Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
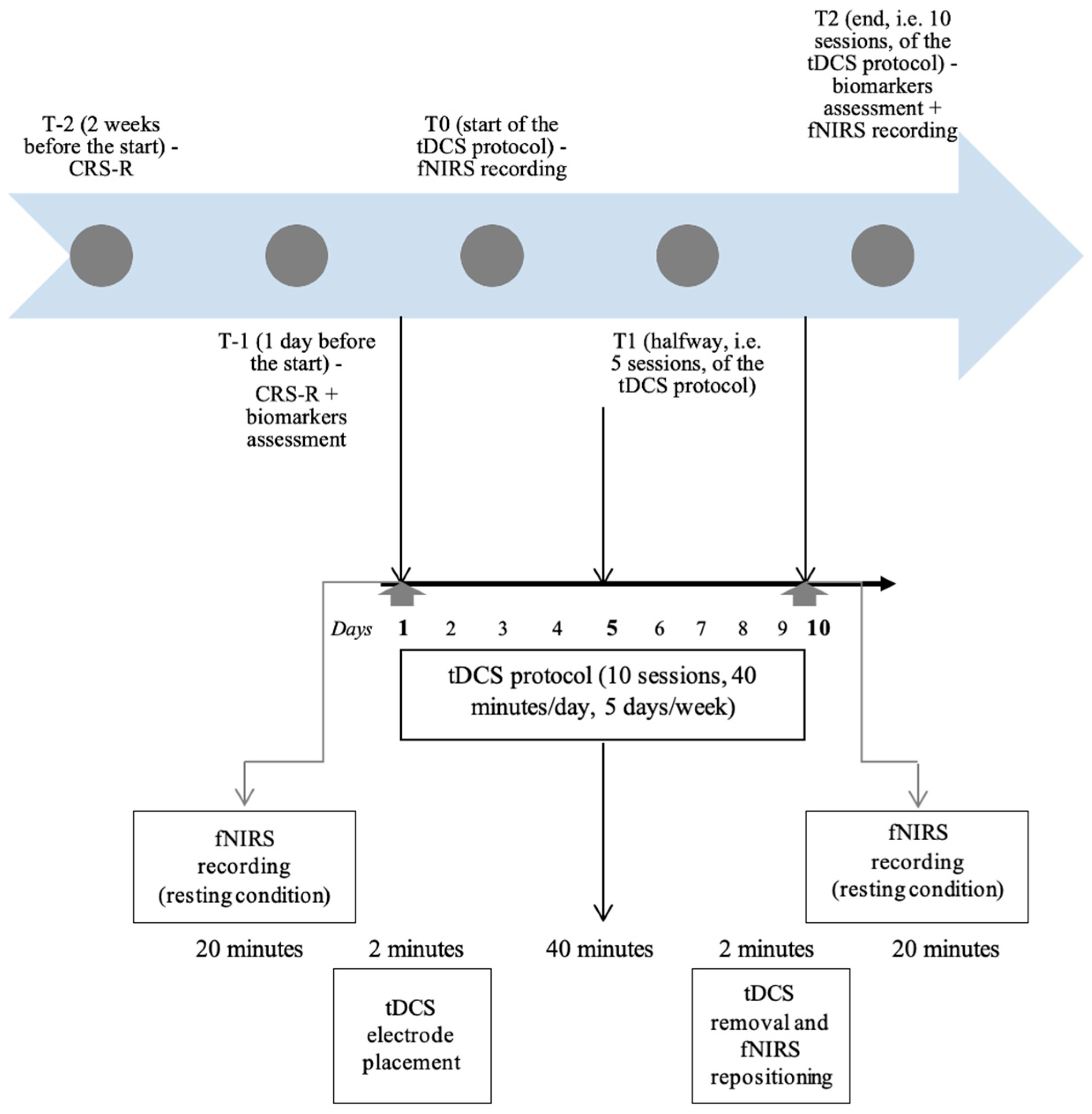
2.2. Intervention
2.3. Outcomes
2.3.1. Clinical and Behavioural Assessments
2.3.2. fNIRS Data Acquisition and Analysis
2.3.3. Circulating Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Brain-Based Haemodynamic Changes after Bilateral M1 Anodal tDCS
3.2. Single-tDCS-Session Effect
3.3. Multiple-tDCS-Sessions Effect
3.4. Circulating Biomarkers
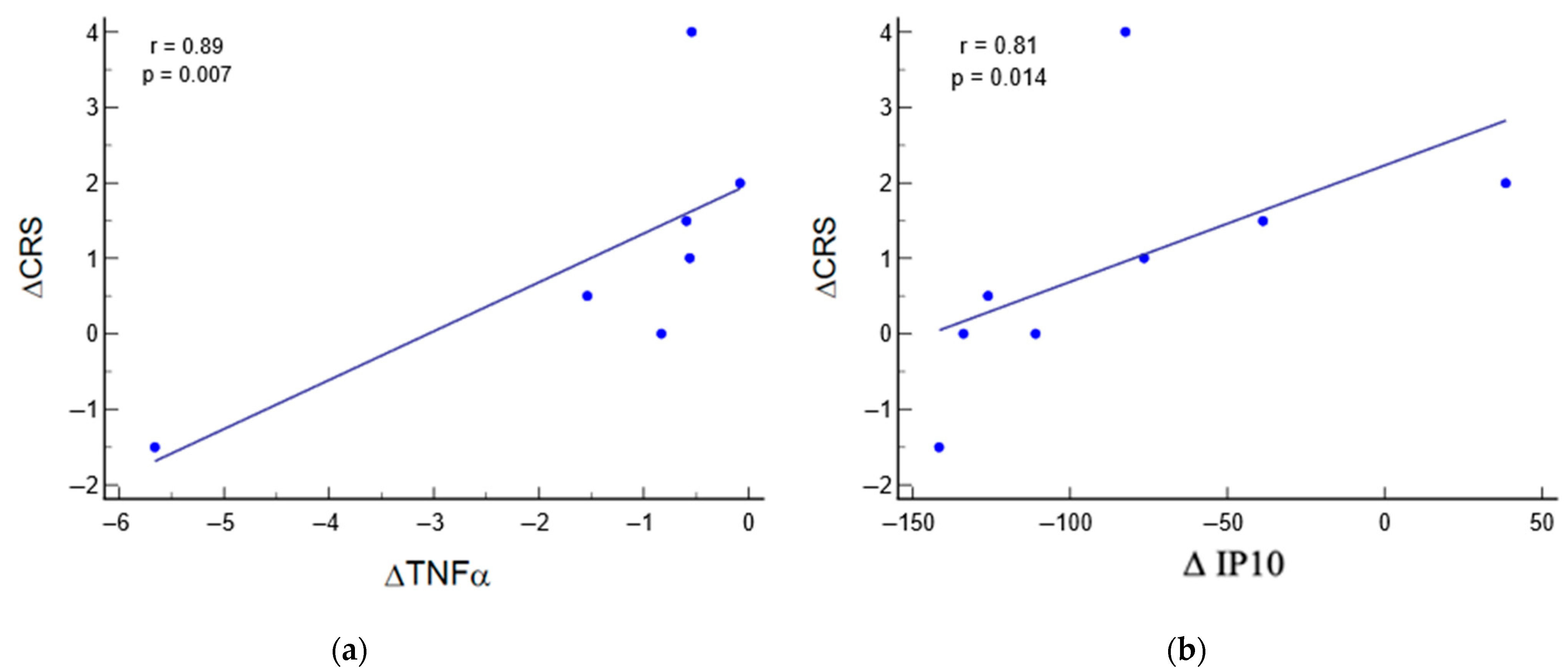
3.5. Relation between Biomarkers and Clinical Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive Wakefulness Syndrome: A New Name for the Vegetative State or Apallic Syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The Minimally Conscious State: Definition and Diagnostic Criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.; Zafonte, R.D. Pharmacological Management of Neurobehavioral Disorders Following Traumatic Brain Injury--a State-of-the-Art Review. J. Rehabil. Res. Dev. 2009, 46, 851–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W. Transcranial Direct Current Stimulation in Disorders of Consciousness: A Review. Int. J. Neurosci. 2018, 128, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Bruno, M.-A.; Ledoux, D.; Demertzi, A.; Laureys, S. tDCS in Patients with Disorders of Consciousness: Sham-Controlled Randomized Double-Blind Study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Liouta, E.; Andreadis, N.; Korfias, S.; Ktonas, P.; Stranjalis, G.; Sakas, D.E. Transcranial Direct Current Stimulation Effects in Disorders of Consciousness. Arch. Phys. Med. Rehabil. 2014, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Fregni, F.; Estraneo, A.; Fiorenza, S.; Noe, E.; Llorens, R.; Ferri, J.; Formisano, R.; Morone, G.; Bender, A.; et al. Sham-Controlled Randomized Multicentre Trial of Transcranial Direct Current Stimulation for Prolonged Disorders of Consciousness. Eur. J. Neurol. 2023, 30, 3016–3031. [Google Scholar] [CrossRef]
- Schiff, N.D.; Giacino, J.T.; Kalmar, K.; Victor, J.D.; Baker, K.; Gerber, M.; Fritz, B.; Eisenberg, B.; Biondi, T.; O’Connor, J.; et al. Behavioural Improvements with Thalamic Stimulation after Severe Traumatic Brain Injury. Nature 2007, 448, 600–603. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating Cortico-Striatal and Thalamo-Cortical Functional Connectivity with Transcranial Direct Current Stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef]
- Aloi, D.; Jalali, R.; Tilsley, P.; Miall, R.C.; Fernández-Espejo, D. tDCS Modulates Effective Connectivity during Motor Command Following; a Potential Therapeutic Target for Disorders of Consciousness. Neuroimage 2022, 247, 118781. [Google Scholar] [CrossRef]
