Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Sources of Information, Research and Selection
2.4. Data Collection Process and Data Characteristics
2.5. Risk of Bias within Individual Studies, Summary Measures, Summary of Results, Risk of Bias across Studies, Publication Bias and Additional Measures
3. Results
3.1. Selection of Studies
3.2. Data Characteristics
3.3. Risk of Bias
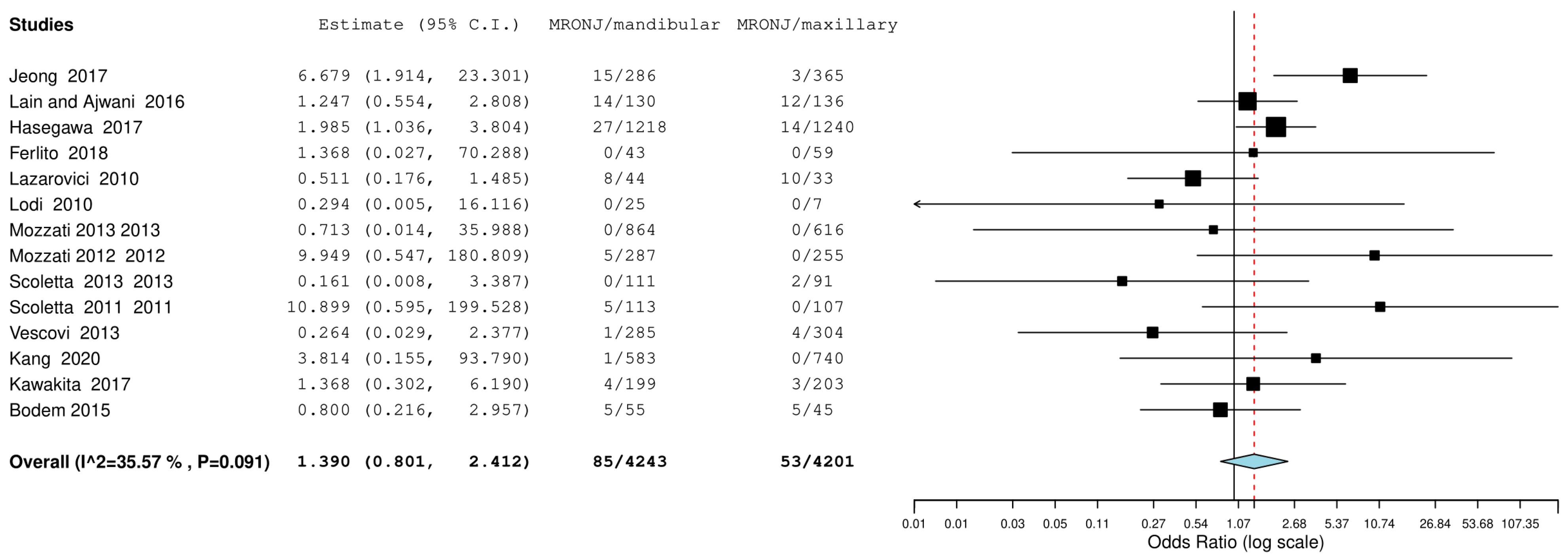
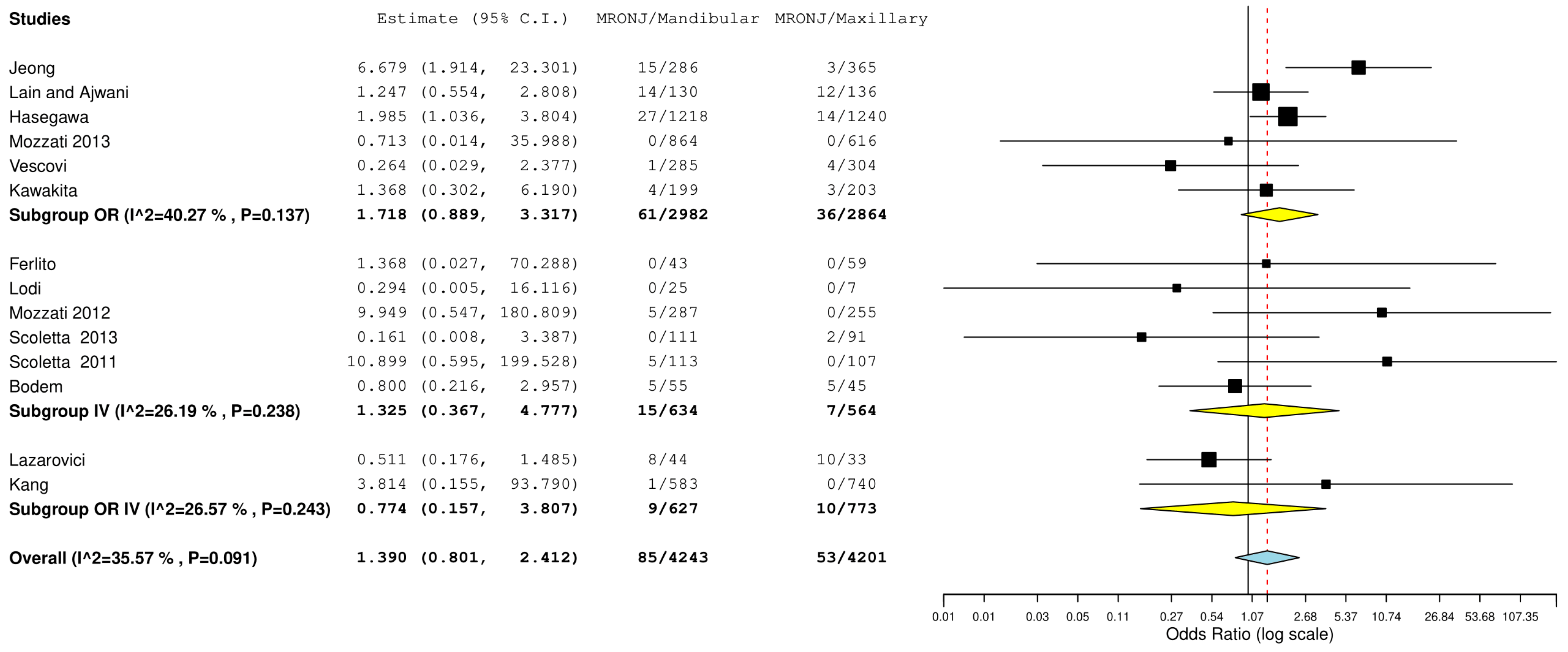
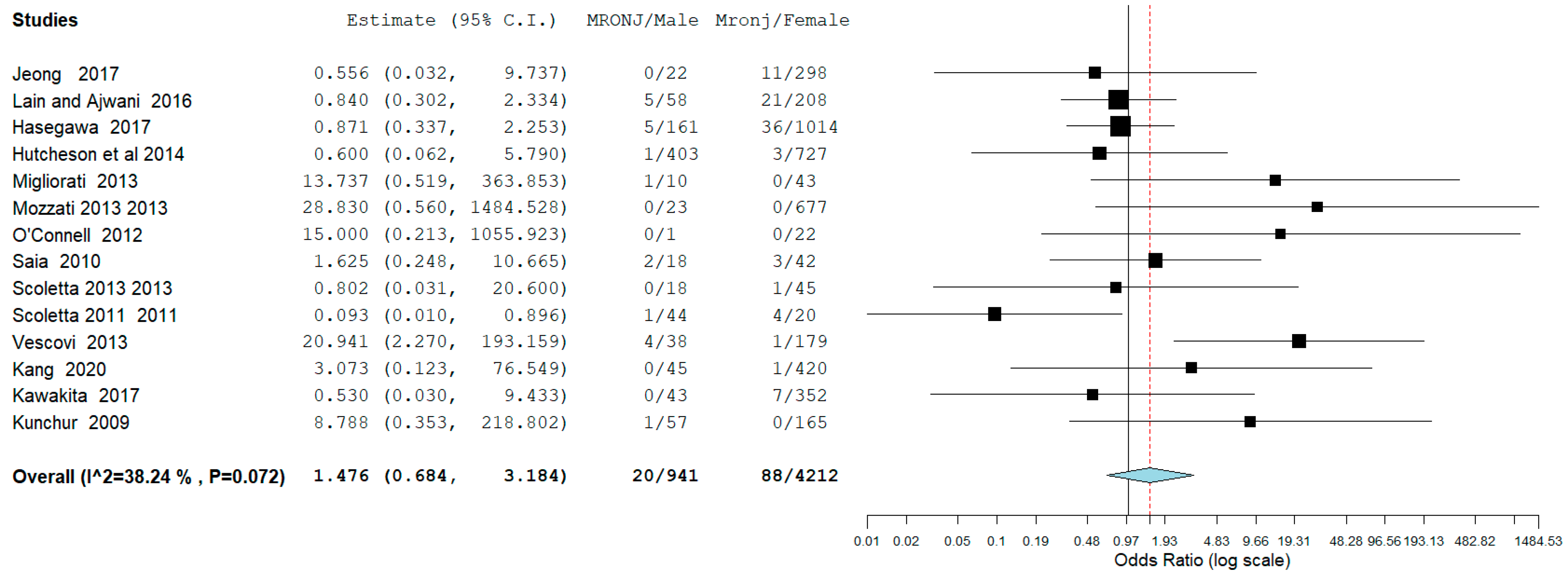
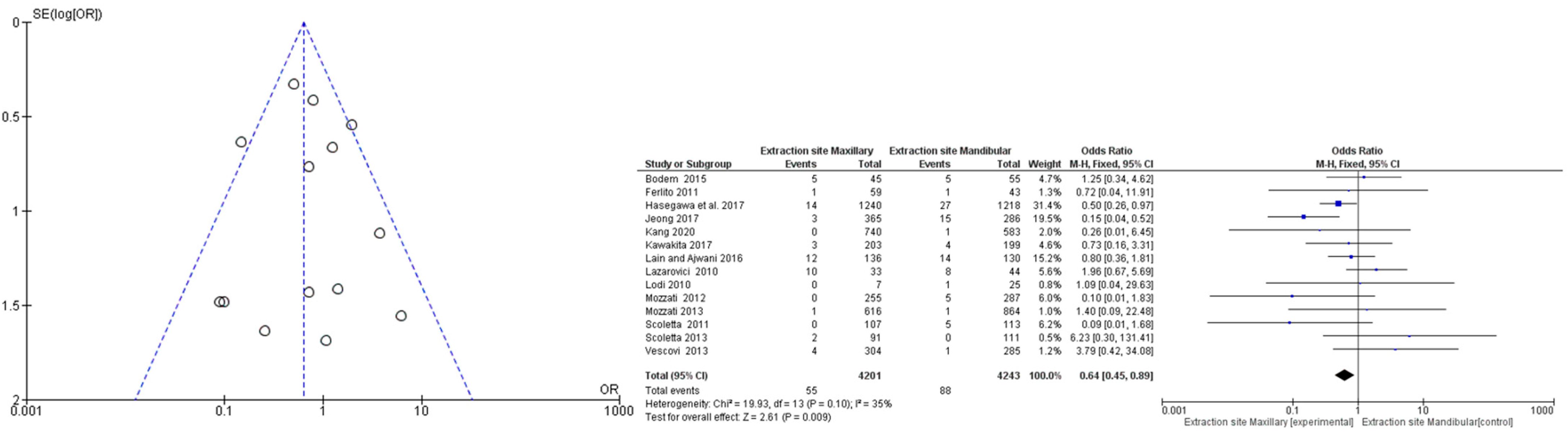
3.4. Meta-Analysis
3.5. Risk of Bias across Studies: Publication Bias
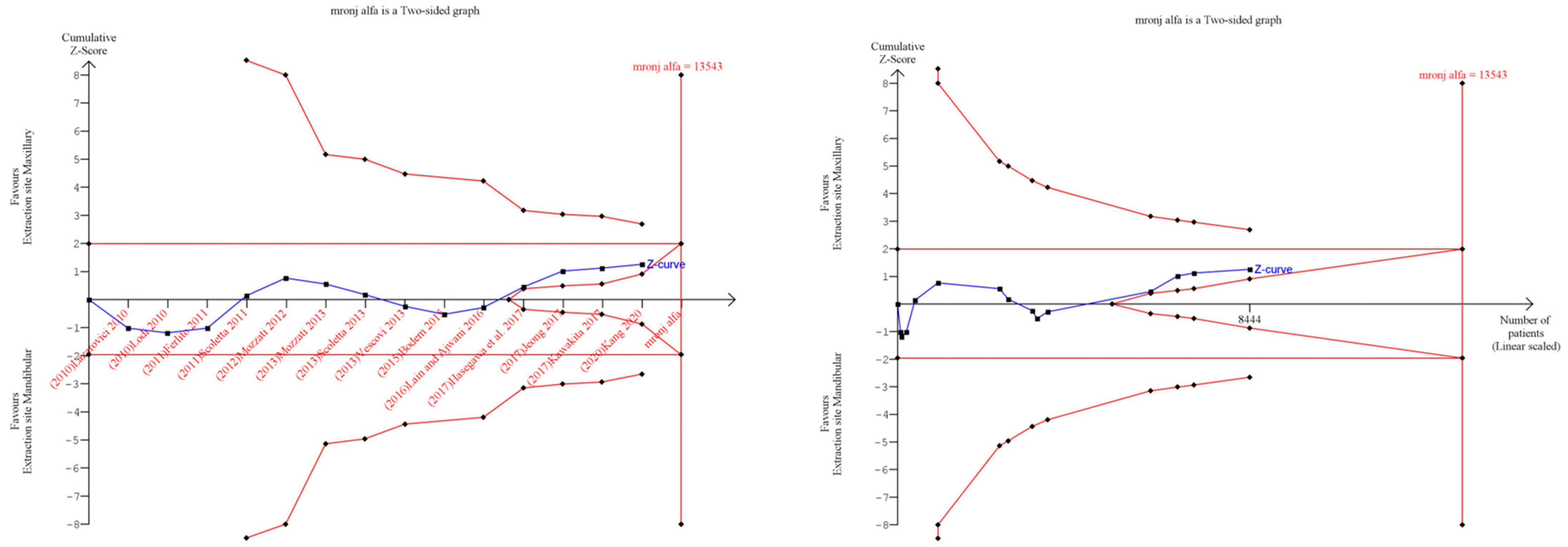
3.6. Trial Sequential Analysis: Grade
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Laino, L.; Dioguardi, M.; Giannatempo, G.; Lo Muzio, L.; Lo Russo, L. Mandibular Class II Furcation Defect Treatment: Effects of the Addition of Platelet Concentrates to Open Flap: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Periodontol. 2016, 87, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Li, W.; Zhang, W.; Yang, C.; Zhang, C.; Liang, X.; Yin, J.; Bai, J.; Ge, G.; Zhang, H.; et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol. Res. 2021, 174, 105967. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Levancini, M. Management of postmenopausal osteoporosis and the prevention of fractures. Panminerva Med. 2014, 56, 115–131. [Google Scholar] [PubMed]
- Zhurakivska, K.; Troiano, G.; Caponio, V.C.A.; Dioguardi, M.; Laino, L.; Maffione, A.B.; Lo Muzio, L. Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Front Physiol. 2019, 10, 1029. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Dioguardi, M.; Alovisi, M.; Crincoli, V.; Aiuto, R.; Malagnino, G.; Quarta, C.; Laneve, E.; Sovereto, D.; Lo Russo, L.; Troiano, G.; et al. Prevalence of the Genus Propionibacterium in Primary and Persistent Endodontic Lesions: A Systematic Review. J. Clin. Med. 2020, 9, 739. [Google Scholar] [CrossRef]
- Papapetrou, P.D. Bisphosphonate-associated adverse events. Hormones 2009, 8, 96–110. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Liu, J.Y.; Pan, J. Progress on medication-related osteonecrosis of the jaw. Hua Xi Kou Qiang Yi Xue Za Zhi 2018, 36, 568–572. [Google Scholar] [CrossRef]
- Grassini, D.; Cascardi, E.; Sarotto, I.; Annaratone, L.; Sapino, A.; Berrino, E.; Marchiò, C. Unusual Patterns of HER2 Expression in Breast Cancer: Insights and Perspectives. Pathobiology 2022, 89, 278–296. [Google Scholar] [CrossRef]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchiò, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Colella, G.; Campisi, G.; Fusco, V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-Related Osteonecrosis of the Jaws-2009 update: The need to refine the BRONJ definition. J. Oral Maxillofac. Surg. 2009, 67, 2698–2699. [Google Scholar] [CrossRef]
- Di Fede, O.; Canepa, F.; Panzarella, V.; Mauceri, R.; Del Gaizo, C.; Bedogni, A.; Fusco, V.; Tozzo, P.; Pizzo, G.; Campisi, G.; et al. The Treatment of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. Int. J. Environ. Res. Public Health 2021, 18, 8432. [Google Scholar] [CrossRef]
- Taguchi, A.; Shiraki, M.; Morrison, A.; Khan, A.A. Antiresorptive agent-related osteonecrosis of the jaw in osteoporosis patients from Asian countries. Osteoporos Sarcopenia 2017, 3, 64–74. [Google Scholar] [CrossRef]
