Roux-en-Y Gastric Bypass after Laparoscopic Sleeve Gastrectomy Failure: Could the Number of Previous Operations Influence the Outcome?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Investigations
2.2. Surgical Technique and Follow-Up
2.3. Statistical Analysis
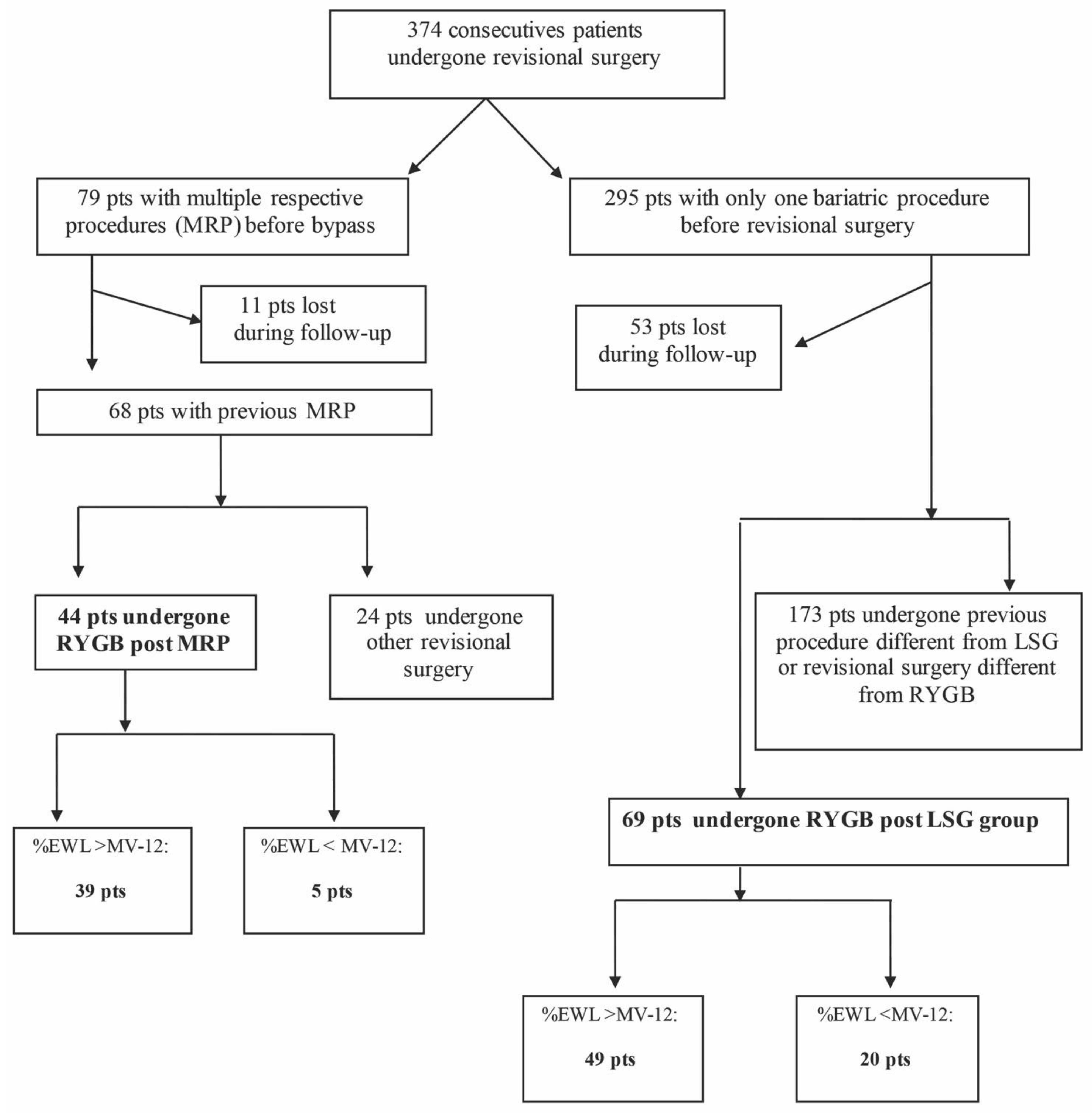
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clementi, M.; Carandina, S.; Zulian, V.; Guadagni, S.; Cianca, G.; Salvatorelli, A.; Sista, F. The role of antral resection in sleeve gastrectomy: An observational comparative study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7204–7210. [Google Scholar] [PubMed]
- Langer, F.B.; Bohdjalian, A.; Shakeri-Leidenmühler, S.; Schoppmann, S.F.; Zacherl, J.; Prager, G. Conversion from sleeve gastrectomy to Roux-en-Y gastric bypass–indications and outcome. Obes. Surg. 2010, 20, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.B.; Bohdjalian, A.; Shakeri-Manesch, S.; Felberbauer, F.X.; Ludvik, B.; Zacherl, J.; Prager, G. Inadequate weight loss vs secondary weight regain: Laparoscopic conversion from gastric banding to Roux-en-Y gastric bypass. Obes. Surg. 2008, 18, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Christou, N.V.; Look, D.; Maclean, L.D. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann. Surg. 2006, 244, 734–740. [Google Scholar] [CrossRef]
- Sista, F.; Carandina, S.; Andreica, A.; Zulian, V.; Pietroletti, R.; Cappelli, S.; Clementi, M. Long-term results of laparoscopic gastric sleeve: The importance of follow-up adherence. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6691–6699. [Google Scholar] [PubMed]
- Ardestani, A.; Lautz, D.B.; Tavakkolizadeh, A. Band revision versus roux-en-Y gastric bypass conversion as salvage operation after laparoscopic adjustable gastric banding. Surg. Obes. Relat. Dis. 2011, 7, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Annaberdyev, S.; Motamarry, I.; Kroh, M.; Schauer, P.R.; Brethauer, S.A. Revisional bariatric surgery for unsuccessful weight loss and complications. Obes. Surg. 2013, 23, 1766–1773. [Google Scholar] [CrossRef]
- Sista, F.; Clementi, M.; Rivkine, E.; Soprani, A.; Fiasca, F.; Cappelli, S.; Carandina, S. Gastric Bypass after multiple restrictive procedures: Roux-en-Y ore One Anastomosis? A Retrospective Multicentric Study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2075–2084. [Google Scholar]
- Debs, T.; Petrucciani, N.; Kassir, R.; Juglard, G.; Gugenheim, J.; Iannelli, A.; Liagre, A. Laparoscopic Conversion of Sleeve Gastrectomy to One Anastomosis Gastric Bypass for Weight Loss Failure: Mid-Term Results. Obes. Surg. 2020, 30, 2259–2265. [Google Scholar] [CrossRef]