- Mashour, G.A.; Alkire, M.T. Consciousness, Anesthesia, and the Thalamocortical System. Anesthesiology 2013, 118, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Martens, G.; Fregni, F.; Carrière, M.; Barra, A.; Laureys, S.; Thibaut, A. Single tDCS Session of Motor Cortex in Patients with Disorders of Consciousness: A Pilot Study. Brain Inj. 2019, 33, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Bonsangue, V.; Mele, S.; Craighero, L.; Montis, A.; Fregni, F.; Lavezzi, S.; Basaglia, N. Bilateral M1 Anodal Transcranial Direct Current Stimulation in Post Traumatic Chronic Minimally Conscious State: A Pilot EEG-tDCS Study. Brain Inj. 2019, 33, 490–495. [Google Scholar] [CrossRef]
- Zheng, X.; Alsop, D.C.; Schlaug, G. Effects of Transcranial Direct Current Stimulation (tDCS) on Human Regional Cerebral Blood Flow. Neuroimage 2011, 58, 26–33. [Google Scholar] [CrossRef]
- Vernieri, F.; Assenza, G.; Maggio, P.; Tibuzzi, F.; Zappasodi, F.; Altamura, C.; Corbetto, M.; Trotta, L.; Palazzo, P.; Ercolani, M.; et al. Cortical Neuromodulation Modifies Cerebral Vasomotor Reactivity. Stroke 2010, 41, 2087–2090. [Google Scholar] [CrossRef]
- Dutta, A. Bidirectional Interactions between Neuronal and Hemodynamic Responses to Transcranial Direct Current Stimulation (tDCS): Challenges for Brain-State Dependent tDCS. Front. Syst. Neurosci. 2015, 9, 107. [Google Scholar] [CrossRef]
- Merzagora, A.C.; Foffani, G.; Panyavin, I.; Mordillo-Mateos, L.; Aguilar, J.; Onaral, B.; Oliviero, A. Prefrontal Hemodynamic Changes Produced by Anodal Direct Current Stimulation. Neuroimage 2010, 49, 2304–2310. [Google Scholar] [CrossRef]
- Khan, B.; Hodics, T.; Hervey, N.; Kondraske, G.; Stowe, A.M.; Alexandrakis, G. Functional Near-Infrared Spectroscopy Maps Cortical Plasticity Underlying Altered Motor Performance Induced by Transcranial Direct Current Stimulation. J. Biomed. Opt. 2013, 18, 116003. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.-G.; Chen, B.-B.; Song, C.-G.; Liu, Y.; Hao, J.-M.; Yuan, F.; Jiang, W. Detecting Residual Brain Networks in Disorders of Consciousness: A Resting-State fNIRS Study. Brain Res. 2023, 1798, 148162. [Google Scholar] [CrossRef] [PubMed]
