Early Re-Exploration versus Conservative Management for Postoperative Bleeding in Stable Patients after Coronary Artery Bypass Grafting: A Propensity Matched Study
Abstract
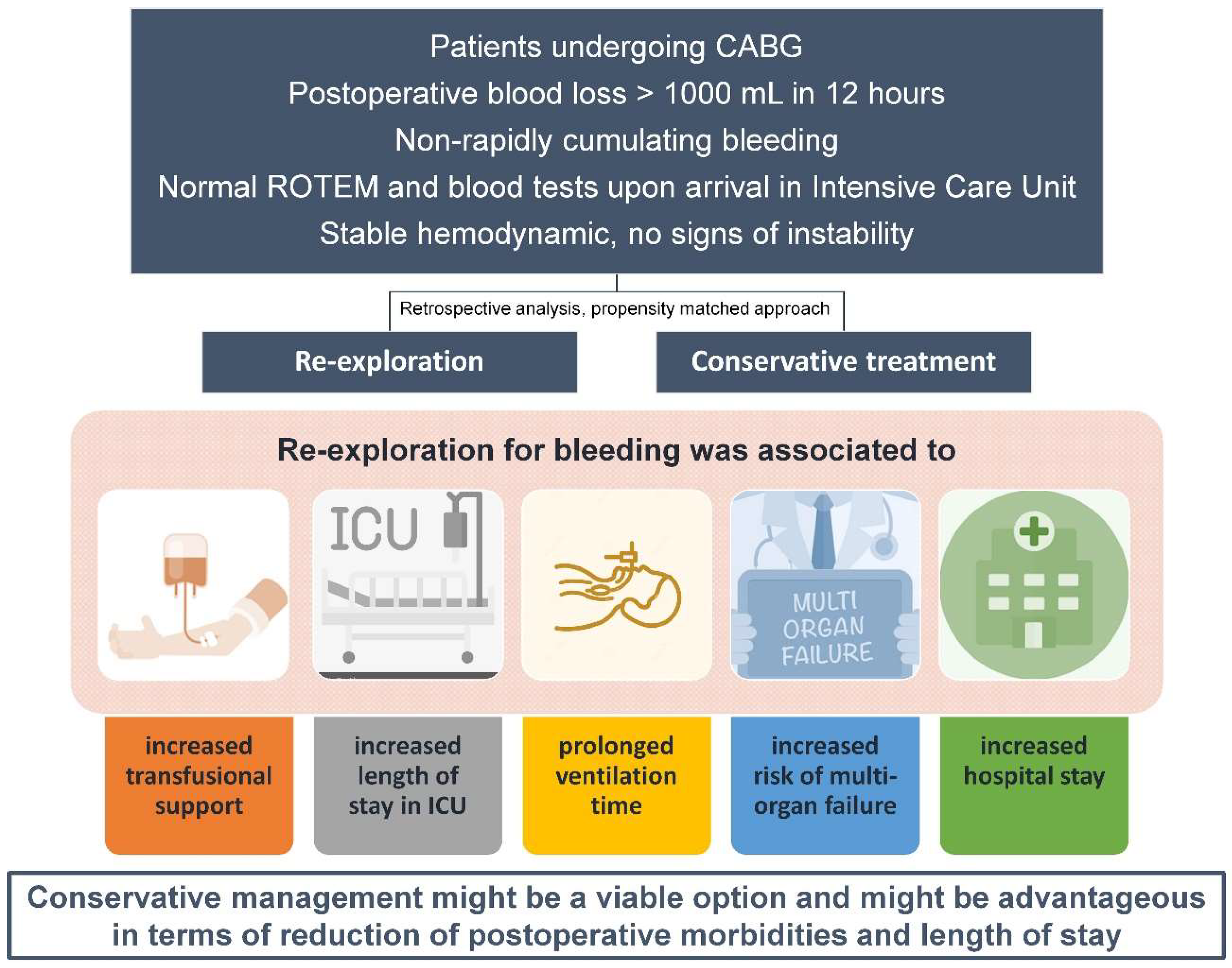
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| BSA | body surface area |
| CABG | coronary artery bypass grafting |
| ICU | intensive care unit |
| ROTEM | rotational thromboelastometry |
References
- Fernandez, F.G.; Shahian, D.M.; Kormos, R.; Jacobs, J.P.; D’agostino, R.S.; Mayer, J.E.; Kozower, B.D.; Higgins, R.S.; Badhwar, V. The Society of Thoracic Surgeons National Database 2019 Annual Report. Ann. Thorac. Surg. 2019, 108, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Ohmes, L.B.; Di Franco, A.; Guy, T.S.; Lau, C.; Munjal, M.; Debois, W.; Li, Z.; Krieger, K.H.; Schwann, A.N.; Leonard, J.R.; et al. Incidence, risk factors, and prognostic impact of re-exploration for bleeding after cardiac surgery: A retrospective cohort study. Int. J. Surg. 2017, 48, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.L.; Rauer, L.J.; Mortensen, P.E.; Kjeldsen, B.J. Reoperation for bleeding in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 709–713. [Google Scholar] [CrossRef]
- Brown, J.A.; Kilic, A.; Aranda-Michel, E.; Navid, F.; Serna-Gallegos, D.; Bianco, V.; Sultan, I. Long-Term Outcomes of Reoperation for Bleeding After Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. 2020, 33, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, A.G.; Van Veghel, D.; Hamad, M.A.S.; Schulz, D.; Stepaniak, P.S.; Van Straten, A.H. Use of an intraoperative checklist to decrease the incidence of re-exploration for postoperative bleeding after cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 555–558. [Google Scholar] [CrossRef]
- Fröjd, V.; Jeppsson, A. Reexploration for Bleeding and Its Association with Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2016, 102, 109–117. [Google Scholar] [CrossRef]
- Ruel, M.; Chan, V.; Boodhwani, M.; McDonald, B.; Ni, X.; Gill, G.; Lam, K.; Hendry, P.; Masters, R.; Mesana, T. How detrimental is reexploration for bleeding after cardiac surgery? J. Thorac. Cardiovasc. Surg. 2017, 154, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Čanádyová, J.; Zmeko, D.; Mokráček, A. Re-exploration for bleeding or tamponade after cardiac operation. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 704–707. [Google Scholar] [CrossRef]
- Biancari, F.; Kinnunen, E.-M.; Kiviniemi, T.; Tauriainen, T.; Anttila, V.; Airaksinen, J.K.; Brascia, D.; Vasques, F. Meta-analysis of the Sources of Bleeding after Adult Cardiac Surgery. J. Cardiothorac. Vasc. Anesthesia 2018, 32, 1618–1624. [Google Scholar] [CrossRef]