- Laino, L.; Troiano, G.; Giannatempo, G.; Graziani, U.; Ciavarella, D.; Dioguardi, M.; Lo Muzio, L.; Lauritano, F.; Cicciù, M. Sinus Lift Augmentation by Using Calcium Sulphate. A Retrospective 12 Months Radiographic Evaluation over 25 Treated Italian Patients. Open Dent. J. 2015, 9, 414–419. [Google Scholar] [CrossRef]
- Smith, S.J.; AlQranei, M.; Alagl, A.S.; Almas, K. Tooth Extraction Protocols for Patients on Bisphosphonate Therapy: An Update. J. Int. Acad. Periodontol. 2017, 20, 38–47. [Google Scholar]
- Troiano, G.; Dioguardi, M.; Cocco, A.; Giuliani, M.; Fabiani, C.; D’Alessandro, A.; Ciavarella, D.; Lo Muzio, L. Centering Ability of ProTaper Next and WaveOne Classic in J-Shape Simulated Root Canals. ScientificWorldJournal 2016, 2016, 1606013. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Dioguardi, M.; Quarta, C.; Sovereto, D.; Troiano, G.; Melillo, M.; Di Cosola, M.; Cazzolla, A.P.; Laino, L.; Lo Muzio, L. Autotransplantation of the Third Molar: A Therapeutic Alternative to the Rehabilitation of a Missing Tooth: A Scoping Review. Bioengineering 2021, 8, 120. [Google Scholar] [CrossRef]
- Kunchur, R.; Need, A.; Hughes, T.; Goss, A. Clinical Investigation of C-Terminal Cross-Linking Telopeptide Test in Prevention and Management of Bisphosphonate-Associated Osteonecrosis of the Jaws. J. Oral Maxillofac. Surg. 2009, 67, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Kawakita, A.; Ueda, N.; Funahara, R.; Tachibana, A.; Kobayashi, M.; Kondou, E.; Takeda, D.; Kojima, Y.; Sato, S.; et al. A multicenter retrospective study of the risk factors associated with medication-related osteonecrosis of the jaw after tooth extraction in patients receiving oral bisphosphonate therapy: Can primary wound closure and a drug holiday really prevent MRONJ? Osteoporos Int. 2017, 28, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Kelleher, M.; Sproat, C.; Kwok, J.; McGurk, M. New cancer therapies and jaw necrosis. Br. Dent. J. 2015, 219, 203–207. [Google Scholar] [CrossRef]
- Aspray, T.J.; Hill, T.R. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019, 91, 453–476. [Google Scholar] [CrossRef]
- Ulm, C.; Tepper, G.; Blahout, R.; Rausch-Fan, X.; Hienz, S.; Matejka, M. Characteristic features of trabecular bone in edentulous mandibles. Clin. Oral Implants Res. 2009, 20, 594–600. [Google Scholar] [CrossRef]
- Lindhe, J.; Cecchinato, D.; Bressan, E.A.; Toia, M.; Araújo, M.G.; Liljenberg, B. The alveolar process of the edentulous maxilla in periodontitis and non-periodontitis subjects. Clin. Oral Implants Res. 2012, 23, 5–11. [Google Scholar] [CrossRef]
- Misch, C.E. Bone classification, training keys to implant success. Dent Today 1989, 8, 39–44. [Google Scholar]
- Monje, A.; Chan, H.L.; Galindo-Moreno, P.; Elnayef, B.; Suarez-Lopez del Amo, F.; Wang, F.; Wang, H.L. Alveolar Bone Architecture: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1231–1248. [Google Scholar] [CrossRef]
- Schwech, N.; Nilsson, J.; Gabre, P. Incidence and risk factors for medication-related osteonecrosis after tooth extraction in cancer patients—A systematic review. Clin. Exp. Dent. Res. 2023, 9, 55–65. [Google Scholar] [CrossRef]
- Aboalela, A.A.; Farook, F.F.; Alqahtani, A.S.; Almousa, M.A.; Alanazi, R.T.; Almohammadi, D.S. The Effect of Antiresorptive Drug Holidays on Medication-Related Osteonecrosis of the Jaw: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e30485. [Google Scholar] [CrossRef]