- Abdalmalak, A.; Milej, D.; Norton, L.; Debicki, D.B.; Owen, A.M.; Lawrence, K.S. The Potential Role of fNIRS in Evaluating Levels of Consciousness. Front. Hum. Neurosci. 2021, 15, 703405. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.J.; Cicchetti, F. Cellular and Molecular Mechanisms of Action of Transcranial Direct Current Stimulation: Evidence from in Vitro and in Vivo Models. Int. J. Neuropsychopharmacol. 2014, 18, pyu047. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, S.; Boccagni, C. Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review. Brain Sci. 2023, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, W.; Zhang, T.; Bao, W. Peripheral Inflammatory Markers in Patients with Prolonged Disorder of Consciousness after Severe Traumatic Brain Injury. Ann. Palliat. Med. 2021, 10, 9114–9121. [Google Scholar] [CrossRef] [PubMed]
- Szade, A.; Grochot-Przeczek, A.; Florczyk, U.; Jozkowicz, A.; Dulak, J. Cellular and Molecular Mechanisms of Inflammation-Induced Angiogenesis. IUBMB Life 2015, 67, 145–159. [Google Scholar] [CrossRef]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from Disorders of Consciousness: Mechanisms, Prognosis and Emerging Therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Lombardi, F.; Gatta, G.; Sacco, S.; Muratori, A.; Carolei, A. The Italian Version of the Coma Recovery Scale-Revised (CRS-R). Funct. Neurol. 2007, 22, 47–61. [Google Scholar]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.N.; Hani, A.; Cheek, J.; Thirumala, P.; Tsuchida, T.N. American Clinical Neurophysiology Society Guideline 2: Guidelines for Standard Electrode Position Nomenclature. J. Clin. Neurophysiol. 2016, 33, 308–311. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M. Quantification in Tissue Near-Infrared Spectroscopy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997, 352, 649–659. [Google Scholar] [CrossRef]
- Lamberti, N.; Manfredini, F.; Nardi, F.; Baroni, A.; Piva, G.; Crepaldi, A.; Basaglia, N.; Casetta, I.; Straudi, S. Cortical Oxygenation during a Motor Task to Evaluate Recovery in Subacute Stroke Patients: A Study with Near-Infrared Spectroscopy. Neurol. Int. 2022, 14, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, S.; Cretaz, E.; Sampaio-Junior, B.; Vieira, É.L.M.; Gattaz, W.; Klein, I.; Lafer, B.; Teixeira, A.L.; Carvalho, A.F.; Lotufo, P.A.; et al. Effects of tDCS on Neuroplasticity and Inflammatory Biomarkers in Bipolar Depression: Results from a Sham-Controlled Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110119. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, N.; Marchetti, G.; Straudi, S.; Tisato, V.; Lavezzi, S.; Manfredini, F.; Basaglia, N.; Bernardi, F. Soluble Neural Cell Adhesion Molecule and Behavioural Recovery in Minimally Conscious Patients Undergoing Transcranial Direct Current Stimulation. Clin. Chim. Acta 2019, 495, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Bragina, O.A.; Lara, D.A.; Nemoto, E.M.; Shuttleworth, C.W.; Semyachkina-Glushkovskaya, O.V.; Bragin, D.E. Increases in Microvascular Perfusion and Tissue Oxygenation via Vasodilatation After Anodal Transcranial Direct Current Stimulation in the Healthy and Traumatized Mouse Brain. Adv. Exp. Med. Biol. 2018, 1072, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gradisek, P.; Carrara, G.; Antiga, L.; Bottazzi, B.; Chieregato, A.; Csomos, A.; Fainardi, E.; Filekovic, S.; Fleming, J.; Hadjisavvas, A.; et al. Prognostic Value of a Combination of Circulating Biomarkers in Critically Ill Patients with Traumatic Brain Injury: Results from the European CREACTIVE Study. J. Neurotrauma. 2021, 38, 2667–2676. [Google Scholar] [CrossRef]
- Molteni, E.; Arrigoni, F.; Bardoni, A.; Galbiati, S.; Villa, F.; Colombo, K.; Strazzer, S. Bedside Assessment of Residual Functional Activation in Minimally Conscious State Using NIRS and General Linear Models. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 3551–3554. [Google Scholar] [CrossRef]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E.D.; Leff, A.P. Functional near Infrared Spectroscopy as a Probe of Brain Function in People with Prolonged Disorders of Consciousness. Neuroimage Clin. 2016, 12, 312–319. [Google Scholar] [CrossRef]
- Piccoli, E.; Cerioli, M.; Castiglioni, M.; Larini, L.; Scarpa, C.; Dell’Osso, B. Recent Innovations in Non-Invasive Brain Stimulation (NIBS) for the Treatment of Unipolar and Bipolar Depression: A Narrative Review. Int. Rev. Psychiatry 2022, 34, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-T.; Jeng, J.-S.; Zeng, B.-S.; Stubbs, B.; Carvalho, A.F.; Brunoni, A.R.; Su, K.-P.; Tu, Y.-K.; Wu, Y.-C.; Chen, T.-Y.; et al. Efficacy of Non-Invasive Brain Stimulation Interventions in Reducing Smoking Frequency in Patients with Nicotine Dependence: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Addiction 2022, 117, 1830–1842. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, Z.; Du, Y.; Xiong, M.; Yang, Z.; Ren, L.; He, L.; Jiang, Y.; McClure, M.A.; Mu, Q. Effects of Noninvasive Brain Stimulation (NIBS) on Cognitive Impairment in Mild Cognitive Impairment and Alzheimer Disease: A Meta-Analysis. Alzheimer. Dis. Assoc. Disord. 2021, 35, 278–288. [Google Scholar] [CrossRef]
