Artificial Intelligence on Diagnostic Aid of Leprosy: A Systematic Literature Review
Abstract
:1. Introduction
2. Background
2.1. Leprosy
2.2. Diagnosis Methods of Leprosy
2.3. Artificial Intelligence in Clinical Medicine
3. Methodology
3.1. Research Questions
- (RQ1) What types of leprosy are targeted by AI models?
- (RQ2) What data types were used to develop AI models?
- (RQ3) What preprocessing techniques were used on the datasets?
- (RQ4) What AI algorithms/architectures were applied to diagnose leprosy?
- (RQ5) How well do the models perform?
3.2. Search Strategy and Selection Criteria
3.3. Quality Assessment
3.4. Data Extraction
4. Results
4.1. Study Selection
4.2. Study Characterization
4.3. Answering the Research Questions
4.3.1. Leprosy Types Targeted by AI Models (RQ1)
4.3.2. Data Types (RQ2)
4.3.3. Preprocessing Techniques (RQ3)
4.3.4. Algorithms and Architectures (RQ4)
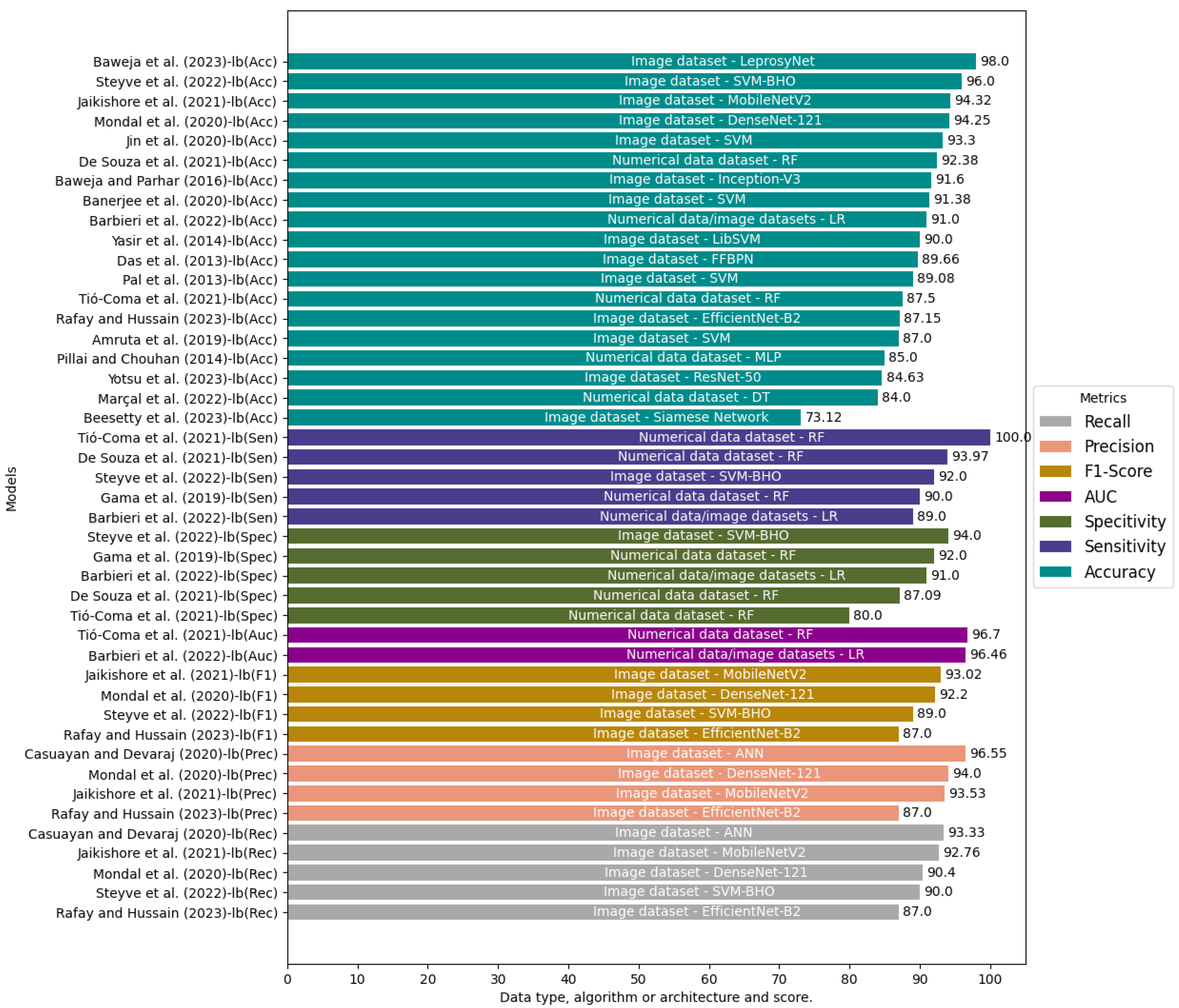
4.3.5. Performance of the Models (RQ5)
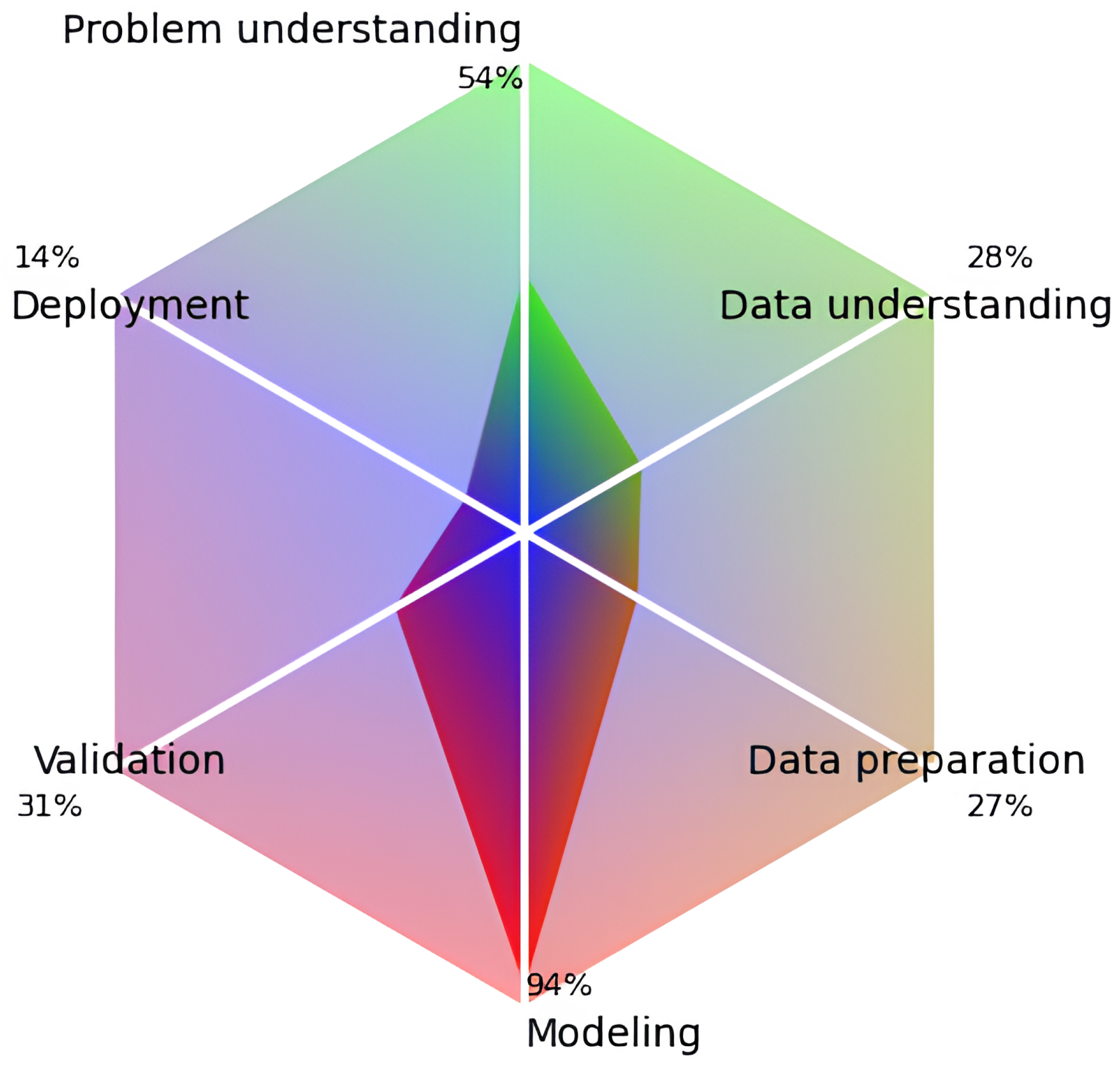
4.4. Study Quality
5. Discussion
5.1. Trends
5.2. Open Issues
5.2.1. Open Science
5.2.2. Data Fusion
5.2.3. Differential Diagnostic
5.2.4. External Validation
5.3. Limitations of the SLR
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ML | Machine Learning |
| DL | Deep Learning |
| CV | Computer Vision |
| NTD | Neglected Tropical Disease |
| CNN | Convolutional Neural Network |
| ANN | Artificial Neural Network |
| WHO | World Health Organization |
| PB | Paucibacillary |
| MB | Multibacillary |
| PGL-1 | Phenolic glycolipid-I |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase Chain Reaction |
| SVM | Support Vector Machine |
| RQ | Research Questions |
| QC | Quality Criteria |
| mR | Minor revision required |
| MR | Major revision required |
| ADAM | Adaptive Moment Estimation |
| SGD | Stochastic Gradient Descent |
| LR | Logistic Regression |
| XGB | XGBoost |
| RF | Random Fores |
| AUC | Area Under Curve |
| DT | Decision Trees |
| LOOCV | Leave-one-out-cross-validation |
| KNN | K-Nearest Neighbors |
| LBP | Local Binary Pattern |
| WLD | Weber Local Descriptor |
| GLCM | Gray-Level Co-Occurrence Matrix |
| riLBP | rotation invariant LBP |
| HOG | Histogram of Oriented Gradients |
| ROI | Region of Interest |
| GCN | Global Contrast Normalization |
| GAN | Generative Adversarial Network |
| ID3 | Iterative Dichotomiser 3 |
| SMO | Sequential Minimal Optimization |
| MLP | Multilayer Perceptron |
| FFBPN | Feed-Forward Back Propagation Network |
| DCT | Discrete Cosine Transform |
| DFT | Discrete Fourier Transform |
| RNA-Seq | RNA sequencing technique |
| RT-qPCR | Real-time Reverse Transcription Polymerase Chain Reaction |
| NGS | Next-generation sequencing |
| TNF | Tumor Necrosis Factor |
| IFN-y | Interferon-gamma |
| IL-4 | Interleukin 4 |
| IL-10 | Interleukin 10 |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
References
- WHO. Leprosy. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 13 March 2023).
- Martins-Melo, F.R.; Carneiro, M.; Ramos, A.N., Jr.; Heukelbach, J.; Ribeiro, A.L.P.; Werneck, G.L. The burden of Neglected Tropical Diseases in Brazil, 1990–2016: A subnational analysis from the Global Burden of Disease Study 2016. PLoS Neglected Trop. Dis. 2018, 12, e0006559. [Google Scholar] [CrossRef] [PubMed]
- Ochola, E.A.; Elliott, S.J.; Karanja, D.M.S. The Impact of Neglected Tropical Diseases (NTDs) on Women’s Health and Wellbeing in Sub-Saharan Africa (SSA): A Case Study of Kenya. Int. J. Environ. Res. Public Health 2021, 18, 2180. [Google Scholar] [CrossRef] [PubMed]