- Axer, S.; Lederhuber, H.; Stiede, F.; Szabo, E.; Näslund, I. Weight-Related Outcomes After Revisional Bariatric Surgery in Patients with Non-response After Sleeve Gastrectomy-a Systematic Review. Obes. Surg. 2023, 33, 2210–2218. [Google Scholar] [CrossRef]
- Boru, C.E.; Marinari, G.M.; Olmi, S.; Gentileschi, P.; Morino, M.; Anselmino, M.; Celiento, M. Trends and safety of bariatric revisional surgery in Italy: Multicenter, prospective, observational study. Surg. Obes. Relat. Dis. 2023, 19, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Chansaenroj, P.; Aung, L.; Lee, W.J.; Chen, S.C.; Chen, J.C.; Ser, K.H. Revision Procedures After Failed Adjustable Gastric Banding: Comparison of Efficacy and Safety. Obes. Surg. 2017, 27, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Dowgiałło-Gornowicz, N.; Janik, M.; Lech, P.; Kowalski, G.; Major, P.; PROSS—Collaborative Study Group. Revisional bariatric surgery after adjustable gastric band: A multicenter Polish Revision Obesity Surgery Study (PROSS). BMC Surg. 2023, 23, 94. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Micheletto, G.; Marin, J.; Bonitta, G.; Lesti, G.; Bona, D. Resleeve for failed laparoscopic sleeve gastrectomy: Systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2020, 16, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Andalib, A.; Alamri, H.; Almuhanna, Y.; Bouchard, P.; Demyttenaere, S.; Court, O. Short-term outcomes of revisional surgery after sleeve gastrectomy: A comparative analysis of re-sleeve, Roux en-Y gastric bypass, duodenal switch (Roux en-Y and single-anastomosis). Surg. Endosc. 2021, 35, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.P.; Jakes, A.D.; Hayden, J.D.; Barth, J.H. Systematic review of definitions of failure in revisional bariatric surgery. Obes. Surg. 2015, 25, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, R.B. Critical analysis of long-term weight loss following gastric bypass. Surg. Gynecol. Obstet. 1982, 155, 385–394. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Cheung, D.; Switzer, N.J.; Gill, R.S.; Shi, X.; Karmali, S. Revisional bariatric surgery following failed primary laparoscopic sleeve gastrectomy: A systematic review. Obes. Surg. 2014, 24, 1757–1763. [Google Scholar] [CrossRef]
- Khoursheed, M.; Al-Bader, I.; Mouzannar, A.; Al-Haddad, A.; Sayed, A.; Mohammad, A.; Fingerhut, A. Sleeve gastrectomy or gastric bypass as revisional bariatric procedures: Retrospective evaluation of outcomes. Surg. Endosc. 2013, 27, 4277–4283. [Google Scholar] [CrossRef]