- Kapetanakis, E.I.; Medlam, D.A.; Boyce, S.W.; Haile, E.; Hill, P.C.; Dullum, M.K.; Bafi, A.S.; Petro, K.R.; Corso, P.J. Clopidogrel administration prior to coronary artery bypass grafting surgery: The cardiologist’s panacea or the surgeon’s headache? Eur. Heart J. 2005, 26, 576–583. [Google Scholar] [CrossRef]
- Karthik, S.; Grayson, A.D.; McCarron, E.E.; Pullan, D.; Desmond, M.J. Reexploration for bleeding after coronary artery bypass surgery: Risk factors, outcomes, and the effect of time delay. Ann. Thorac. Surg. 2004, 78, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Castelvecchio, S.; Pelissero, G. Major Bleeding, Transfusions, and Anemia: The Deadly Triad of Cardiac Surgery. Ann. Thorac. Surg. 2013, 96, 478–485. [Google Scholar] [CrossRef]
- Ranucci, M.; Bozzetti, G.; Ditta, A.; Cotza, M.; Carboni, G.; Ballotta, A. Surgical Reexploration After Cardiac Operations: Why a Worse Outcome? Ann. Thorac. Surg. 2008, 86, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Choong, C.K.; Gerrard, C.; Goldsmith, K.A.; Dunningham, H.; Vuylsteke, A. Delayed re-exploration for bleeding after coronary artery bypass surgery results in adverse outcomes? Eur. J. Cardio-Thorac. Surg. 2007, 31, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Tirilomis, T.; Bougioukas, I.G.; Friedrich, M.G.; Danner, B.C.; Schoendube, F.A. Re-exploration Early after Cardiac Surgery in Adults: The Importance of Bleeding-Related Complications. Hear. Surg. Forum 2020, 23, E174–E177. [Google Scholar] [CrossRef] [PubMed]
- Petricevic, M.; Petricevic, M.; Pasalic, M.; Cepulic, B.G.; Raos, M.; Dujmic, D.; Kalamar, V.; Mestrovic, V.; Gasparovic, H.; Vasicek, V.; et al. Cost Analysis of Transfusion Therapy in Coronary Artery Surgery. Thorac. Cardiovasc. Surg. 2021, 69, 621–629. [Google Scholar] [CrossRef]
- Senage, T.; Gerrard, C.; Moorjani, N.; Jenkins, D.P.; Ali, J.M. Early postoperative bleeding impacts long-term survival following first-time on-pump coronary artery bypass grafting. J. Thorac. Dis. 2021, 13, 5670–5682. [Google Scholar] [CrossRef]
- Tran, Z.; Williamson, C.; Hadaya, J.; Verma, A.; Sanaiha, Y.; Chervu, N.; Gandjian, M.; Benharash, P. Trends and Outcomes of Surgical Reexploration After Cardiac Operations in the United States. Ann. Thorac. Surg. 2021, 113, 783–792. [Google Scholar] [CrossRef]