- Barra, A.; Monti, M.; Thibaut, A. Noninvasive Brain Stimulation Therapies to Promote Recovery of Consciousness: Where We Are and Where We Should Go. Semin. Neurol. 2022, 42, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, H.; Dang, H.; Zhang, X.; Yue, S.; Zhang, H. Corrigendum: Efficacy of Non-Invasive Brain Stimulation for Disorders of Consciousness: A Systematic Review and Meta-Analysis. Front. Neurosci. 2023, 17, 1293703. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dawidziuk, A.; Darzi, A.; Singh, H.; Leff, D.R. Systematic Review of Combined Functional Near-Infrared Spectroscopy and Transcranial Direct-Current Stimulation Studies. Neurophotonics 2020, 7, 020901. [Google Scholar] [CrossRef] [PubMed]
- Muthalib, M.; Besson, P.; Rothwell, J.; Perrey, S. Focal Hemodynamic Responses in the Stimulated Hemisphere During High-Definition Transcranial Direct Current Stimulation. Neuromodulation 2018, 21, 348–354. [Google Scholar] [CrossRef]
- Kumar, R.G.; Boles, J.A.; Wagner, A.K. Chronic Inflammation After Severe Traumatic Brain Injury: Characterization and Associations with Outcome at 6 and 12 Months Postinjury. J. Head. Trauma Rehabil. 2015, 30, 369–381. [Google Scholar] [CrossRef]
- Deshetty, U.M.; Periyasamy, P. Potential Biomarkers in Experimental Animal Models for Traumatic Brain Injury. J. Clin. Med. 2023, 12, 3923. [Google Scholar] [CrossRef]
- Nwafor, D.C.; Brichacek, A.L.; Foster, C.H.; Lucke-Wold, B.P.; Ali, A.; Colantonio, M.A.; Brown, C.M.; Qaiser, R. Pediatric Traumatic Brain Injury: An Update on Preclinical Models, Clinical Biomarkers, and the Implications of Cerebrovascular Dysfunction. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221098125. [Google Scholar] [CrossRef]
- Musso, N.; Bivona, D.; Bonomo, C.; Bonacci, P.; D’Ippolito, M.E.; Boccagni, C.; Rubino, F.; De Tanti, A.; Lucca, L.F.; Pingue, V.; et al. Investigating microRNAs as Biomarkers in Disorders of Consciousness: A Longitudinal Multicenter Study. Sci. Rep. 2023, 13, 18415. [Google Scholar] [CrossRef] [PubMed]
- Rodney, T.; Osier, N.; Gill, J. Pro- and Anti-Inflammatory Biomarkers and Traumatic Brain Injury Outcomes: A Review. Cytokine 2018, 110, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-I.; Hsu, L.-J.; Wang, H.-C. Effects of Ankle Proprioceptive Interference on Locomotion after Stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.H.; Hadad, R.; Taylor, R.R.; Rodríguez Pilar, J.; Salazar, O.; Llompart-Pou, J.A.; Dietrich, W.D.; Keane, R.W.; Pérez-Bárcena, J.; de Rivero Vaccari, J.P. Inflammatory Biomarkers of Traumatic Brain Injury. Pharmaceuticals 2022, 15, 660. [Google Scholar] [CrossRef]