- Van Wezenbeek, M.R.; van Oudheusden, T.R.; de Zoete, J.P.; Smulders, J.F.; Nienhuijs, S.W. Conversion to Gastric Bypass After Either Failed Gastric Band or Failed Sleeve Gastrectomy. Obes. Surg. 2017, 27, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Mahawar, K.K.; Maureen Boyle, M.; Schroeder, N.; Balupuri, S.; Small, P.K. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass is Effective for Gastro-Oesophageal Reflux Disease but not for Further Weight Loss. Obes. Surg. 2017, 27, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Rafols, J.P.; Al Abbas, A.I.; Devriendt, S.; Guerra, A.; Herrera, M.F.; Himpens, J.; Van Wagensveld, B. Roux-en-Y gastric bypass, sleeve gastrectomy, or one anastomosis gastric bypass as rescue therapy after failed adjustable gastric banding: A multicenter comparative study. Surg. Obes. Relat. Dis. 2018, 14, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Bonaldi, M.; Rubicondo, C.; Giorgi, R.; Cesana, G.; Ciccarese, F.; Uccelli, M.; Olmi, S. Re-sleeve gastrectomy: Weight loss, comorbidities and gerd evaluation in a large series with 5 years of follow-up. Updates Surg. 2023, 75, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, S.M. Laparoscopic sleeve gastrectomy as revisional surgery for adjustable gastric band erosion. J. Laparoendosc. Adv. Surg. Tech. 2014, 24, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, K.K.; Himpens, J.M.; Shikora, S.A.; Ramos, A.C.; Torres, A.; Somers, S.; Small, P.K. The first consensus statement on revisional bariatric surgery using a modified Delphi approach. Surg. Endosc. 2020, 34, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Felsenreich, D.M.; Langer, F.B.; Kefurt, R.; Panhofer, P.; Schermann, M.; Beckerhinn, P.; Prager, G. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Arman, G.A.; Himpens, J.; Dhaenens, J.; Ballet, T.; Vilallonga, R.; Leman, G. Long-term (11+ years) outcomes in weight, patient satisfaction, comorbidities, and gastroesophageal reflux treatment after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 1778–1786. [Google Scholar] [CrossRef]
- Silecchia, G.; De Angelis, F.; Rizzello, M.; Albanese, A.; Longo, F.; Foletto, M. Residual fundus or neofundus after laparoscopic sleeve gastrectomy: Is fundectomy safe and effective as revision surgery? Surg. Endosc. 2015, 29, 2899–2903. [Google Scholar] [CrossRef]
- Coakley, B.A.; Deveney, C.W.; Spight, D.H.; Thompson, S.K.; Le, D.; Jobe, B.A.; O’Rourke, R.W. Revisional bariatric surgery for failed restrictive procedures. Surg. Obes. Relat. Dis. 2008, 4, 581–586. [Google Scholar] [CrossRef]
- Maleckas, A.; Venclauskas, L.; Wallenius, V.; Lönroth, H.; Fändriks, L. Surgery in the treatment of type 2 diabetes mellitus. Scand. J. Surg. 2015, 104, 40–47. [Google Scholar] [CrossRef] [PubMed]