- Elassal, A.A.; Al-Ebrahim, K.E.; Debis, R.S.; Ragab, E.S.; Faden, M.S.; Fatani, M.A.; Allam, A.R.; Abdulla, A.H.; Bukhary, A.M.; Noaman, N.A.; et al. Re-exploration for bleeding after cardiac surgery: Revaluation of urgency and factors promoting low rate. J. Cardiothorac. Surg. 2021, 16, 166. [Google Scholar] [CrossRef]
- Biancari, F. Favoring Prompt Re-exploration for Excessive Bleeding After Adult Cardiac Surgery. Ann. Thorac. Surg. 2022. [Google Scholar] [CrossRef]
- Salna, M.; Takayama, H. Prolene or Products: When Is the Right Time to Bite the Bullet and Takeback? Ann. Thorac. Surg. 2023, 115, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Shou, B.L.; Aravind, P.; Ong, C.S.; Alejo, D.; Canner, J.K.; Etchill, E.W.; DiNatale, J.; Prokupets, R.; Esfandiary, T.; Lawton, J.S.; et al. Early Reexploration for Bleeding Is Associated with Improved Outcome in Cardiac Surgery. Ann. Thorac. Surg. 2023, 115, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal definition of perioperative bleeding in adult cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Pagano, D.; Milojevic, M.; Meesters, M.I.; Benedetto, U.; Bolliger, D.; Von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2018, 53, 79–111. [Google Scholar] [CrossRef]
- Moulton, M.J.; Creswell, L.L.; Mackey, M.E.; Cox, J.L.; Rosenbloom, M. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J. Thorac. Cardiovasc. Surg. 1996, 111, 1037–1046. [Google Scholar] [CrossRef]
- Biancari, F.; Mikkola, R.; Heikkinen, J.; Lahtinen, J.; Airaksinen, K.J.; Juvonen, T. Estimating the risk of complications related to re-exploration for bleeding after adult cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. Surg. 2012, 41, 50–55. [Google Scholar] [CrossRef]
- Haneya, A.; Diez, C.; Kolat, P.; von Suesskind-Schwendi, M.; Ried, M.; Schmid, C.; Hirt, S.W. Re-Exploration for Bleeding or Tamponade after Cardiac Surgery: Impact of Timing and Indication on Outcome. Thorac. Cardiovasc. Surg. 2015, 63, 051–057. [Google Scholar] [CrossRef]
- Demal, T.J.; Fehr, S.; Mariscalco, G.; Reiter, B.; Bibiza, E.; Reichenspurner, H.; Gatti, G.; Onorati, F.; Faggian, G.; Salsano, A.; et al. Coronary Artery Bypass Grafting in Patients with High Risk of Bleeding. Hear. Lung Circ. 2021, 31, 263–271. [Google Scholar] [CrossRef]
- Bastopcu, M.; Özhan, A.; Erdoğan, S.B.; Kehlibar, T. Factors associated with excessive bleeding following elective on-pump coronary artery bypass grafting. J. Card. Surg. 2021, 36, 1277–1281. [Google Scholar] [CrossRef]
- Loor, G.; Vivacqua, A.; Sabik, J.F.; Li, L.; Hixson, E.D.; Blackstone, E.H.; Koch, C.G. Process improvement in cardiac surgery: Development and implementation of a reoperation for bleeding checklist. J. Thorac. Cardiovasc. Surg. 2013, 146, 1028–1032. [Google Scholar] [CrossRef]