- Licastro, F.; Hrelia, S.; Porcellini, E.; Malaguti, M.; Di Stefano, C.; Angeloni, C.; Carbone, I.; Simoncini, L.; Piperno, R. Peripheral Inflammatory Markers and Antioxidant Response during the Post-Acute and Chronic Phase after Severe Traumatic Brain Injury. Front. Neurol. 2016, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Incio, J.; Soares, R. Angiogenesis and Chronic Inflammation: Cause or Consequence? Angiogenesis 2007, 10, 149–166. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Angiogenesis, Neurogenesis and Brain Recovery of Function Following Injury. Curr. Opin. Investig. Drugs 2010, 11, 298–308. [Google Scholar]
- Badoiu, A.; Mitran, S.I.; Catalin, B.; Balseanu, T.A.; Popa-Wagner, A.; Gherghina, F.L.; Albu, C.V.; Sandu, R.E. From Molecule to Patient Rehabilitation: The Impact of Transcranial Direct Current Stimulation and Magnetic Stimulation on Stroke-A Narrative Review. Neural Plast. 2023, 2023, 5044065. [Google Scholar] [CrossRef]
- Callai, E.M.M.; Zin, L.E.F.; Catarina, L.S.; Ponzoni, D.; Gonçalves, C.A.S.; Vizuete, A.F.K.; Cougo, M.C.; Boff, J.; Puricelli, E.; Fernandes, E.K.; et al. Evaluation of the Immediate Effects of a Single Transcranial Direct Current Stimulation Session on Astrocyte Activation, Inflammatory Response, and Pain Threshold in Naïve Rats. Behav. Brain Res. 2022, 428, 113880. [Google Scholar] [CrossRef]
- Ethridge, V.T.; Gargas, N.M.; Sonner, M.J.; Moore, R.J.; Romer, S.H.; Hatcher-Solis, C.; Rohan, J.G. Effects of Transcranial Direct Current Stimulation on Brain Cytokine Levels in Rats. Front. Neurosci. 2022, 16, 1069484. [Google Scholar] [CrossRef]
- Evans, C.; Zich, C.; Lee, J.S.A.; Ward, N.; Bestmann, S. Inter-Individual Variability in Current Direction for Common tDCS Montages. Neuroimage 2022, 260, 119501. [Google Scholar] [CrossRef] [PubMed]

| Patient ID | Sex | Age | Time Since TBI | Medications | Implantable Devices | CRS-R Baseline |
|---|---|---|---|---|---|---|
| 1 | M | 35 | 11 y | - | ITB | 15 |
| 2 | M | 36 | 8 y, 9 m | - | VPS | 11 |
| 3 | M | 47 | 4 y, 7m | Levetiracetam | ITB | 13 |
| 4 | M | 34 | 19 y | - | - | 12 |
| 5 | F | 24 | 2 y | Levetiracetam, Amantadin | - | 11 |
| 6 | F | 27 | 7 y, 6 m | Levetiracetam | ITB | 9 |
| 7 | M | 42 | 2 y, 7 m | - | VPS | 9 |
| 8 | M | 26 | 1 y, 3 m | Lamictal, Fenobarbital | - | 9 |
| 9 | M | 63 | 1 y | Carbamazepine | VPS | 8 |
| 10 | M | 21 | 1 y, 1 m | - | VPS | 9 |
| Healthy Subjects (N = 8) | MCS Patients (N = 10) | p Value | |
|---|---|---|---|
| Angiopoietin-2 | 1616 (1121–2111) | 1870 (1140–2601) | 0.514 |
| BMP9 | 228 (155–301) | 174 (86–262) | 0.288 |
| Endoglin | 931 (720–1141) | 553 (332–773) | 0.011 |
| HbEFG | 32 (20–45) | 26 (15–36) | 0.387 |
| HGF | 181 (145–217) | 247 (167–327) | 0.180 |
| IL8 | 3.3 (2.6–4.1) | 10.5 (0.1–22.2) | <0.001 |