- Pescarini, J.M.; Strina, A.; Nery, J.S.; Skalinski, L.M.; Andrade, K.V.F.d.; Penna, M.L.F.; Brickley, E.B.; Rodrigues, L.C.; Barreto, M.L.; Penna, G.O. Socioeconomic risk markers of leprosy in high-burden countries: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2018, 12, e0006622. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Del Prete, R.; Charitos, I.A.; Bottalico, L. Mycobacterium leprae: A historical study on the origins of leprosy and its social stigma. Infez. Med. 2021, 29, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Y.; Silva, F.J. On the Age of Leprosy. PLoS Neglected Trop. Dis. 2014, 8, e2544. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Liu, H.; Zhang, F. Advances in the immunology and genetics of leprosy. Front. Immunol. 2020, 11, 567. [Google Scholar] [CrossRef]
- Vieira, M.C.A.; Nery, J.S.; Paixão, E.S.; de Andrade, K.V.F.; Penna, G.O.; Teixeira, M.G. Leprosy in children under 15 years of age in Brazil: A systematic review of the literature. PLoS Neglected Trop. Dis. 2018, 12, e0006788. [Google Scholar] [CrossRef]
- Nunzi, E.; Massone, C. Leprosy: A Practical Guide; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Ghorpade, A. Inoculation (tattoo) leprosy: A report of 31 cases. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 494–499. [Google Scholar] [CrossRef]
- Patrocínio, L.G.; Goulart, I.M.B.; Goulart, L.R.; Patrocínio, J.A.; Ferreira, F.R.; Fleury, R.N. Detection of Mycobacterium leprae in nasal mucosa biopsies by the polymerase chain reaction. FEMS Immunol. Med. Microbiol. 2005, 44, 311–316. [Google Scholar] [CrossRef]
- Barbieri, R.R.; Xu, Y.; Setian, L.; Souza-Santos, P.T.; Trivedi, A.; Cristofono, J.; Bhering, R.; White, K.; Anna M, S.; Miller, G.; et al. Reimagining leprosy elimination with AI analysis of a combination of skin lesion images with demographic and clinical data. Lancet Reg. Health-Am. 2022, 9, 100192. [Google Scholar] [CrossRef]
- Martins, P.V.; Iriart, J.A.B. Itinerários terapêuticos de pacientes com diagnóstico de hanseníase em Salvador, Bahia. Physis Rev. Saúde Coletiva 2014, 24, 273–289. [Google Scholar] [CrossRef]
- Marçal, P.H.F.; de Souza, M.L.M.; Gama, R.S.; de Oliveira, L.B.P.; Gomes, M.d.S.; do Amaral, L.R.; Pinheiro, R.O.; Sarno, E.N.; Moraes, M.O.; Fairley, J.K.; et al. Algorithm Design for a Cytokine Release Assay of Antigen-Specific In Vitro Stimuli of Circulating Leukocytes to Classify Leprosy Patients and Household Contacts. Open Forum Infect. Dis. 2022, 9, ofac036. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Cockerell, C.J.; Patil, A.; Pietkiewicz, P.; Giulini, M.; Grabbe, S.; Goldust, M. Machine Learning and Its Application in Skin Cancer. Int. J. Environ. Res. Public Health 2021, 18, 13409. [Google Scholar] [CrossRef]
- Young, A.T.; Xiong, M.; Pfau, J.; Keiser, M.J.; Wei, M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020, 140, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Dildar, M.; Akram, S.; Irfan, M.; Khan, H.U.; Ramzan, M.; Mahmood, A.R.; Alsaiari, S.A.; Saeed, A.H.M.; Alraddadi, M.O.; Mahnashi, M.H. Skin Cancer Detection: A Review Using Deep Learning Techniques. Int. J. Environ. Res. Public Health 2021, 18, 5479. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, K.; Garcia, R. Computerized analysis of pigmented skin lesions: A review. Artif. Intell. Med. 2012, 56, 69–90. [Google Scholar] [CrossRef]
- Oliveira, R.B.; Filho, M.E.; Ma, Z.; Papa, J.P.; Pereira, A.S.; Tavares, J.M.R. Computational methods for the image segmentation of pigmented skin lesions: A review. Comput. Methods Programs Biomed. 2016, 131, 127–141. [Google Scholar] [CrossRef]
- Pai, V.V.; Pai, R.B. Artificial intelligence in dermatology and healthcare: An overview. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 457–467. [Google Scholar] [CrossRef]
- Li, Z.; Koban, K.C.; Schenck, T.L.; Giunta, R.E.; Li, Q.; Sun, Y. Artificial Intelligence in Dermatology Image Analysis: Current Developments and Future Trends. J. Clin. Med. 2022, 11, 6826. [Google Scholar] [CrossRef]