- Ali, J.M.; Gerrard, C.; Clayton, J.; Moorjani, N. Hemostasis Checklist Reduces Bleeding and Blood Product Consumption After Cardiac Surgery. Ann. Thorac. Surg. 2021, 111, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Ak, K.; Isbir, C.S.; Tetik, S.; Atalan, N.; Tekeli, A.; Aljodi, M.; Civelek, A.; Arsan, S. Thromboelastography-Based Transfusion Algorithm Reduces Blood Product Use after Elective CABG: A Prospective Randomized Study. J. Card. Surg. 2009, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Di Franco, A.; Rahouma, M.; Tam, D.Y.; Iannaccone, M.; Deb, S.; D’Ascenzo, F.; Abouarab, A.A.; Girardi, L.N.; Taggart, D.P.; et al. Unmeasured Confounders in Observational Studies Comparing Bilateral Versus Single Internal Thoracic Artery for Coronary Artery Bypass Grafting: A Meta-Analysis. J. Am. Hear. Assoc. 2018, 7, e008010. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Hall, J.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; McGuinness, S.; et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2017, 377, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Sef, D.; Gaudino, M.; Taggart, D.P. Postcardiac surgery myocardial ischemia: Why, when, and how to intervene. J. Thorac. Cardiovasc. Surg. 2023, 165, 687–695. [Google Scholar] [CrossRef]
- Rudolph, D.A.; Hald, S.M.; Rodríguez, L.A.G.; Möller, S.; Hallas, J.; Goldstein, L.B.; Gaist, D. Association of Long-term Statin Use with the Risk of Intracerebral Hemorrhage: A Danish Nationwide Case-Control Stu. Neurology 2022, 99, e711–e719. [Google Scholar] [CrossRef] [PubMed]
- Nenna, A.; Lusini, M.; Spadaccio, C.; Nappi, F.; Prestipino, F.; Barbato, R.; Casacalenda, A.; Pugliese, G.; Barberi, F.; Giacinto, O.; et al. Preoperative atorvastatin reduces bleeding and blood products use in patients undergoing on-pump coronary artery bypass grafting. J. Cardiovasc. Med. 2017, 18, 976–982. [Google Scholar] [CrossRef]
- Nenna, A.; Spadaccio, C.; Lusini, M.; Nappi, F.; Mastroianni, C.; Giacinto, O.; Pugliese, G.; Casacalenda, A.; Barbato, R.; Barberi, F.; et al. Preoperative atorvastatin reduces bleeding and blood transfusions in patients undergoing elective isolated aortic valve replacement. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 51–58. [Google Scholar] [CrossRef]
- Nenna, A.; Nappi, F.; Lusini, M.; Satriano, U.M.; Schilirò, D.; Spadaccio, C.; Chello, M. Effect of Statins on Platelet Activation and Function: From Molecular Pathways to Clinical Effects. BioMed Res. Int. 2021, 2021, 6661847. [Google Scholar] [CrossRef]
