL-17E contributed to the infiltration of Th17 cells and DC through the production of CCL20 by NPDF |
| Nonaka (2010) | To assess if NPDF produced CCL20 | 3 atopic patients, 2 of which with asthma | No comment | No | IL-1β TNF-α TLRs | RT-PCR | ELISA | CCL20 (MIP-3α) | NPDF recruiting DCs via the TLR/proinflammatory cytokine induced the production of CCL20 |
| Nonaka (2010) | To assess the expression of TSLP on NPDF | No comment | No comment | No | IL-4 TNF-α IFN-ɣ IL-10 IL-13 | RT-PCR | ELISA | TSLP | TNF-α and Th2 cytokine (IL-4 or IL-13) synergistically induced TSLP for the development and regulation of Th2 cells |
| Shun (2011) | Hypoxia activates NPDF which induce Cyr61, VEGF, and IL-8 | No history of nasal allergy, asthma, or aspirin sensitivity | No regular topical or oral medication within 3 weeks | No | Cyr61 | No | Inmunohistochemistry Western Blot ELISA | IL-8 | Under hypoxia, NPDF may induce VEGF and IL-8 |
| Yoshikawa (2013) | To assess the expression of CXCL10 | 3 different groups of CRS patients:
| No comment | Middle turbinate mucosa and middle meatus mucosa | TNF-α IFN-ɣ IFN-β poly I:C | qRT-PCR | ELISA | CXCL10 | The ATA and AIA groups showed a significantly enhanced expression of CXCL 10 after poly IC |
| Homma (2013) | To assess the level of cytokine and chemokine release from the NPDF and its effect on IL-17A |
| No systemic or topical corticosteroids or other immune-modulating drugs for at least 1 month before | Sphenoid mucosa | IL-17A | qRT-PCR | Cytokine assay system | IL-6 IL-8 IL-9 CCL2 (MCP-1) G-CSF | NPDF showed an enhanced basal secretion of MCP-1 and IL-17A stimulated secretion of IL-6, IL-9, and G-CSF. |
| Niu (2014) | To assess the expression of IL-8 and CXCL1 by NPDF | No comment | Not regular topical or oral medication at least 1 month before | No | IL-17 | RT-PCR | No | CXCL1 IL-8 | NPDF produced CXCL1 and IL-8 which play a role in neutrophil recruitment |
| Cho (2014) | To determine PGE2 effect on the levels of IL-6 and IL-8 | No comment | No comment | No | PGE2 | RT-PCR | Western blot ELISA | IL-6 IL-8 | PGE2 increased IL-6 expression via the EP2 and EP4 receptors and IL-8 expression via the EP4 receptor in NPDF |
| Cho (2014) | To assess proinflammatory cytokines and MMPs | No history of allergy, asthma, or aspirin sensitivity | No oral antiallergic agents for at least 2 months | No | LPS | Microarray RT-PCR | Western blot ELISA | IL-6 IL-8 | LPS induced the production and expression of IL-6, IL-8, and MMPs in NPDF |
| Shin (2016) | To assess the role of PPRs and TLRs in the production of cytokines by NPDF stimulated with airborne fungi | No history of allergy or asthma | No oral or topical medications at least 4 weeks before | No | Alternaria and Aspergillus | RT-PCR | ELISA | IL-6 IL-8 | Alternaria induced the production of IL-6 and IL-8 from NPDF, through the interaction of TLRs |
| Shin (2016) | To assess if airborne fungi activate NPDF to produce TSLP | No history of allergy or asthma | No oral or topical medications at least 4 weeks before | No | Alternaria and Aspergillus | RT-PCR | No | TSLP | Fungi induced TSLP production by NPDF |
| Cho (2016) | To investigate the mechanism of IL-32 expression induced by LPS in NPDF | No history of allergy, asthma, or aspirin sensitivity | No oral or topical medications during the previous 8 weeks before | Inferior turbinate mucosa | TNF-α IFN-ɣ TGF-β1 IL-1β LPS | RT-PCR | Western blot | IL-32 | LPS induced IL-32 expression in NPDF through the TLR4-JNK-AKT-CREB signalling pathway |
| Shimizu (2017) | To assess if coagulation factors can produce the release of cytokines and extracellular matrix proteins from NPDF | No comment | No comment | No | Thrombin FXa PAR agonist peptides | RT-PCR | Inmunoassay kit | TGF-β1 CCL11(eotaxin-1) IL-6 IL-8 | Coagulation factors induced the release of cytokines and ECM protein by NPDF |
| Imoto (2022) | To assess if leptin release is related to severity of eosinophilic CRSwNP | Eosinophilic CRSwNP | No oral steroids intake for at least 4 weeks before | No CRS | Leptin IL-4 IL-13 | RT-PCR | Western blot ELISA | CCL26 (eotaxin-3) | Leptin significantly augmented eotaxin-3 expression in NPDF |
| Cheng (2022) | To assess the role of IL-17 in pathogenesis od CRSwNP | No comment | No nasal decongestants or antihistamines were administered for 4 weeks before | Untreated fibroblasts | TNF-α | RT-PCR | Western blot ELISA | IL-17A | TNF-α increased levels of IL-17A in NPDF through the PI3K/Akt/HIF-1 α pathway |
| Lee (2022) | To assess the inflammatory effects of PM10 in NPDF | No comment | No oral or nasal corticosteroids or antibiotics for 4 weeks before | No | PM10 | RT-PCR | Western blot ELISA | IL-4 IL-6 IL-33 CXCL1 | Increased expression of IL-6, IL-4, IL-33, and CXCL1 on PM10-treated human NPDF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-García, J.; Porras-González, C.; Moreno-Luna, R.; Maza-Solano, J.; Polo-Padillo, J.; Muñoz-Bravo, J.L.; Sánchez-Gómez, S. Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps—A Systematic Review. J. Clin. Med. 2023, 12, 3280. https://doi.org/10.3390/jcm12093280
Palacios-García J, Porras-González C, Moreno-Luna R, Maza-Solano J, Polo-Padillo J, Muñoz-Bravo JL, Sánchez-Gómez S. Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps—A Systematic Review. Journal of Clinical Medicine. 2023; 12(9):3280. https://doi.org/10.3390/jcm12093280
Chicago/Turabian StylePalacios-García, José, Cristina Porras-González, Ramón Moreno-Luna, Juan Maza-Solano, Juan Polo-Padillo, José Luis Muñoz-Bravo, and Serafín Sánchez-Gómez. 2023. "Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps—A Systematic Review" Journal of Clinical Medicine 12, no. 9: 3280. https://doi.org/10.3390/jcm12093280
APA StylePalacios-García, J., Porras-González, C., Moreno-Luna, R., Maza-Solano, J., Polo-Padillo, J., Muñoz-Bravo, J. L., & Sánchez-Gómez, S. (2023). Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps—A Systematic Review. Journal of Clinical Medicine, 12(9), 3280. https://doi.org/10.3390/jcm12093280