- Cabras, M.; Gambino, A.; Broccoletti, R.; Sciascia, S.; Arduino, P.G. Lack of evidence in reducing risk of MRONJ after teeth extractions with systemic antibiotics. J. Oral Sci. 2021, 63, 217–226. [Google Scholar] [CrossRef]
- Troiano, G.; Dioguardi, M.; Cocco, A.; Laino, L.; Cervino, G.; Cicciu, M.; Ciavarella, D.; Lo Muzio, L. Conservative vs. Radical Approach for the Treatment of Solid/Multicystic Ameloblastoma: A Systematic Review and Meta-analysis of the Last Decade. Oral Health Prev. Dent. 2017, 15, 421–426. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Shudo, A.; Kishimoto, H.; Takaoka, K.; Noguchi, K. Long-term oral bisphosphonates delay healing after tooth extraction: A single institutional prospective study. Osteoporos. Int. 2018, 29, 2315–2321. [Google Scholar] [CrossRef]
- Jeong, H.G.; Hwang, J.J.; Lee, J.H.; Kim, Y.H.; Na, J.Y.; Han, S.S. Risk factors of osteonecrosis of the jaw after tooth extraction in osteoporotic patients on oral bisphosphonates. Imaging Sci. Dent. 2017, 47, 45–50. [Google Scholar] [CrossRef]
- Lain, R.; Ajwani, S. Minor post-extraction complications other than BRONJ in older patients on oral bisphosphonates—A retrospective study. Gerodontology 2017, 34, 171–179. [Google Scholar] [CrossRef]
- Ferlito, S.; Puzzo, S.; Liardo, C. Preventive protocol for tooth extractions in patients treated with zoledronate: A case series. J. Oral Maxillofac. Surg. 2011, 69, e1–e4. [Google Scholar] [CrossRef]
- Hutcheson, A.; Cheng, A.; Kunchar, R.; Stein, B.; Sambrook, P.; Goss, A. A C-terminal crosslinking telopeptide test-based protocol for patients on oral bisphosphonates requiring extraction: A prospective single-center controlled study. J. Oral Maxillofac. Surg. 2014, 72, 1456–1462. [Google Scholar] [CrossRef]
- Lazarovici, T.S.; Mesilaty-Gross, S.; Vered, I.; Pariente, C.; Kanety, H.; Givol, N.; Yahalom, R.; Taicher, S.; Yarom, N. Serologic bone markers for predicting development of osteonecrosis of the jaw in patients receiving bisphosphonates. J. Oral Maxillofac. Surg. 2010, 68, 2241–2247. [Google Scholar] [CrossRef]
- Lodi, G.; Sardella, A.; Salis, A.; Demarosi, F.; Tarozzi, M.; Carrassi, A. Tooth extraction in patients taking intravenous bisphosphonates: A preventive protocol and case series. J. Oral Maxillofac. Surg. 2010, 68, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, C.A.; Saunders, D.; Conlon, M.S.; Ingstad, H.K.; Vaagen, P.; Palazzolo, M.J.; Herlofson, B.B. Assessing the association between bisphosphonate exposure and delayed mucosal healing after tooth extraction. J. Am. Dent. Assoc. 2013, 144, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth extraction in osteoporotic patients taking oral bisphosphonates. Osteoporos. Int. 2013, 24, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth extraction in patients on zoledronic acid therapy. Oral Oncol. 2012, 48, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G.; Carossa, S. A dental extraction protocol with plasma rich in growth factors (PRGF) in patients on intravenous bisphosphonate therapy: A case-control study. Joint Bone Spine 2011, 78, 648–649. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Ikeagwani, O.; Kearns, G.J. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? Ir. J. Med. Sci. 2012, 181, 237–242. [Google Scholar] [CrossRef]
- Saia, G.; Blandamura, S.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Bedogni, A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J. Oral Maxillofac. Surg. 2010, 68, 797–804. [Google Scholar] [CrossRef]