| All Patients N = 1642 | Unmatched Cohort, Not Reoperated N = 1390 | Unmatched Cohort, Reoperated N = 252 | p Value | Matched Cohort, Not Reoperated N = 251 | Matched Cohort, Reoperated N = 251 | p Value | |
|---|---|---|---|---|---|---|---|
| Age | 65.1 ± 9.6 | 64.9 ± 9.5 | 65.8 ± 10.1 | 0.226 | 66.1 ± 10.0 | 65.8 ± 10.1 | 0.667 |
| Male sex | 1397 (85.1) | 1181 (84.9) | 216 (85.7) | 0.758 | 216 (86.1) | 216 (86.1) | 0.999 |
| Body mass index (kg/m2) | 26.7 ± 5.0 | 26.7 ± 5.2 | 26.7 ± 3.8 | 0.952 | 26.9 ± 3.8 | 26.7 ± 3.8 | 0.629 |
| Body surface area (m2) | 1.94 ± 0.30 | 1.94 ± 0.30 | 0.96 ± 0.29 | 0.322 | 1.97 ± 0.34 | 1.96 ± 0.29 | 0.879 |
| Preoperative angina class | |||||||
| 0 | 204 (12.4) | 169 (12.2) | 35 (13.9) | 0.397 | 30 (11.9) | 35 (13.9) | 0.237 |
| 1 | 247 (15.0) | 203 (14.6) | 44 (17.5) | 37 (14.7) | 44 (17.5) | ||
| 2 | 542 (33.0) | 458 (32.9) | 84 (33.3) | 81 (32.2) | 83 (33.1) | ||
| 3 | 442 (26.9) | 377 (27.2) | 65 (25.8) | 62 (24.7) | 65 (25.9) | ||
| 4 | 207 (12.6) | 183 (13.2) | 24 (9.5) | 41 (16.3) | 24 (9.6) | ||
| Preoperative NYHA class | |||||||
| 0 | 230 (14.0) | 191 (13.7) | 39 (15.5) | 0.065 | 43 (17.1) | 39 (15.5) | 0.158 |
| 1 | 634 (38.6) | 524 (37.7) | 110 (43.6) | 84 (33.5) | 109 (43.4) | ||
| 2 | 468 (28.5) | 396 (28.5) | 72 (28.6) | 77 (30.7) | 71 (28.3) | ||
| 3 | 277 (16.9) | 249 (17.9) | 28 (11.1) | 40 (15.9) | 28 (11.1) | ||
| 4 | 33 (2.0) | 30 (2.2) | 3 (1.2) | 7 (2.8) | 4 (1.6) | ||
| Preoperative myocardial infarction | |||||||
| no | 795 (48.2) | 683 (49.1) | 112 (44.4) | 0.337 | 120 (47.8) | 112 (44.6) | 0.620 |
| <6 h | 726 (44.2) | 608 (43.7) | 118 (46.8) | 114 (45.4) | 117 (46.6) | ||
| 6–24h | 121 (7.4) | 99 (7.1) | 22 (8.7) | 17 (6.8) | 22 (8.7) | ||
| Previous percutaneous coronary intervention | 130 (7.9) | 104 (7.5) | 26 (10.3) | 0.125 | 18 (7.2) | 28 (11.1) | 0.122 |
| Diabetes | |||||||
| no | 1386 (84.4) | 1174 (84.5) | 212 (84.1) | 0.844 | 218 (86.8) | 211 (84.1) | 0.567 |
| diet treatment | 63 (3.8) | 51 (3.7) | 12 (4.7) | 14 (5.6) | 12 (4.8) | ||
| oral treatment | 121 (7.4) | 104 (7.5) | 17 (6.7) | 11 (4.4) | 17 (6.8) | ||
| insulin | 72 (4.4) | 61 (4.4) | 11 (4.4) | 8 (3.2) | 11 (4.4) | ||
| Smoking | |||||||
| never | 646 (39.3) | 555 (39.9) | 91 (36.1) | 0.428 | 91 (36.2) | 90 (35.8) | 0.771 |
| past | 827 (50.3) | 696 (50.1) | 131 (52.0) | 135 (53.8) | 131 (52.2) | ||
| current | 169 (10.3) | 139 (10.0) | 30 (11.9) | 25 (10.0) | 30 (11.9) | ||