- Brinker, T.J.; Hekler, A.; Utikal, J.S.; Grabe, N.; Schadendorf, D.; Klode, J.; Berking, C.; Steeb, T.; Enk, A.H.; von Kalle, C. Skin cancer classification using convolutional neural networks: Systematic review. J. Med. Internet Res. 2018, 20, e11936. [Google Scholar] [CrossRef]
- Popescu, D.; El-Khatib, M.; El-Khatib, H.; Ichim, L. New trends in melanoma detection using neural networks: A systematic review. Sensors 2022, 22, 496. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, B.; Zeng, A.; Pan, D.; Wang, R.; Zhao, S. Skin cancer classification with deep learning: A systematic review. Front. Oncol. 2022, 12, 893972. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient Intell. Humaniz. Comput. 2022, 14, 8459–8486. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Syed, M.N.; Bernardis, E.; Gelfand, J.M. Machine learning applications in the evaluation and management of psoriasis: A systematic review. J. Psoriasis Psoriatic Arthritis 2020, 5, 147–159. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Franco-Paredes, C. Leprosy in the 21st century. Clin. Microbiol. Rev. 2015, 28, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Manta, F.S.d.N.; Leal-Calvo, T.; Moreira, S.J.M.; Marques, B.L.C.; Ribeiro-Alves, M.; Rosa, P.S.; Nery, J.A.C.; Rampazzo, R.d.C.P.; Costa, A.D.T.; Krieger, M.A.; et al. Ultra-sensitive detection of Mycobacterium leprae: “DNA” extraction and “PCR” assays. PLoS Neglected Trop. Dis. 2020, 14, e0008325. [Google Scholar] [CrossRef] [PubMed]
- Makhakhe, L. Leprosy review. S. Afr. Fam. Pract. 2021, 63, e1–e6. [Google Scholar] [CrossRef]
- Moet, F.J.; Meima, A.; Oskam, L.; Richardus, J.H. Risk factors for the development of clinical leprosy among contacts, and their relevance for targeted interventions. Lepr. Rev. 2004, 75, 310–326. [Google Scholar] [CrossRef]
- Job, C.K.; Jayakumar, J.; Kearney, M.; Gillis, T.P. Transmission of leprosy: A study of skin and nasal secretions of household contacts of leprosy patients using PCR. Am. J. Trop. Med. Hyg. 2008, 78, 518–521. [Google Scholar] [CrossRef]
- Hambridge, T.; Nanjan Chandran, S.L.; Geluk, A.; Saunderson, P.; Richardus, J.H. Mycobacterium leprae transmission characteristics during the declining stages of leprosy incidence: A systematic review. PLoS Neglected Trop. Dis. 2021, 15, e0009436. [Google Scholar] [CrossRef]
- Lockwood, D.N.J.; Nicholls, P.; Smith, W.C.S.; Das, L.; Barkataki, P.; van Brakel, W.; Suneetha, S. Comparing the Clinical and Histological Diagnosis of Leprosy and Leprosy Reactions in the INFIR Cohort of Indian Patients with Multibacillary Leprosy. PLoS Neglected Trop. Dis. 2012, 6, e1702. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.S.; Penna, G.O.; Cardoso, L.P.V.; de Andrade Pontes, M.A.; Cruz, R.; de Sá Gonçalves, H.; Fernandes Penna, M.L.; de Araújo Stefani, M.M.; Bührer-Sékula, S. Description of leprosy classification at baseline among patients enrolled at the uniform multidrug therapy clinical trial for leprosy patients in Brazil. Am. J. Trop. Med. Hyg. 2015, 92, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G. Hanseníase no Brasil. Rev. Soc. Bras. Med. Trop. 2003, 36, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bührer-Sékula, S.; Visschedijk, J.; Grossi, M.A.F.; Dhakal, K.P.; Namadi, A.U.; Klatser, P.R.; Oskam, L. The ML flow test as a point of care test for leprosy control programmes: Potential effects on classification of leprosy patients. Lepr. Rev. 2007, 78, 70–79. [Google Scholar] [CrossRef]
- Eichelmann, K.; González, S.G.; Salas-Alanis, J.; Ocampo-Candiani, J. Leprosy. An Update: Definition, Pathogenesis, Classification, Diagnosis, and Treatment. Actas Dermo-Sifiliográficas Engl. Ed. 2013, 104, 554–563. [Google Scholar] [CrossRef]
- Ridley, D.S.; Jopling, W.H. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966, 34, 255–273. Available online: https://pubmed.ncbi.nlm.nih.gov/5950347/ (accessed on 1 November 2023).