- Scoletta, M.; Arata, V.; Arduino, P.G.; Lerda, E.; Chiecchio, A.; Gallesio, G.; Scully, C.; Mozzati, M. Tooth extractions in intravenous bisphosphonate-treated patients: A refined protocol. J. Oral Maxillofac. Surg. 2013, 71, 994–999. [Google Scholar] [CrossRef]
- Scoletta, M.; Arduino, P.G.; Pol, R.; Arata, V.; Silvestri, S.; Chiecchio, A.; Mozzati, M. Initial experience on the outcome of teeth extractions in intravenous bisphosphonate-treated patients: A cautionary report. J. Oral Maxillofac. Surg. 2011, 69, 456–462. [Google Scholar] [CrossRef]
- Vescovi, P.; Meleti, M.; Merigo, E.; Manfredi, M.; Fornaini, C.; Guidotti, R.; Nammour, S. Case series of 589 tooth extractions in patients under bisphosphonates therapy. Proposal of a clinical protocol supported by Nd:YAG low-level laser therapy. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e680–e685. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Jensen, S.S.; Kofod, T.; Gotfredsen, K. Tooth extractions in patients with cancer receiving high-dose antiresorptive medication: A randomized clinical feasibility trial of drug holiday versus drug continuation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 165–173. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, S.J.; Kim, M.K. The effect of bisphosphonate discontinuation on the incidence of postoperative medication-related osteonecrosis of the jaw after tooth extraction. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 78–83. [Google Scholar] [CrossRef]
- Kawakita, A.; Yanamoto, S.; Morishita, K.; Naruse, T.; Hayashida, S.; Soutome, S.; Rokutanda, S.; Inokuchi, S.; Matsuo, T.; Umeda, M. Discontinuing oral bisphosphonate therapy during dental extraction does not prevent osteonecrosis of the jaw: A multicenter retrospective study of 341 patients with propensity score matching analysis. J. Oral Maxillofac. Surg. Med. Pathol. 2017, 29, 522–526. [Google Scholar] [CrossRef]
- Fujieda, Y.; Doi, M.; Asaka, T.; Ota, M.; Hisada, R.; Ohnishi, N.; Kono, M.; Kameda, H.; Nakazawa, D.; Kato, M.; et al. Incidence and risk of antiresorptive agent-related osteonecrosis of the jaw (ARONJ) after tooth extraction in patients with autoimmune disease. J. Bone Miner. Metab. 2020, 38, 581–588. [Google Scholar] [CrossRef]
- Bodem, J.P.; Kargus, S.; Eckstein, S.; Saure, D.; Engel, M.; Hoffmann, J.; Freudlsperger, C. Incidence of bisphosphonate-related osteonecrosis of the jaw in high-risk patients undergoing surgical tooth extraction. J. Cranio-Maxillofac. Surg. 2015, 43, 510–514. [Google Scholar] [CrossRef]
- O’Ryan, F.S.; Lo, J.C. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: Clinical course and outcomes. J. Oral Maxillofac. Surg. 2012, 70, 1844–1853. [Google Scholar] [CrossRef]
- Troiano, G.; Perrone, D.; Dioguardi, M.; Buonavoglia, A.; Ardito, F.; Lo Muzio, L. In vitro evaluation of the cytotoxic activity of three epoxy resin-based endodontic sealers. Dent. Mater. J. 2018, 37, 374–378. [Google Scholar] [CrossRef]
- Laino, L.; Troiano, G.; Dioguardi, M.; Perillo, L.; Laino, G.; Lo Muzio, L.; Cicciù, M. Patient Discomfort During and After Surgically Assisted Rapid Maxillary Expansion Under Local Anaesthesia. J. Craniofac. Surg. 2016, 27, 772–775. [Google Scholar] [CrossRef]
- Rosales, H.D.; Garcia Guevara, H.; Requejo, S.; Jensen, M.D.; Acero, J.; Olate, S. Medication-Related Osteonecrosis of the Jaws (MRONJ) in Children and Young Patients-A Systematic Review. J. Clin. Med. 2023, 12, 1416. [Google Scholar] [CrossRef]