| Hypertension | 932 (56.8) | 782 (56.2) | 150 (59.5) | 0.336 | 154 (61.3) | 150 (59.8) | 0.715 |
| Hypercholesterolemia | 909 (55.4) | 783 (56.3) | 126 (50.0) | 0.063 | 126 (50.2) | 126 (50.2) | 0.999 |
| Preoperative dialysis | 18 (1.1) | 17 (1.2) | 1 (0.4) | 0.246 | 2 (0.8) | 1 (0.4) | 0.563 |
| Preoperative pulmonary disease | 143 (8.7) | 119 (8.6) | 24 (9.5) | 0.618 | 23 (9.2) | 24 (9.5) | 0.878 |
| Preoperative stroke | |||||||
| transient | 79 (4.8) | 65 (4.7) | 14 (5.6) | 0.727 | 16 (6.4) | 14 (5.6) | 0.932 |
| stroke | 26 (1.6) | 23 (1.6) | 3 (1.2) | 3 (1.2) | 3 (1.2) | ||
| Preoperative extracardiac arteriopathy | 246 (15.0) | 212 (15.2) | 34 (13.5) | 0.471 | 36 (14.3) | 33 (13.1) | 0.697 |
| Number of diseased vessels | |||||||
| 1 | 62 (3.8) | 53 (3.8) | 9 (3.6) | 0.425 | 9 (3.6) | 9 (3.6) | 0.951 |
| 2 | 322 (19.6) | 265 (19.1) | 57 (22.6) | 60 (23.9) | 37 (22.7) | ||
| 3 | 1258 (76.6) | 1072 (77.1) | 186 (73.8) | 182 (72.5) | 185 (73.7) | ||
| Left main disease | 378 (23.0) | 321 (23.1) | 57 (22.6) | 0.869 | 54 (21.5) | 57 (22.7) | 0.747 |
| Left ventricular ejection fraction, category | |||||||
| good (>50%) | 1244 (75.8) | 1050 (75.5) | 194 (77.0) | 0.723 | 191 (76.1) | 193 (76.9) | 0.975 |
| fair (31–50%) | 324 (19.7) | 275 (19.8) | 49 (19.4) | 51 (20.3) | 49 (19.5) | ||
| poor (21–30%) | 74 (4.5) | 65 (4.7) | 9 (3.6) | 9 (3.6) | 9 (3.6) | ||
| Preoperative nitrates or heparin | 52 (3.2) | 40 (2.9) | 12 (4.7) | 0.116 | 9 (3.6) | 12 (4.8) | 0.504 |
| Endoscopic vein harvesting | 96 (5.9) | 80 (5.7) | 16 (6.3) | 0.712 | 19 (7.6) | 16 (6.4) | 0.599 |
| Skeletonized internal mammary artery | 1642 (100) | 1390 (100) | 252 (100) | 0.999 | 251 (100) | 251 (100) | 0.999 |
| Number of distal anastomoses | |||||||
| 1 | 50 (3.0) | 42 (3.0) | 8 (3.2) | 0.140 | 10 (4.0) | 8 83.2) | 0.895 |
| 2 | 310 (18.9) | 248 (17.8) | 62 (24.6) | 63 (25.1) | 62 (24.7) | ||
| 3 | 734 (44.7) | 633 (45.5) | 101 (40.1) | 102 (40.6) | 100 (39.8) | ||
| 4 | 465 (28.2) | 395 (28.4) | 70 (27.8) | 62 (24.7) | 70 (27.9) | ||
| 5 | 83 (5.0) | 72 (5.2) | 11 (4.4) | 14 (5.6) | 11 (4.4) | ||
| On pump surgery | 1374 (83.7) | 1169 (84.1) | 205 (81.3) | 0.277 | 213 (84.8) | 204 (81.3) | 0.284 |
| mean cardiopulmonary bypass time | 88.5 ± 31.0 | 88.7 ± 30.7 | 87.6 ± 32.7 | 0.657 | 85.7 ± 31.8 | 87.7 ± 32.8 | 0.528 |
| mean aortic cross clamp time | 50.6 ± 22.6 | 50.3 ± 22.4 | 52.1 ± 24.0 | 0.287 | 49.6 ± 24.0 | 51.3 ± 23.9 | 0.473 |
| Unmatched Cohort Not Reoperated N = 1390 | Unmatched Cohort Reoperated N = 252 | p Value | Matched Cohort Not Reoperated N = 251 | Matched Cohort Reoperated N = 251 | p Value | |