- AlSabah, S.; Al Haddad, E.; Al-Subaie, S.; Ekrouf, S.; Alenezi, K.; Almulla, A.; Alhaddad, M. Short-Term Results of Revisional Single-Anastomosis Gastric Bypass After Sleeve Gastrectomy for Weight Regain. Obes. Surg. 2018, 28, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Stroh, C.; Benedix, D.; Weiner, R.; Benedix, F.; Wolff, S.; Knoll, C.; Manger, T.; Obesity Surgery Working Group Competence Network Obesity. Is a one-step sleeve gastrectomy indicated as a revision procedure after gastric banding? Data analysis from a quality assurance study of the surgical treatment of obesity in Germany. Obes. Surg. 2014, 24, 9–14. [Google Scholar] [CrossRef] [PubMed]


| Total n = 113 | MRP n = 44 (38.94%) | LSG n = 69 (61.06%) | p-Value | |
|---|---|---|---|---|
| Age, mean ± SD | 43.50 ± 10.95 | 45.73 ± 10.70 | 42.07 ± 10.95 | 0.084 * |
| Sex, n (%) | 0.674 ** | |||
| Female | 101 (89.38) | 40 (90.91) | 61 (88.41) | |
| Male | 12 (10.62) | 4 (9.09) | 8 (11.59) | |
| BMI before LSG, mean ± SD | 46.08 ± 6.47 | 45.36 ± 6.95 | 46.53 ± 6.16 | 0.351 * |
| BMI before RYGB, mean ± SD | 40.71 ± 5.32 | 41.53 ± 6.25 | 40.19 ± 4.60 | 0.196 * |
| MRP/LSG–RYGB timing, mean ± SD | 49.62 ± 28.88 | 48.91 ± 24.25 | 50.07 ± 31.64 | 0.836 * |
| Type 2 diabetes | 11/9.7% | 6/13.6% | 5/7.2% | 0.33 |
| OSAS | 32/28.% | 1431.8% | 18/26.% | 0.52 |
| Hypertension | 22/19.5% | 10/22.7% | 12/17.4% | 0.64 |
| %EWL before RYGB | 7.22 ± 52.32 | 22.40 ± 73.72 | 25.67 ± 14.56 | <0.001 |
| Weight condition before RYGB, n (%) | ||||
| IWL | 99 (87.61) | 30 (68.18) | 69 (100.00) | <0.001 ** |
| %TWL-24M | 21.7 ± 1.1 | 23.8 ± 9.4 | 0.163 * | |
| WR | 14 (12.39) | 14 (31.82) | 0 (0.00) | <0.001 ** |
| %TWL-24M | 29.4 ± 1.4 | |||
| %EWL after RYGB (3M), mean ± SD | 29.34 ± 17.07 | 28.94 ± 14.87 | 29.59 ± 18.44 | 0.844 * |
| %EWL after RYGB (6M), mean ± SD | 43.98 ± 20.81 | 42.76 ± 21.46 | 44.75 ± 20.51 | 0.623 * |
| %EWL after RYGB (12M), mean ± SD | 57.66 ± 24.93 | 55.54 ± 27.37 | 59.01 ± 23.35 | 0.474 * |
| %EWL after RYGB (24M), mean ± SD | 62.73 ± 25.13 | 63.07 ± 26.10 | 62.51 ± 24.68 | 0.909 * |
| %TWL after RYGB (3M), mean ± SD | 11.00 ± 6.03 | 11.03 ± 6.26 | 10.97 ± 5.92 | 0.958 * |
| %TWL after RYGB (6M), mean ± SD | 16.40 ± 8.08 | 16.39 ± 9.11 | 16.40 ± 7.42 | 0.995 * |
| %TWL after RYGB (12M), mean ± SD | 21.30 ± 9.43 | 21.43 ± 11.56 | 21.22 ± 7.87 | 0.912 * |
| %TWL after RYGB (24M), mean ± SD | 23.30 ± 9.91 | 24.22 ± 11.31 | 22.71 ± 8.93 | 0.909 * |
| Linear Regression | |||
|---|---|---|---|
| β | 95% CI | p-Value | |
| MRP group | |||
| MRP–RYGB timing | 0.53 | 0.23–0.82 | 0.001 |
| %EWL before RYGB | −0.12 | −0.22–−0.01 | 0.031 |
| LSG group | |||
| LSG–RYGB timing | 0.08 | −0.11–0.27 | 0.404 |