- Dioguardi, M.; Gioia, G.D.; Caloro, G.A.; Capocasale, G.; Zhurakivska, K.; Troiano, G.; Russo, L.L.; Muzio, L.L. The Association between Tooth Loss and Alzheimer’s Disease: A Systematic Review with Meta-Analysis of Case Control Studies. Dent. J. 2019, 7, 49. [Google Scholar] [CrossRef]
- Ottesen, C.; Schiodt, M.; Gotfredsen, K. Efficacy of a high-dose antiresorptive drug holiday to reduce the risk of medication-related osteonecrosis of the jaw (MRONJ): A systematic review. Heliyon 2020, 6, e03795. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, E.; Seidel, L.; Bacevic, M.; Rompen, E.; Lambert, F. Occurrence and risk indicators of medication-related osteonecrosis of the jaw after dental extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Nogueras Gonzalez, G.M.; Geng, Y.; Won, A.M.; Myers, J.; Li, Y.; Chambers, M.S. Medication-Related Osteonecrosis of the Jaw in Patients Treated Concurrently with Antiresorptive and Antiangiogenic Agents: Systematic Review and Meta-Analysis. J. Immunother. Precis. Oncol. 2021, 4, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Santini, D.; Armento, G.; Tonini, G.; Campisi, G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert. Opin. Drug. Saf. 2016, 15, 925–935. [Google Scholar] [CrossRef]
- Yong, E.L.; Logan, S. Menopausal osteoporosis: Screening, prevention and treatment. Singapore Med. J. 2021, 62, 159–166. [Google Scholar] [CrossRef]
- Suryani, I.R.; Ahmadzai, I.; Shujaat, S.; Ma, H.; Jacobs, R. Non-antiresorptive drugs associated with the development of medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 2269–2279. [Google Scholar] [CrossRef]


| First Autor, Data | Country | Study Design | Population (F, M) | Mean Age (y), DS, Range Age (y) | Primary Disease | Type of Administration (OR, IV) | Duration of Administration, Mean DS (m), Range (m) | Extraction\Procedure Site |
|---|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | Japan | PS | 132 (112, 20) | 71.9 ± 11.4, (40–94) | Primary osteoporosis, prevention osteoporosis | OR: Alendronate (59), Risedronate (37), Minodronate (31), Ibandronato (5). | 40.4 ± 38.0, 1–162 | 274 |
| Jeong et al., 2017 [36] | Korea | PS | 320 (298, 22) | 111 patients < 65 y | Osteoporosis | OR: Alendronate (161) Risedronate (73), Ibandronato (20). | 140 patients < 3 y | 651 |
| Lain and Ajwani, 2016 [37] | Australia | RS | 266 (OR) (208, 58) | 73.3 ± 6.9 | Osteoporosis and cancer | OR: 266 Alendronate (203), Risedronate (55), Etidronate (1) Conbination (3), Unknown (4) IV:9 | \ | 266 |
| Hasegawa et al., 2017 [22] | Japan | RMS | 1175 (1014, 161) | 70.7 ± 11.7 (23–102) | Osteoporosis and cancer | OR: Alendronate (695), Risedronate (304), Minodronate (106), Others (8), Alendronate/Risedronate (27), Alendronate/Minodronate (19), Alendronate/Others (1), Risedronate/Minodronate (3), Minodronate/Others (1), Unknown (11). | 38.5 ± 37.7, 1–246 | 2458 |
| Ferlito et al., 2011 [38] | Italy | OLNS | 43 | 56.4 ± 5.8 | Multiple myeloma, breast cancer, prostate cancer, lung cancer | IV: Zolendronato | 16.2 ± 3.2 | 102 |
| Hutcheson et al., 2014 [39] | Australia | PS | 950 (727, 403) | 71 | Osteoporosis | OR: Alendronate (560) Risedronate (373), Other combinations (17). | 199 patients >5 y | 2461 |