|---|---|---|---|---|---|---|
| RPC use(1+ units) | 703 (50.6) | 223 (88.5) | 0.001 | 132 (52.6) | 222 (88.4) | 0.001 |
| Transfused RPC units | 1 (0–2) | 3 (2–4) | 0.001 | 1 (0–2) | 3 (2–4) | 0.001 |
| Transfused FFP units | 0 (0–2) | 2 (0–4) | 0.001 | 0 (0–2) | 0 (2–4) | 0.001 |
| Transfused PLT units | 0 (0–0) | 4 (0–4) | 0.001 | 0 (0–1) | 4 (0–4) | 0.001 |
| Total blood loss | 1220 (1080–1450) | 1820 (1450–2290) | 0.001 | 1240 (1100–1500) | 1820 (1440–2300) | 0.001 |
| Stroke | 16 (1.1) | 2 (0.8) | 0.616 | 4 (1.6) | 2 (0.8) | 0.411 |
| Dialysis | 12 (0.8) | 7 (2.8) | 0.009 | 3 (1.2) | 7 (2.8) | 0.201 |
| Prolonged ventilation | 157 (11.4) | 111 (44.2) | 0.001 | 37 (14.8) | 110 (44.0) | 0.001 |
| Pulmonary complications | 172 (12.4) | 35 (13.9) | 0.505 | 27 (10.7) | 35 (13.9) | 0.278 |
| Gastrointestinal complications | 26 (1.9) | 6 (2.4) | 0.590 | 5 (2.0) | 6 (2.4) | 0.760 |
| Pericardial effusion * | 161 (11.6) | 32 (12.7) | 0.613 | 29 (11.5) | 32 (12.7) | 0.682 |
| CSAAKI | 41 (2.9) | 16 (6.3) | 0.007 | 9 (3.6) | 16 (6.4) | 0.151 |
| MOF | 6 (0.4) | 5 (2.0) | 0.005 | 0 (0.0) | 5 (2.0) | 0.025 |
| In-hospital mortality | 9 (0.6) | 6 (2.4) | 0.008 | 1 (0.4) | 6 (2.4) | 0.057 |
| In-hospital cardiac mortality | 2 (0.1) | 1 (0.4) | 0.387 | 0 (0.0) | 1 (0.4) | 0.317 |
| Intensive care unit stay, days | 1 (1–1) | 1 (1–1) | 0.001 | mean 1.28 ± 2.56 median 1 (1–1) | mean 1.61 ± 3.19 median 1 (1–1) | 0.001 |
| Postoperative hospital stay, days | 6 (5–8) | 7 (6–9) | 0.001 | 6 (5–8) | 7 (6–9) | 0.004 |
| Regression Method | Unadjusted Regression | Propensity Score Adjusted Regression | Matched Cohort Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect * | 95% CI | p value | Effect * | 95% CI | p Value | Effect * | 95% CI | p Value | ||
| RPC use (1+ units) | logistic | 7.51 | 5.03–11.22 | 0.001 | 7.72 | 5.15–11.56 | 0.001 | 6.90 | 4.35–10.92 | 0.001 |
| RPC units | zero-inflated | 1.97 | 1.72–2.23 | 0.001 | 2.01 | 1.75–2.28 | 0.001 | 1.72 | 1.38–2.07 | 0.001 |
| FFP units | zero-inflated | 1.48 | 1.05–1.90 | 0.044 | 1.53 | 1.10–1.95 | 0.048 | 1.37 | 0.75–1.98 | 0.156 |
| PLT units | zero-inflated | 1.55 | 1.29–1.81 | 0.001 | 1.51 | 1.24–1.77 | 0.001 | 1.39 | 0.74–2.05 | 0.220 |
| Total blood loss | linear | 590.2 | 523.8–656.7 | 0.001 | 596.5 | 529.4–663.7 | 0.001 | 546.5 | 436.8–656.2 | 0.001 |
| Stroke | logistic | 0.68 | 0.15–3.00 | 0.618 | 0.627 | 0.14–2.77 | 0.539 | 0.49 | 0.09–2.73 | 0.421 |