- Santana, J.S.; Silva, R.A.N.; Lima, T.O.S.; Basso, N.; Machado, L.B.; dos Santos, D.S.; Reginaldo, M.P.; de Sá Junior, J.X.; Bandeira, M.; Abrão, R.K. The role of nurses in leprosy control in primary care. Res. Soc. Dev. 2022, 11, e51811427664. [Google Scholar] [CrossRef]
- Britton, W.J.; Lockwood, D.N. Leprosy. Lancet 2004, 363, 1209–1219. [Google Scholar] [CrossRef]
- Moschella, S.L. An update on the diagnosis and treatment of leprosy. J. Am. Acad. Dermatol. 2004, 51, 417–426. [Google Scholar] [CrossRef]
- Saunderson, P.; Groenen, G. Which physical signs help most in the diagnosis of leprosy? A proposal based on experience in the AMFES project, ALERT, Ethiopia. Lepr. Rev. 2000, 71, 34–42. [Google Scholar] [CrossRef]
- Naaz, F.; Mohanty, P.; Bansal, A.; Kumar, D.; Gupta, U. Challenges beyond elimination in leprosy. Int. J. Mycobacteriol. 2017, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zha, S.; Shui, T.J. Presenting symptoms of leprosy at diagnosis: Clinical evidence from a cross-sectional, population-based study. PLoS Neglected Trop. Dis. 2021, 15, e0009913. [Google Scholar] [CrossRef] [PubMed]
- Wexler, R.; Melchior, H. Dorsal sensory impairment in hands and feet of people affected by Hansen’s disease in Israel. Lepr. Rev. 2007, 78, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Somensi, D.N.; de Jesus Soares de Sousa, E.; Lopes, G.L.; de Sousa, G.C.; Xavier, M.B. Clinical and electrophysiological characteristics of neuropathic pain in leprosy patients: A prospective cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2021, 88, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N. Leprosy: The challenges ahead for India. J. Ski. Sex. Transm. Dis. 2021, 3, 106–110. [Google Scholar] [CrossRef]
- Maymone, M.B.; Laughter, M.; Venkatesh, S.; Dacso, M.M.; Rao, P.N.; Stryjewska, B.M.; Hugh, J.; Dellavalle, R.P.; Dunnick, C.A. Leprosy: Clinical aspects and diagnostic techniques. J. Am. Acad. Dermatol. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Antunes, S.L.G.; Jardim, M.R.; Vital, R.T.; Pascarelli, B.M.d.O.; Nery, J.A.d.C.; Amadeu, T.P.; Sales, A.M.; da Costa, E.A.F.; Sarno, E.N. Fibrosis: A distinguishing feature in the pathology of neural leprosy. Mem. Inst. Oswaldo Cruz 2019, 114, e190056. [Google Scholar] [CrossRef]
- Alecrim, E.S.d.; Chaves, A.T.; Pôrto, L.A.B.; Grossi, M.A.d.F.; Lyon, S.; Rocha, M.O.d.C. Reading of the Mitsuda test: Comparison between diameter and total area by means of a computerized method. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e5. [Google Scholar] [CrossRef]
- Young, D.B.; Buchanan, T.M. A Serological Test for Leprosy with a Glycolipid Specific for Mycobacterium leprae. Science 1983, 221, 1057–1059. [Google Scholar] [CrossRef]
- Barbieri, R.R.; Manta, F.S.N.; Moreira, S.J.M.; Sales, A.M.; Nery, J.A.C.; Nascimento, L.P.R.; Hacker, M.A.; Pacheco, A.G.; Machado, A.M.; Sarno, E.M.; et al. Quantitative polymerase chain reaction in paucibacillary leprosy diagnosis: A follow-up study. PLoS Neglected Trop. Dis. 2019, 13, e0007147. [Google Scholar] [CrossRef]
- Martinez, A.N.; Talhari, C.; Moraes, M.O.; Talhari, S. PCR-Based Techniques for Leprosy Diagnosis: From the Laboratory to the Clinic. PLoS Neglected Trop. Dis. 2014, 8, e2655. [Google Scholar] [CrossRef]
- Araujo, S.; Freitas, L.O.; Goulart, L.R.; Goulart, I.M.B. Molecular Evidence for the Aerial Route of Infection of Mycobacterium leprae and the Role of Asymptomatic Carriers in the Persistence of Leprosy. Clin. Infect. Dis. 2016, 63, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Manta, F.S.N.; Barbieri, R.R.; Moreira, S.J.M.; Santos, P.T.S.; Nery, J.A.C.; Duppre, N.C.; Sales, A.M.; Pacheco, A.G.; Hacker, M.A.; Machado, A.M.; et al. Quantitative PCR for leprosy diagnosis and monitoring in household contacts: A follow-up study, 2011–2018. Sci. Rep. 2019, 9, 16675. [Google Scholar] [CrossRef] [PubMed]
- Rayan, Z.; Alfonse, M.; Salem, A.B.M. Machine Learning Approaches in Smart Health. Procedia Comput. Sci. 2019, 154, 361–368. [Google Scholar] [CrossRef]
- Fourcade, A.; Khonsari, R. Deep learning in medical image analysis: A third eye for doctors. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 279–288. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Yunchao, G.; Jiayao, Y. Application of Computer Vision and Deep Learning in Breast Cancer Assisted Diagnosis. In Proceedings of the ICMLSC 2019: 3rd International Conference on Machine Learning and Soft Computing, New York, NY, USA, 25–28 January 2019; pp. 186–191. [Google Scholar] [CrossRef]
- Maglogiannis, I.; Doukas, C.N. Overview of Advanced Computer Vision Systems for Skin Lesions Characterization. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Chomutare, T.; Tejedor, M.; Svenning, T.O.; Marco-Ruiz, L.; Tayefi, M.; Lind, K.; Godtliebsen, F.; Moen, A.; Ismail, L.; Makhlysheva, A.; et al. Artificial Intelligence Implementation in Healthcare: A Theory-Based Scoping Review of Barriers and Facilitators. Int. J. Environ. Res. Public Health 2022, 19, 16359. [Google Scholar] [CrossRef]
- Abbas, A.; Afzal, M.; Hussain, J.; Ali, T.; Bilal, H.S.M.; Lee, S.; Jeon, S. Clinical Concept Extraction with Lexical Semantics to Support Automatic Annotation. Int. J. Environ. Res. Public Health 2021, 18, 10564. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]
- Hao, Z.; Ma, J.; Sun, W. The Technology-Oriented Pathway for Auxiliary Diagnosis in the Digital Health Age: A Self-Adaptive Disease Prediction Model. Int. J. Environ. Res. Public Health 2022, 19, 12509. [Google Scholar] [CrossRef] [PubMed]
- Briganti, G.; Le Moine, O. Artificial Intelligence in Medicine: Today and Tomorrow. Front. Med. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, S.M.D.A.C.; Ganegoda, G.U. Involvement of machine learning tools in healthcare decision making. J. Healthc. Eng. 2021, 2021, 6679512. [Google Scholar] [CrossRef] [PubMed]
- Diniz, E.J.S.; Fontenele, J.E.; de Oliveira, A.C.; Bastos, V.H.; Teixeira, S.; Rabêlo, R.L.; Calçada, D.B.; dos Santos, R.M.; de Oliveira, A.K.; Teles, A.S. Boamente: A Natural Language Processing-Based Digital Phenotyping Tool for Smart Monitoring of Suicidal Ideation. Healthcare 2022, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.; Teles, A.; Viana, D.; Marques, J.; Coutinho, L.; Silva, F. Digital Phenotyping of Mental Health using multimodal sensing of multiple situations of interest: A Systematic Literature Review. J. Biomed. Inform. 2023, 138, 104278. [Google Scholar] [CrossRef]
- Du-Harpur, X.; Watt, F.; Luscombe, N.; Lynch, M. What is AI? Applications of artificial intelligence to dermatology. Br. J. Dermatol. 2020, 183, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Yassin, N.I.; Omran, S.; El Houby, E.M.; Allam, H. Machine learning techniques for breast cancer computer aided diagnosis using different image modalities: A systematic review. Comput. Methods Programs Biomed. 2018, 156, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Reddy, V.; Myers, B.; Thibodeaux, Q.; Brownstone, N.; Liao, W. Machine learning in dermatology: Current applications, opportunities, and limitations. Dermatol. Ther. 2020, 10, 365–386. [Google Scholar] [CrossRef]
- Jaradat, A.S.; Al Mamlook, R.E.; Almakayeel, N.; Alharbe, N.; Almuflih, A.S.; Nasayreh, A.; Gharaibeh, H.; Gharaibeh, M.; Gharaibeh, A.; Bzizi, H. Automated Monkeypox Skin Lesion Detection Using Deep Learning and Transfer Learning Techniques. Int. J. Environ. Res. Public Health 2023, 20, 4422. [Google Scholar] [CrossRef]
- Zafar, M.; Sharif, M.I.; Sharif, M.I.; Kadry, S.; Bukhari, S.A.C.; Rauf, H.T. Skin Lesion Analysis and Cancer Detection Based on Machine/Deep Learning Techniques: A Comprehensive Survey. Life 2023, 13, 146. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372. [Google Scholar] [CrossRef]
- Parsif.all, v2.2.0. 2022. Available online: https://parsif.al/ (accessed on 2 January 2023).