| Lazarovici et al., 2010 [40] | Israel | PS | 78 (63, 15) | F 64.2, (20–89); M 62.63, (9–81). | Osteoporosis, breast carcinoma, multiple myeloma, prostate carcinoma, neurogenic carcinoma | OR: Alendronate (44), Risedronate (3), Zoledronic acid (10), Pamidronate (7). IV: Alendronate and Risendronate (4), Zoledronic acid and Clodronate (2), Pamidronate and Clodronate (1). | Or: 42–144, IV: 24–61 | 78 |
| Lodi et al., 2010 [41] | Italy | PS | 23 | 68.2, (44–83) | Multiple myeloma, bone metastasis of breast cancer or other solid tumors and severe osteoporosis | IV: Zoledronate (20), Pamidronate (2), Clodronate (1). | 17.5, 3–36 | 38 |
| Migliorati et al., 2013 [42] | Canada, Norway, USA | PS | 53 (43, 10) | 70, (40–92) | Osteoporosis metastatic bone cancer | IV 13\45, OR 32\45 | 60 | 53 |
| Mozzati et al., 2013 [43] | Italy | PS | 700 (677, 23) | (52–79) | Osteoporosis, rheumatoid arthritis, and Paget’s disease. | OR: Alendronate | \ | \ |
| Mozzati et al., 2012 [44] | Italy | CCS | 176 (101, 75) | (44–83) | Prostatic carcinoma, breast carcinoma, multiple myeloma, lung carcinoma, ovarian carcinoma | IV: Zoledronic acid | \ | \ |
| Mozzati et al., 2011 [45] | Italy | CCS | 100 (75, 25) | (44–83) | Prostatic carcinoma, breast carcinoma, multiple myeloma, lung carcinoma, ovarian carcinoma | IV: Zoledronic acid (53), Pamidronate (47) | \ | \ |
| O’Connell et al., 2012 [46] | Ireland | PS | 23 (22, 1) | 59, (44–78) | Osteoporosis | OR: Acid Alendronic (19), Risendronate (2); IV: Zoledronic Acid (2) | 30 (8–72) | 23 ? |
| Saia et al., 2010 [47] | Italy | PS | 60 (42, 18) | 65 ± 13, (17–84) | Cancer | IV: Zoledronate (38), Pamidronate (24), Neridronate (4), OR: Risedronate (2); | \ | 185 |
| Scoletta et al., 2013 [48] | Italy | PS | 63 (45, 18) | 65.82 ± 8.82 | Cancer and osteoporosis | IV: Zoledronic acid (54), Pamidronate (4), Ibandronate (5) | 19.03 months | 202 |
| Scoletta et al., 2011 [49] | Italy | PS | 64 (44, 20) | 64.81 ± 10.98 | Cancer and osteoporosis | IV: Zoledronic acid (57), Pamidronate (2), Zoledronic acid + Pamidronate (5) | 16.20 | 220 |
| Vescovi et al., 2013 [50] | Italy | CS | 217 (179, 38) | 68.72, (30–83) | Cancer and osteoporosis | Zoledronate (87), Zoledronate + Pamidronate (1), Alendronate 54, Risedronate 18, Alendronate + Zoledronate (3), Clodronate (24), in 30 cases different association of BPs. | 17 (cancer) 53 (osteoporosis) | 589 |
| Ottesen et al., 2021 [51] | Denmark | RTC | 23 (12, 11) | 69 (59–77), 67 (56–78) | Cancer | IV: Denosumab (13), Bisphosphonate (10). | 9 (range 2–30) 17.5 (range 4–96) | 31 |
| Kang et al., 2020 [52] | Korea | RS | 465 (420, 45) | M 3.7 ± 10.5; F 69.3 ± 8.8 | Osteoporosis and cancer | OR: 410 Alendronate IV and OR: 30 Ibandronate and Alendronate. IV: Ibandronate 26 | OR: 39.0 ± 35.5; IV:(40.0 ± 35.6) | 1323 |
| Kawakita et al., 2017 [53] | Japan | RS | 341 (352, 43) | 72.4 ± 10.6, 74.1 ± 9.62 | Osteoporosis and cancer | OR | 43.4 ± 36.3; 31.3 ± 31.0 | 850 |
| Fujieda et al., 2020 [54] | Japan | RS | 232 (202, 30) | 71 (24–94) | Autoimmune disease | Alendronate (111), Risedronate (80), Minodronate (23), Ibantronate (4), Denousumab (14) | 37 (17–51) | \ |
| Bodem et al., 2015 [55] | Germany | PS | 61 (42, 19) | 65.65 ± 12.69 (34–87) | Cancer | IV: Zoledronic acid (38), Ibandronate (17), and Pamidronate (6). | 40.25 ± 32.91; (4–245) | 102 (184) 1 |
| O’Ryan and Lo, 2012 [56] | USA | RS | 30 (26, 4) | (54–89) | Osteoporosis | \ | \ | \ |