| Dialysis | logistic | 3.28 | 1.28–8.41 | 0.013 | 3.05 | 1.17–7.93 | 0.022 | 2.37 | 0.60–9.28 | 0.215 |
| Prolonged ventilation | logistic | 6.16 | 4.57–8.31 | 0.001 | 5.80 | 4.29–7.86 | 0.001 | 4.52 | 2.94–6.94 | 0.001 |
| Pulmonary complications | logistic | 1.14 | 0.77–1.69 | 0.505 | 1.06 | 0.71–1.58 | 0.765 | 1.34 | 0.78–2.29 | 0.279 |
| Gastroint. complications | logistic | 1.27 | 0.52–3.14 | 0.591 | 1.34 | 0.54–3.31 | 0.527 | 1.20 | 0.36–4.00 | 0.761 |
| Pericardial effusion | logistic | 1.15 | 0.49–2.67 | 0.627 | 1.19 | 0.52–2.72 | 0.657 | 1.21 | 0.39–3.95 | 0.734 |
| CSAAKI | logistic | 2.23 | 1.23–4.04 | 0.008 | 2.07 | 1.13–3.78 | 0.017 | 1.83 | 0.79–4.22 | 0.156 |
| Multiorgan failure | logistic | 4.66 | 1.41–15.41 | 0.011 | 4.59 | 1.37–15.42 | 0.014 | collinearity | - | - |
| In-hospital mortality | logistic | 3.74 | 1.32–10.61 | 0.013 | 3.12 | 1.08–8.99 | 0.035 | 6.12 | 0.73–51.22 | 0.095 |
| Cardiac mortality | logistic | 2.76 | 0.25–30.60 | 0.407 | 2.12 | 0.18–24.40 | 0.544 | collinearity | - | - |
| Intensive care unit stay | linear | 1.76 | 0.75–2.76 | 0.001 | 1.66 | 0.64–2.67 | 0.001 | 1.72 | 0.27–3.16 | 0.020 |
| Postoperative stay | linear | 2.75 | 1.00–4.50 | 0.002 | 2.16 | 0.42–3.91 | 0.015 | 2.34 | 0.19–4.50 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaccio, C.; Rose, D.; Nenna, A.; Taylor, R.; Bittar, M.N. Early Re-Exploration versus Conservative Management for Postoperative Bleeding in Stable Patients after Coronary Artery Bypass Grafting: A Propensity Matched Study. J. Clin. Med. 2023, 12, 3327. https://doi.org/10.3390/jcm12093327
Spadaccio C, Rose D, Nenna A, Taylor R, Bittar MN. Early Re-Exploration versus Conservative Management for Postoperative Bleeding in Stable Patients after Coronary Artery Bypass Grafting: A Propensity Matched Study. Journal of Clinical Medicine. 2023; 12(9):3327. https://doi.org/10.3390/jcm12093327
Chicago/Turabian StyleSpadaccio, Cristiano, David Rose, Antonio Nenna, Rebecca Taylor, and Mohamad Nidal Bittar. 2023. "Early Re-Exploration versus Conservative Management for Postoperative Bleeding in Stable Patients after Coronary Artery Bypass Grafting: A Propensity Matched Study" Journal of Clinical Medicine 12, no. 9: 3327. https://doi.org/10.3390/jcm12093327
APA StyleSpadaccio, C., Rose, D., Nenna, A., Taylor, R., & Bittar, M. N. (2023). Early Re-Exploration versus Conservative Management for Postoperative Bleeding in Stable Patients after Coronary Artery Bypass Grafting: A Propensity Matched Study. Journal of Clinical Medicine, 12(9), 3327. https://doi.org/10.3390/jcm12093327