| Leptin | 5850 (2392–9308) | 16,590 (7697–25,482) | 0.027 |
| PLGF | N.D. | 11.6 (4.1–19.1) | N.A. |
| VEGF-A | N.D. | 121 (15–258) | N.A. |
| VEGF-C | 300 (212–387) | 330 (259–401) | 0.545 |
| GM-CSF | 9.89 (5.59–14.19) | 3.49 (1.10–5.89) | 0.011 |
| IFNg | 10.15 (0.37–24.07) | 5.58 (2.70–8.47) | <0.001 |
| IP10 | 222 (153–292) | 507 (61–953) | <0.001 |
| MCP1 | 281 (234–328) | 373 (223–523) | 0.008 |
| TNFα | 2.97 (1.52–4.43) | 4.03 (1.61–6.45) | 0.39 |
| Day 1 | Day 10 | p Value | |
|---|---|---|---|
| Angiopoietin-2 | 1870 (1140–2601) | 1535 (955–2113) | 0.040 |
| BMP9 | 174 (86–262) | 185 (104–265) | 0.39 |
| Endoglin | 553 (332–773) | 585 (369–800) | 0.22 |
| HbEFG | 26 (15–36) | 36 (19–52) | 0.14 |
| HGF | 247 (167–327) | 234 (155–312) | 0.33 |
| IL8 | 10.5 (0.1–22.2) | 11.0 (2.3–24.0) | 0.66 |
| Leptin | 16,590 (7697–25,482) | 14,682 (7155–22,210) | 0.060 |
| PLGF | 11.6 (4.1–19.1) | 10.6 (1.6–19.5) | 0.56 |
| VEGF-A | 121 (15–258) | 130 (22–247) | 0.73 |
| VEGF-C | 330 (259–401) | 388 (307–469) | 0.041 |
| GM-CSF | 3.49 (1.10–5.89) | 4.40 (2.17–6.61) | 0.30 |
| IFNg | 5.58 (2.70–8.47) | 5.16 (1.75–8.57) | 0.75 |
| IP10 | 507 (61–953) | 423 (1–846) | 0.006 |
| MCP1 | 373 (223–523) | 355 (236–474) | 0.56 |
| TNFα | 4.03 (1.61–6.45) | 2.63 (1.63–3.62) | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straudi, S.; Antonioni, A.; Baroni, A.; Bonsangue, V.; Lavezzi, S.; Koch, G.; Tisato, V.; Ziliotto, N.; Basaglia, N.; Secchiero, P.; et al. Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study. J. Clin. Med. 2024, 13, 108. https://doi.org/10.3390/jcm13010108
Straudi S, Antonioni A, Baroni A, Bonsangue V, Lavezzi S, Koch G, Tisato V, Ziliotto N, Basaglia N, Secchiero P, et al. Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study. Journal of Clinical Medicine. 2024; 13(1):108. https://doi.org/10.3390/jcm13010108
Chicago/Turabian StyleStraudi, Sofia, Annibale Antonioni, Andrea Baroni, Valentina Bonsangue, Susanna Lavezzi, Giacomo Koch, Veronica Tisato, Nicole Ziliotto, Nino Basaglia, Paola Secchiero, and et al. 2024. "Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study" Journal of Clinical Medicine 13, no. 1: 108. https://doi.org/10.3390/jcm13010108
APA StyleStraudi, S., Antonioni, A., Baroni, A., Bonsangue, V., Lavezzi, S., Koch, G., Tisato, V., Ziliotto, N., Basaglia, N., Secchiero, P., Manfredini, F., & Lamberti, N. (2024). Anti-Inflammatory and Cortical Responses after Transcranial Direct Current Stimulation in Disorders of Consciousness: An Exploratory Study. Journal of Clinical Medicine, 13(1), 108. https://doi.org/10.3390/jcm13010108