| Kunchur et al., 2009 [21] | Australia | PS | 222 (165, 57) | OR 71 ± 11.6, IV 61 ± 11 | Osteoporosis and cancer | OR: Alendronate (139) Risedronate (76); IV: Pamidronate (6), Zoledronic acid (1) | OR: 50.5 ± 32 (2–180) IV: 26.9 ± 25 (21–72) | 194 procedure and 21 endodontic therapy 2 |
| First Autor, Data | Population (F, M) | Extraction Site Total | Extraction Site Maxillary\MRONJ Site | Extraction Site Mandibular\MRONJ | Extraction Site Anterior\MRONJ | Extraction Site Posterior\MRONJ | MRONJ Total |
|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | 132 (112, 20) | 274 | 165\0 | 109\0 | 97 | 177 | 0 |
| Jeong et al., 2017 [36] | 320 (298, 22) | 651 | 365\3 | 286\15 | 168\5 | 483\13 | 11 patients, 18 sites |
| Lain and Ajwani 2016 [37] | 266 (208, 58) | 266 | 136\12 | 130\14 | 93\5 | 173\21 | 26 sites |
| Hasegawa et al., 2017 [22] | 1175 (1014, 161) | 2458 | 1240\14 | 1218\27 | 1231\5 | 1591\36 | 41 sites |
| Ferlito et al., 2011 [38] | 43 | 102 | 59\0 | 43\0 | \ | \ | 102 sites |
| Hutcheson et al., 2014 [39] | 950 (727, 403) | 2461 | \ | \ | \ | \ | 4 |
| Lazarovici et al., 2010 [40] | 78 (63, 15) | 78 | 33 1\10 | 44\8 | 20\7 | 57\11 | 18 patients |
| Lodi et al., 2010 [41] | 23 (15, 8) | 38 | 7 2\0 | 25\0 | 4\0 | 33\0 | 0 |
| Migliorati et al., 2013 [42] | 53 (43, 10) | 53 | \ | \ | \ | \ | 1 |
| Mozzati et al., 2013 [43] | 700 (677, 23) | 1480 | 616 | 864 | \ | \ | 0 |
| Mozzati et al., 2012 [44] | 176 (101, 75) | 542 | 255\0 | 287\5 | \ | \ | 5 |
| Mozzati et al., 2011 [45] | 100 | 222 | 108 | 114 | \ | \ | 2 |
| O’Connell et al., 2012 [46] | 23 (22, 1) | 23 ? | \ | \ | \ | \ | 0 |
| Saia et al., 2010 [47] | 60 (42,18) | 185 | 82 | 103 | \ | \ | 5 patients |
| Scoletta et al., 2013 [48] | 63 (45, 18) | 202 | 91\2 | 111\0 | \ | \ | 2 |
| Scoletta et al., 2011 [49] | 64 (44, 20) | 220 | 107\0 | 113\5 | \ | \ | 5 |
| Vescovi et al., 2013 [50] | 217 (179, 38) | 589 | 304\4 | 285\1 | \ | \ | 5 |
| Ottesen et al., 2021 [51] | 23 (12, 11) | 31 | 18 | 13 | \ | \ | 4 |
| Kang et al., 2020 [52] | 465 (420, 45) | 1323 | 740\0 | 583\1 | \ | \ | 1 |
| Kawakita et al., 2017 [53] | 341 (352, 43) | 850 | 203\3 | 199\4 | \ | \ | 7 |
| Fujieda et al., 2020 [54] | 232 (202, 30) | \ | \ | \ | \ | \ | 10 |
| Bodem et al., 2015 [55] | 61 (42, 19) | 102 | 45\5 | 55\5 | \ | \ | 10 (8 3) |
| First Autor, Data | Male (MRONJ) | Male Total | Female (MRONJ) | Female Total |
|---|---|---|---|---|
| Shudo et al., 2018 [35] | 0 | 20 | 0 | 212 |
| Jeong et al., 2017 [36] | 0 | 22 | 11 | 298 |
| Lain and Ajwani, 2016 [37] | 5 | 58 | 21 | 208 |
| Hasegawa et al., 2017 [22] | 5 | 161 | 36 | 1014 |
| Ferlito et al., 2011 [38] | \ | \ | \ | \ |
| Hutcheson et al., 2014 [39] | 1 | 403 | 3 | 727 |
| Lazarovici et al., 2010 [40] | \ | 15 | \ | 63 |
| Lodi et al., 2010 [41] | 0 | \ | 0 | \ |
| Migliorati et al., 2013 [42] | 1 | 10 | 0 | 43 |
| Mozzati et al., 2013 [43] | 0 | 23 | 0 | 677 |
| Mozzati et al., 2012 [44] | \ | 75 | \ | 101 |
| Mozzati et al., 2011 [45] | \ | \ | \ | \ |
| O’Connell et al., 2012 [46] | 0 | 1 | 0 | 22 |
| Saia et al., 2010 [47] | 2 | 18 | 3 | 42 |
| Scoletta et al., 2013 [48] | 0 | 18 | 1 | 45 |
| Scoletta et al., 2011 [49] | 1 | 44 | 4 | 20 |
| Vescovi et al., 2013 [50] | 4 | 38 | 1 | 179 |
| Ottesen et al., 2021 [51] | \ | 11 | \ | 12 |
| Kang et al., 2020 [52] | 0 | 45 | 1 | 420 |
| Kawakita et al., 2017 [53] | 0 | 43 | 7 | 352 |
| Fujieda et al., 2020 [54] | \ | 30 | \ | 202 |
| Bodem et al., 2015 [55] | \ | 19 | \ | 42 |
| Kunchur et al., 2009 [21] | 1 | 57 | 0 | 165 |
| O’Ryan and Lo, 2012 [56] | \ | 4 | \ | 26 |