- Tió-Coma, M.; Kiełbasa, S.M.; van den Eeden, S.J.; Mei, H.; Roy, J.C.; Wallinga, J.; Khatun, M.; Soren, S.; Chowdhury, A.S.; Alam, K.; et al. Blood RNA signature RISK4LEP predicts leprosy years before clinical onset. eBioMedicine 2021, 68, 103379. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, New York, NY, USA, 13–14 May 2014; Number 38 in EASE ’14. p. 10. [Google Scholar] [CrossRef]
- Kitchenham, B.; Brereton, P. A systematic review of systematic review process research in software engineering. Inf. Softw. Technol. 2013, 55, 2049–2075. [Google Scholar] [CrossRef]
- Cabitza, F.; Campagner, A. The need to separate the wheat from the chaff in medical informatics: Introducing a comprehensive checklist for the (self)-assessment of medical AI studies. Int. J. Med. Inform. 2021, 153, 104510. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Beesetty, R.; Reddy, S.A.; Modali, S.; Sunkara, G.; Dalal, J.; Damagathla, J.; Banerjee, D.; Venkatachalapathy, M. Leprosy Skin Lesion Detection: An AI Approach Using Few Shot Learning in a Small Clinical Dataset. Indian J. Lepr. 2023, 95, 89–102. [Google Scholar]
- Baweja, A.K.; Aditya, S.; Kanchana, M. Leprosy Diagnosis using Explainable Artificial Intelligence Techniques. In Proceedings of the 2023 International Conference on Sustainable Computing and Data Communication Systems (ICSCDS), Erode, India, 23–25 March 2023; pp. 551–556. [Google Scholar] [CrossRef]
- Rafay, A.; Hussain, W. EfficientSkinDis: An EfficientNet-based classification model for a large manually curated dataset of 31 skin diseases. Biomed. Signal Process. Control 2023, 85, 104869. [Google Scholar] [CrossRef]
- Yotsu, R.R.; Ding, Z.; Hamm, J.; Blanton, R.E. Deep learning for AI-based diagnosis of skin-related neglected tropical diseases: A pilot study. PLoS Neglected Trop. Dis. 2023, 17, e0011230. [Google Scholar] [CrossRef]
- Steyve, N.; Steve, P.; Ghislain, M.; Ndjakomo, S.; pierre, E. Optimized real-time diagnosis of neglected tropical diseases by automatic recognition of skin lesions. Inform. Med. Unlocked 2022, 33, 101078. [Google Scholar] [CrossRef]
- De Souza, M.L.M.; Lopes, G.A.; Branco, A.C.; Fairley, J.K.; Fraga, L.A.D.O. Leprosy screening based on artificial intelligence: Development of a cross-platform app. JMIR MHealth UHealth 2021, 9, e23718. [Google Scholar] [CrossRef] [PubMed]
- Jaikishore, C.; Udutalapally, V.; Das, D. AI Driven Edge Device for Screening Skin Lesion and Its Severity in Peripheral Communities. In Proceedings of the 2021 IEEE 18th India Council International Conference (INDICON), Guwahati, India, 19–21 December 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Banerjee, A.; Das, N.; Nasipuri, M. Skin Diseases Detection using LBP and WLD- An Ensembling Approach. arXiv 2020, arXiv:2004.04122. [Google Scholar]
- Jin, B.; Cruz, L.; Gonçalves, N. Deep Facial Diagnosis: Deep Transfer Learning From Face Recognition to Facial Diagnosis. IEEE Access 2020, 8, 123649–123661. [Google Scholar] [CrossRef]
- Mondal, B.; Das, N.; Santosh, K.; Nasipuri, M. Improved Skin Disease Classification Using Generative Adversarial Network. In Proceedings of the 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS), Rochester, MN, USA, 28–30 July 2020; pp. 520–525. [Google Scholar] [CrossRef]
- Casuayan de Goma, J.; Devaraj, M. Recognizing Common Skin Diseases in the Philippines Using Image Processing and Machine Learning Classification. In Proceedings of the ICCBD ’20: 2020 the 3rd International Conference on Computing and Big Data, New York, NY, USA, 5–7 August 2020; pp. 68–72. [Google Scholar] [CrossRef]
- Joshi, A.D.; Manerkar, S.S.; Nagvekar, V.U.; Naik, K.P.; Palekar, C.G.; Pugazhenthi, V.; Naik, S. Skin disease detection and classification. Int. J. Adv. Eng. Res. Sci. 2019, 6, 396–400. [Google Scholar] [CrossRef]
- Gama, R.S.; Souza, M.L.M.d.; Sarno, E.N.; Moraes, M.O.d.; Gonçalves, A.; Stefani, M.M.A.; Garcia, R.M.G.; Fraga, L.A.d.O. A novel integrated molecular and serological analysis method to predict new cases of leprosy amongst household contacts. PLoS Neglected Trop. Dis. 2019, 13, e0007400. [Google Scholar] [CrossRef] [PubMed]
- Baweja, H.S.; Parhar, T. Leprosy lesion recognition using convolutional neural networks. In Proceedings of the 2016 International Conference on Machine Learning and Cybernetics (ICMLC), Jeju, Republic of Korea, 10–13 July 2016; Volume 1, pp. 141–145. [Google Scholar] [CrossRef]
- Pillai, L.; Chouhan, U. Comparative analysis of machine learning algorithms for mycobacterium tuberculosis protein sequences on the basis of physicochemical parameters. J. Med. Imaging Health Inform. 2014, 4, 212–219. [Google Scholar] [CrossRef]
- Yasir, R.; Rahman, M.A.; Ahmed, N. Dermatological disease detection using image processing and artificial neural network. In Proceedings of the 8th International Conference on Electrical and Computer Engineering, Dhaka, Bangladesh, 20–22 December 2014; pp. 687–690. [Google Scholar] [CrossRef]