| %EWL before RYGB | 0.18 | −0.23–0.59 | 0.378 |
| %EWL after RYGB (24M) | Univariate Logistic Model | ||||
|---|---|---|---|---|---|
| >MV-12M n (%) 88 (77.88) | ≤MV-12M n (%) 25 (22.12) | OR | 95% CI | p-Value | |
| Age, mean ± SD | 44.26 ± 10.87 | 40.80 ± 11.04 | 0.97 | 0.93–1.01 | 0.165 |
| Sex, n (%) | |||||
| Female | 78 (88.64) | 23 (92.00) | 1 | ||
| Male | 10 (11.36) | 2 (8.00) | 0.68 | 0.14–3.32 | 0.632 |
| BMI LSG, mean ± SD | 46.47 ± 6.85 | 44.68 ± 4.77 | 0.95 | 0.88–1.03 | 0.222 |
| Duration LSG, mean ± SD | 48.76 ± 28.91 | 52.64 ± 29.15 | 1.00 | 0.99–1.02 | 0.553 |
| BMI, RYGB mean ± SD | 40.93 ± 5.37 | 39.94 ± 5.12 | 0.96 | 0.88–1.05 | 0.408 |
| MRP n (%) | 39 (44.32) | 5 (20.00) | 1 | ||
| LSG n (%) | 49 (55.68) | 20 (80.00) | 3.18 | 1.10–9.25 | 0.033 |
| MRP | LSG | p-Value | MRP | LSG | p-Value | MRP | LSG | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| I | I | R | R | S | S | ||||
| T2D | 4/6 | 3/5 | 1 | / | / | / | 2/6 | 2/5 | 0.56 |
| AHT | 5/10 | 2/12 | 0.17 | 3/10 | 6/12 | 0.41 | 2/10/ | 4/12 | 0.64 |
| OSAS | 14/14 | 16/18 | 1 | / | / | / | 2/14 | 2/18 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sista, F.; Carandina, S.; Soprani, A.; Rivkine, E.; Montana, L.; Fiasca, F.; Cappelli, S.; Grasso, A.; Nedelcu, M.; Tucceri Cimini, I.; et al. Roux-en-Y Gastric Bypass after Laparoscopic Sleeve Gastrectomy Failure: Could the Number of Previous Operations Influence the Outcome? J. Clin. Med. 2024, 13, 293. https://doi.org/10.3390/jcm13010293
Sista F, Carandina S, Soprani A, Rivkine E, Montana L, Fiasca F, Cappelli S, Grasso A, Nedelcu M, Tucceri Cimini I, et al. Roux-en-Y Gastric Bypass after Laparoscopic Sleeve Gastrectomy Failure: Could the Number of Previous Operations Influence the Outcome? Journal of Clinical Medicine. 2024; 13(1):293. https://doi.org/10.3390/jcm13010293
Chicago/Turabian StyleSista, Federico, Sergio Carandina, Antoine Soprani, Emmanuel Rivkine, Laura Montana, Fabiana Fiasca, Sonia Cappelli, Antonella Grasso, Marius Nedelcu, Irene Tucceri Cimini, and et al. 2024. "Roux-en-Y Gastric Bypass after Laparoscopic Sleeve Gastrectomy Failure: Could the Number of Previous Operations Influence the Outcome?" Journal of Clinical Medicine 13, no. 1: 293. https://doi.org/10.3390/jcm13010293
APA StyleSista, F., Carandina, S., Soprani, A., Rivkine, E., Montana, L., Fiasca, F., Cappelli, S., Grasso, A., Nedelcu, M., Tucceri Cimini, I., & Clementi, M. (2024). Roux-en-Y Gastric Bypass after Laparoscopic Sleeve Gastrectomy Failure: Could the Number of Previous Operations Influence the Outcome? Journal of Clinical Medicine, 13(1), 293. https://doi.org/10.3390/jcm13010293