| First Autor, Data | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Classification of Interventions | Bias Due to Deviations from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | |
|---|---|---|---|---|---|---|---|---|
| Shudo et al., 2018 [35] | + | + | + | + | + | + | + | + |
| Jeong et al., 2017 [36] | - | + | + | + | + | + | + | + |
| Lain and Ajwani, 2016 [37] | + | + | + | + | + | + | + | + |
| Hasegawa et al., 2017 [22] | + | + | + | + | + | + | + | + |
| Ferlito et al., 2011 [38] | + | + | + | + | ? | + | ? | + |
| Hutcheson et al., 2014 [39] | + | + | + | + | ? | + | + | + |
| Lazarovici et al., 2010 [40] | + | + | + | + | + | + | + | + |
| Lodi et al., 2010 [41] | + | + | + | + | + | + | + | + |
| Migliorati et al., 2013 [42] | + | + | ? | + | ? | + | ? | + |
| Mozzati et al., 2013 [43] | + | + | ? | + | ? | + | ? | + |
| Mozzati et al., 2012 [44] | + | + | + | + | ? | + | + | + |
| Mozzati et al., 2011 [45] | + | + | + | + | ? | + | ? | + |
| O’Connell et al., 2012 [46] | + | + | + | + | ? | + | ? | + |
| Saia et al., 2010 [47] | + | + | + | + | ? | + | ? | + |
| Scoletta et al., 2013 [48] | + | + | + | + | ? | + | ? | + |
| Scoletta et al., 2011 [49] | + | + | + | + | ? | + | ? | + |
| Vescovi et al., 2013 [50] | + | + | + | + | ? | + | ? | + |
| Ottesen et al., 2021 [51] | + | + | + | + | + | + | + | + |
| Kang et al., 2020 [52] | + | + | + | + | + | + | + | + |
| Kawakita et al., 2017 [53] | + | + | + | + | ? | + | ? | + |
| Fujieda et al., 2020 [54] | + | + | + | + | ? | + | ? | + |
| Bodem et al., 2015 [55] | + | + | + | + | ? | + | ? | + |
| Kunchur et al., 2009 [21] | + | + | + | + | ? | + | + | + |
| O’Ryan and Lo, 2012 [56] | + | - | + | + | ? | + | + | + |
| Certainty Assessment | No. of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | MRONJ Maxillary | MRONJ Mandibular | Relative (95% CI) | Absolute (95% CI) | |
| 14 | Observational studies | Not serious | not serious | not serious | not serious | none | 55/4201 (1.4%) | 88/4243 (2.1%) | OR 0.68 (0.48 to 0.95) | 7 fewer per 1.000 (from 11 fewer to 1 fewer) | ⨁⨁◯◯ Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dioguardi, M.; Spirito, F.; Alovisi, M.; Aiuto, R.; Garcovich, D.; Crincoli, V.; Ballini, A.; Caloro, G.A.; Lo Muzio, L. Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis. J. Clin. Med. 2023, 12, 3299. https://doi.org/10.3390/jcm12093299
Dioguardi M, Spirito F, Alovisi M, Aiuto R, Garcovich D, Crincoli V, Ballini A, Caloro GA, Lo Muzio L. Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis. Journal of Clinical Medicine. 2023; 12(9):3299. https://doi.org/10.3390/jcm12093299
Chicago/Turabian StyleDioguardi, Mario, Francesca Spirito, Mario Alovisi, Riccardo Aiuto, Daniele Garcovich, Vito Crincoli, Andrea Ballini, Giorgia Apollonia Caloro, and Lorenzo Lo Muzio. 2023. "Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis" Journal of Clinical Medicine 12, no. 9: 3299. https://doi.org/10.3390/jcm12093299
APA StyleDioguardi, M., Spirito, F., Alovisi, M., Aiuto, R., Garcovich, D., Crincoli, V., Ballini, A., Caloro, G. A., & Lo Muzio, L. (2023). Location and Gender Differences in Osteonecrosis of the Jaws in Patients Treated with Antiresorptive and Antineoplastic Drugs Undergoing Dentoalveolar Surgical, Systematic Review with Meta-Analysis and Trial Sequential Analysis. Journal of Clinical Medicine, 12(9), 3299. https://doi.org/10.3390/jcm12093299