- Das, N.; Pal, A.; Mazumder, S.; Sarkar, S.; Gangopadhyay, D.; Nasipuri, M. An SVM Based Skin Disease Identification Using Local Binary Patterns. In Proceedings of the 2013 Third International Conference on Advances in Computing and Communications, Cochin, India, 29–31 August 2013; pp. 208–211. [Google Scholar] [CrossRef]
- Pal, A.; Das, N.; Sarkar, S.; Gangopadhyay, D.; Nasipuri, M. A New Rotation Invariant Weber Local Descriptor for Recognition of Skin Diseases. In Proceedings of the Pattern Recognition and Machine Intelligence, Kolkata, India, 10–14 December 2013; Maji, P., Ghosh, A., Murty, M.N., Ghosh, K., Pal, S.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 355–360. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Astrand, M.; Speed, T. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Deng, J.; Bi, L.; Zhou, L.; Guo, S.J.; Fleming, J.; Jiang, H.W.; Zhou, Y.; Gu, J.; Zhong, Q.; Wang, Z.X.; et al. Mycobacterium tuberculosis proteome microarray for global studies of protein function and immunogenicity. Cell Rep. 2014, 9, 2317–2329. [Google Scholar] [CrossRef]
- van Hooij, A.; Tjon Kon Fat, E.M.; Batista da Silva, M.; Carvalho Bouth, R.; Cunha Messias, A.C.; Gobbo, A.R.; Lema, T.; Bobosha, K.; Li, J.; Weng, X.; et al. Evaluation of immunodiagnostic tests for leprosy in Brazil, China and Ethiopia. Sci. Rep. 2018, 8, 17920. [Google Scholar] [CrossRef]
- Minion, J.; Pai, M.; Ramsay, A.; Menzies, D.; Greenaway, C. Comparison of LED and Conventional Fluorescence Microscopy for Detection of Acid Fast Bacilli in a Low-Incidence Setting. PLoS ONE 2011, 6, e22495. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Raschka, S.; Liu, Y.; Mirjalili, V. Machine Learning with PyTorch and Scikit-Learn: Develop Machine Learning and Deep Learning Models with Python, 1st ed.; Packt Publishing: Birmingham, UK, 2022. [Google Scholar]
- Russell, S.J.; Norvig, P. Artificial Intelligence: A Modern Approach, 4th ed.; Pearson Education: London, UK, 2021. [Google Scholar]
- Dinga, R.; Penninx, B.W.; Veltman, D.J.; Schmaal, L.; Marquand, A.F. Beyond accuracy: Measures for assessing machine learning models, pitfalls and guidelines. bioRxiv 2019. [Google Scholar] [CrossRef]
- Gundersen, O.E.; Gil, Y.; Aha, D.W. On Reproducible AI: Towards Reproducible Research, Open Science, and Digital Scholarship in AI Publications. AI Mag. 2018, 39, 56–68. [Google Scholar] [CrossRef]
- Novelli, C.; Taddeo, M.; Floridi, L. Accountability in artificial intelligence: What it is and how it works. AI Soc. 2023. [Google Scholar] [CrossRef]
- Meng, T.; Jing, X.; Yan, Z.; Pedrycz, W. A survey on machine learning for data fusion. Inf. Fusion 2020, 57, 115–129. [Google Scholar] [CrossRef]
- Zheng, Y. Methodologies for Cross-Domain Data Fusion: An Overview. IEEE Trans. Big Data 2015, 1, 16–34. [Google Scholar] [CrossRef]
- Stingl, P. Die Differentialdiagnose der Lepra in Entwicklungsländern–Haut und Mundhöhle [Differential diagnosis of leprosy in developing countries–the skin and oral cavity. Z. Hautkrankh. 1987, 62, 227–231. [Google Scholar]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External validation of prognostic models: What, why, how, when and where? Clin. Kidney J. 2021, 14, 49–58. [Google Scholar] [CrossRef]
- Collins, G.S.; de Groot, J.A.; Dutton, S.; Omar, O.; Shanyinde, M.; Tajar, A.; Voysey, M.; Wharton, R.; Yu, L.M.; Moons, K.G.; et al. External validation of multivariable prediction models: A systematic review of methodological conduct and reporting. BMC Med. Res. Methodol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Bleeker, S.; Moll, H.; Steyerberg, E.; Donders, A.; Derksen-Lubsen, G.; Grobbee, D.; Moons, K. External validation is necessary in prediction research:: A clinical example. J. Clin. Epidemiol. 2003, 56, 826–832. [Google Scholar] [CrossRef] [PubMed]
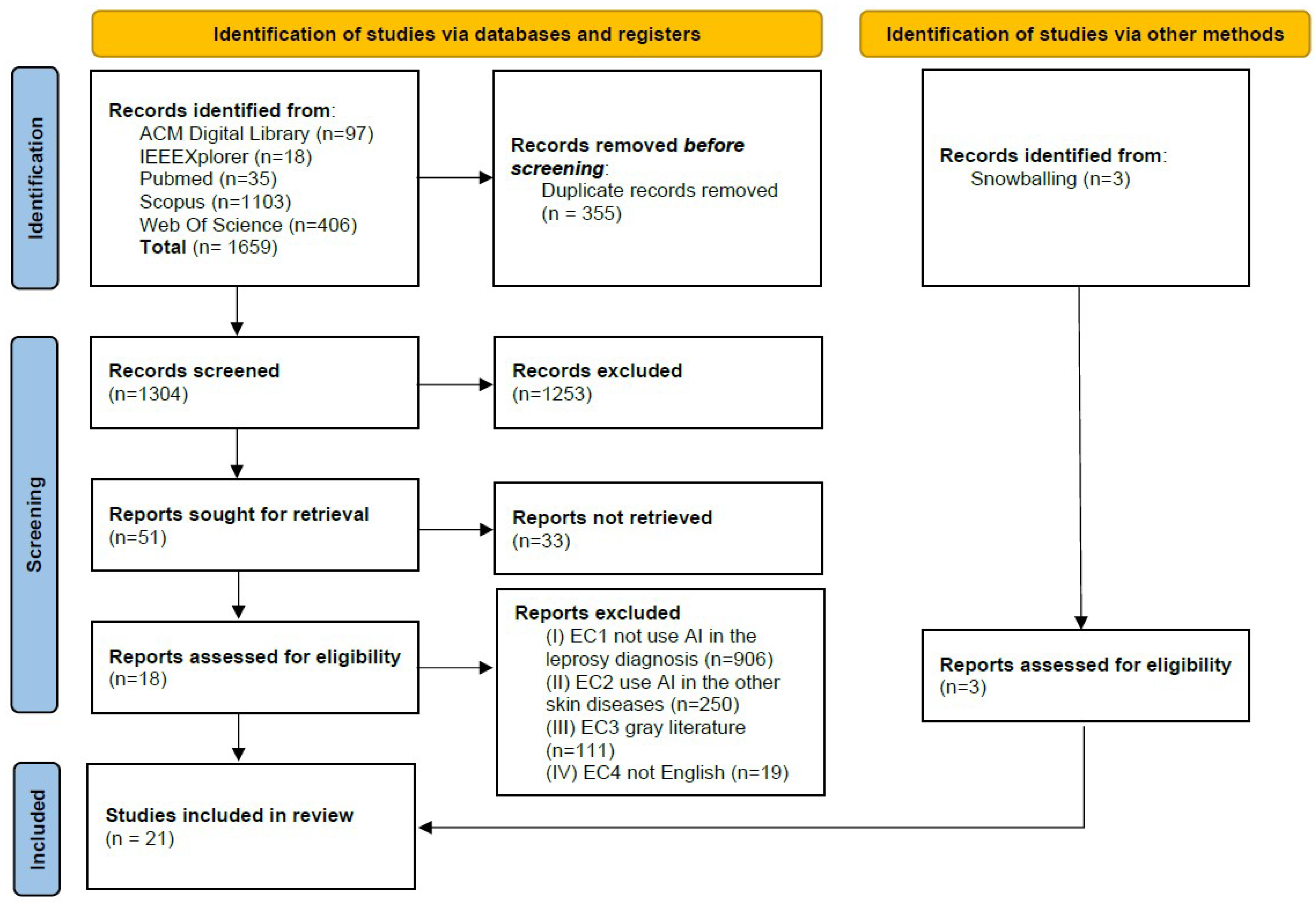

| Inclusion Criteria (IC) | Exclusion Criteria (EC) |
|---|---|
| (IC1) Studies that used AI to aid in the leprosy diagnosis. | (EC1) Studies that did not use AI in the leprosy diagnosis. |
| (IC2) Full articles. | (EC2) Studies that used AI to diagnose other skin diseases. |
| (IC3) Articles in English. | (EC3) Gray literature: reviews, reports, short papers, conference abstracts, communications, theses, and dissertations. |
| (IC4) Peer-reviewed articles. | (EC4) Articles in a language other than English. |
| Item | Problem Understanding |
|---|---|
| (QC1) | Is the study population described, also in terms of inclusion/exclusion criteria? |
| (QC2) | Is the study design described? |
| (QC3) | Is the study setting described? |
| (QC4) | Is the source of data described? |
| (QC5) | Is the medical task reported?? |
| (QC6) | Is the data collection process described, also in terms of setting-specific data collection strategies? |
| Item | Data Understanding |
| (QC7) | Are the subject demographics described in terms of average age, age variability, gender breakdown, main comorbidities, ethnic group, socioeconomic status? |
| (QC8) | If the task is supervised, is the gold standard described? |
| (QC9) | In the case of tabular data, are the features described? |
| Item | Data Preparation |
| (QC10) | Is outlier detection and analysis performed and reported? |
| (QC11) | Is missing-value management described? |
| (QC12) | Is feature pre-processing performed and described? |
| (QC13) | Is data imbalance analysis and adjustment performed and reported? |
| Item | Modeling |
| (QC14) | Is the model task reported? |
| (QC15) | Is the model output specified? |
| (QC16) | Is the model architecture or type described? |
| Item | Validation |
| (QC17) | Is the data splitting described (e.g., no data splitting; k-fold cross-validation (CV); nested k-fold CV; repeated CV; bootstrap validation; leave-one-out CV; 80%/10%10% train/validation/test)? |
| (QC18) | Are the model training and selection described? |
| (QC19) | (classification models) Is the model calibration described? |
| (QC20) | Is the internal/internal-external model validation procedure described, (e.g., internal 10-fold CV, time-based cross-validation)? |
| (QC21) | Has the model been externally validated? |
| (QC22) | Are the main error-based metrics used? |
| (QC23) | Are some relevant errors described? |
| Item | Deployment |
| (QC24) | Is the target user indicated? |
| (QC25) | (Classification models) Is the utility of the model discussed? |
| (QC26) | Is information regarding model interpretability and explainability available? |
| (QC27) | Is there any discussion regarding model fairness, ethical concerns, or bias risks, (for a list of clinically relevant biases, refer to)? |
| (QC28) | Is any point made about the environmental sustainability of the model, the carbon footprint, of either the training phase or inference phase (use) of the model? |
| (QC29) | Is code and data shared with the community? |
| (QC30) | Is the system already adopted in daily practice? |
| Research Questions | Form Questions |
|---|---|
| (RQ1) | What types of leprosy were targeted? |
| (RQ2) | What data types are used in the dataset? |
| (RQ3) | What data preparation techniques? |
| (RQ3) | What was the data preparation process? |
| (RQ4) | What algorithm/architecture was used to develop models? |
| (RQ5) | How was the model evaluated? |
| (RQ5) | What performance metrics were used? |
| (RQ5) | How well did the models perform? |
| Study | Diseases | Data Types | Data Preparation | Algorithm/Architecture | Model Evaluation | Performance Metrics |
|---|---|---|---|---|---|---|
| Beesetty et al. (2023) [82] | Leprosy and other skin lesions | Images | Not Available | Siamese Network and Inception-V3, Adaptive Moment Estimation (ADAM) | Not Available | Accuracy (73.12%) |
| Baweja et al. (2023) [83] | Leprosy and other skin lesions | Images | Data augmented by Rotation, Scale Transformation, Blurring | AlexNet, ResNet, and LeprosyNet, optimized by ADAM | 80/20 | Accuracy (98.00%) |
| Rafay and Hussain (2023) [84] | Leprosy Borderline, Leprosy Lepromatous, Leprosy Tuberculoid, Basal Cell Carcinoma, Dariers’s Disease, Epidermolysis Bullosa Pruriginosa, Hailey-Hailey Disease, Herpes Simplex, Impetigo, Larva Migrans, Lichen Planus, Lupus, Melanoma, Molluscum Contagiosum, Mycosis Fungoides, Neurofibromatosis, Papilomatosis Confluentes And Reticulate, Pediculosis Capitis, Pityriases Rosea, Porokeratosis Actinic, Psoriasis, Tinea Corporis, Tinea Nigra, Tungiasis, Actinic Keratosis, Dermatofibrona, Nevus, Pigmented Benign Keratosis, Squamous Cell Carcinoma and Vascular Lesion | Images | Data augmented by Rotation, Shear, Center Zoom, Horizontal Flip, Vertical Flip, Brightness | ResNet, VGG and EfficientNet-B2 | 80/20, 10-fold Cross-Validation | Accuracy (87.15%), Precision (87.00%), Recall (87.00%), and F1 score (87.00%) |
| Yotsu et al. (2023) [85] | Leprosy, Buruli Ulcer, Mycetoma, Scabies, and Yaws | Images | Images resized to 224 × 224, Data augmentation and normalization | ResNet-50 and VGG-16, optimized by Stochastic Gradient Descent (SGD) | 70/30 | Accuracy (84.63%) |
| Barbieri et al. (2022) [12] | Leprosy and other skin diseases | Images, Numerical Data | Numeric data: normalization. Images: tuning strategy or freeze | Inception-V4, ResNet-50, Elastic-net Logistic Regression (LR), XGBoost (XGB) and Random Forest (RF) | Dataset split into 80% training, 20% test (80/20); 5-fold and 10-fold cross-validation | Accuracy (90.00%), Area Under Curve (AUC) (96.46%), Sensitivity (89.00%), and Specificity (91.00%) |
| Marçal et al. (2022) [14] | Paucibacillary or Multibacillary Leprosy | Numerical Data | Not Available | Decision Trees (DT) | Leave-one-out-cross-validation (LOOCV) | Accuracy (84.00%) |
| Steyve et al. (2022) [86] | Leprosy, Leishmaniasis, Buruli Ulcer | Images | OTSU thresholding and filters Canny, Sober, Gabor, and Robert | Support Vector Machine (SVM), SVM optimized by Black Hole Algorithm (BHO), K-Nearest Neighbors (KNN), DT | Not Available | Accuracy (96.00%), Specificity (94.00%), F-Score (89.00%), Recall (90.00%), and Sensitivity (92.00%) |
| De Souza et al. (2021) [87] | Paucibacillary or Multibacillary Leprosy | Numerical Data | Not Available | RF | 10-fold cross-validation | Accuracy (92.38%), Sensitivity (93.97%), and Specificity (87.09%) |
| Tió-Coma et al. (2021) [76] | Leprosy | Numerical Data | Not Available | RF | 80/20; LOOCV | Accuracy (87.50%), Sensitivity (100.0%), Specificity (80.0%), and AUC (96.70%) |
| Jaikishore et al. (2021) [88] | Leprosy, Eczema, and Measles | Images | Re-scaling to normalize the image, zoom in and zoom out, width shift, height shift, and rotation angle of 45° | MobileNet-V2, VGG16, Inception-V3, Xception | 80/20 | Accuracy (94.32%), F1 score (93.02%), Precision (93.53%), and Recall (92.76%) |
| Banerjee et al. (2020) [89] | Leprosy, Vitiligo, and Tinea versicolor | Images | Local Binary Pattern (LBP), Weber Local Descriptor (WLD), Gray-Level Co-Occurrence Matrix (GLCM), riLBP (rotation invariant LBP) and WLDRI (rotation invariant WLD) | GoogLeNet, MobileNet-V1, ResNet-152, DenseNet-121, ResNet-101 and SVM | 80/20 | Accuracy (91.38%) |
| Jin et al. (2020) [90] | Leprosy, Thalassemia, Hyperthyroidism, and Down’s syndrome | Images | OpenCV, Histogram of Oriented Gradients (HOG), Dlib library, ResNet50, VGG16 | SVM Linear | 80/20 | Accuracy (93.30%) |
| Mondal et al. (2020) [91] | Leprosy, Tinea versicolor, and vitiligo | Images | Images cropped and centered a Region of Interest (ROI) manually, Global Contrast Normalization (GCN), Generative Adversarial Network (GAN), Wasserstein GAN with gradient penalty (WGAN-GP) | ResNet-101, DenseNet-169 and DenseNet-121 | 80/20 | Accuracy (94.00%), Recall (90.00%), and F1 score (92.00%) |
| Casuayan and Devaraj (2020) [92] | Leprosy, Acne Vulgaris, Atopic Dermatitis, Keratosis Pilaris (Chicken Skin), Psoriasis and Warts | Images | Dull Razor Algorithm, GLCM, Sharpening filter, Median filter, Smoothing filter, Binary mask, Sobel Operator | ANN and SVM | 70/30; 10-fold cross-validation | Precision (96.55%), and Recall (93.33%) |
| Amruta et al. (2019) [93] | Leprosy, Melanoma, Eczema | Images | Histogram equalization, Global Thresholding, Thresholding, GLCM | Iterative Dichotomiser 3 (ID3) | Not Available | Accuracy (87.00%) |
| Gama et al. (2019) [94] | Paucibacillary or Multibacillary Leprosy | Numerical Data | Not Available | RF | Not Available | Sensitivity (90.50%), and Specificity (92.50%) |
| Baweja and Parhar (2016) [95] | Leprosy | Images | Not Available | Inception-V3 | 80/20; Dataset into 50% positive and negative images | Accuracy (91.60%) |
| Pillai and Chouhan (2014) [96] | Leprosy | Numerical Data | Not Available | Sequential Minimal Optimization (SMO), LibSVM and Multilayer Perceptron (MLP) | 80/20; Stratified 5–fold and 10-fold cross-validation | Accuracy (85.00%) |
| Yasir et al. (2014) [97] | Leprosy, Eczema, Acne, Psoriasis, Scabies, Foot ulcer, Vitiligo, Tinea Corporis, Pityriasis rosea | Images | Filters sharpening, median, smooth, binary mask, histogram, YCbCr algorithm, Sobel operator | Feed-Forward Back Propagation Network (FFBPN) | 85/15; 10-fold cross-validation | Accuracy (90.00%) |
| Das et al. (2013) [98] | Leprosy, Tinea versicolor, Vitiligo | Images | LBP, GLCM, Discrete Cosine Transform (DCT), and Discrete Fourier Transform (DFT) | LibSVM | 80/20 | Accuracy (89.66%) |
| Pal et al. (2013) [99] | Leprosy, Tinea versicolor, Vitiligo | Images | Differential Excitation, WLD Histogram, WLD, WLDRI | SVM | 80/20 | Accuracy (86.78%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.R.N.; Teles, A.S.; Fernandes, T.R.S.; Lima, L.D.B.; Balhara, S.; Gupta, N.; Teixeira, S. Artificial Intelligence on Diagnostic Aid of Leprosy: A Systematic Literature Review. J. Clin. Med. 2024, 13, 180. https://doi.org/10.3390/jcm13010180
Fernandes JRN, Teles AS, Fernandes TRS, Lima LDB, Balhara S, Gupta N, Teixeira S. Artificial Intelligence on Diagnostic Aid of Leprosy: A Systematic Literature Review. Journal of Clinical Medicine. 2024; 13(1):180. https://doi.org/10.3390/jcm13010180
Chicago/Turabian StyleFernandes, Jacks Renan Neves, Ariel Soares Teles, Thayaná Ribeiro Silva Fernandes, Lucas Daniel Batista Lima, Surjeet Balhara, Nishu Gupta, and Silmar Teixeira. 2024. "Artificial Intelligence on Diagnostic Aid of Leprosy: A Systematic Literature Review" Journal of Clinical Medicine 13, no. 1: 180. https://doi.org/10.3390/jcm13010180







