Keratosis Pilaris-like Eruption during Treatment of Chronic Myeloid Leukemia with Tyrosine Kinase Inhibitors: Literature Review and Report of a Case Related to Imatinib
Abstract
:1. Introduction
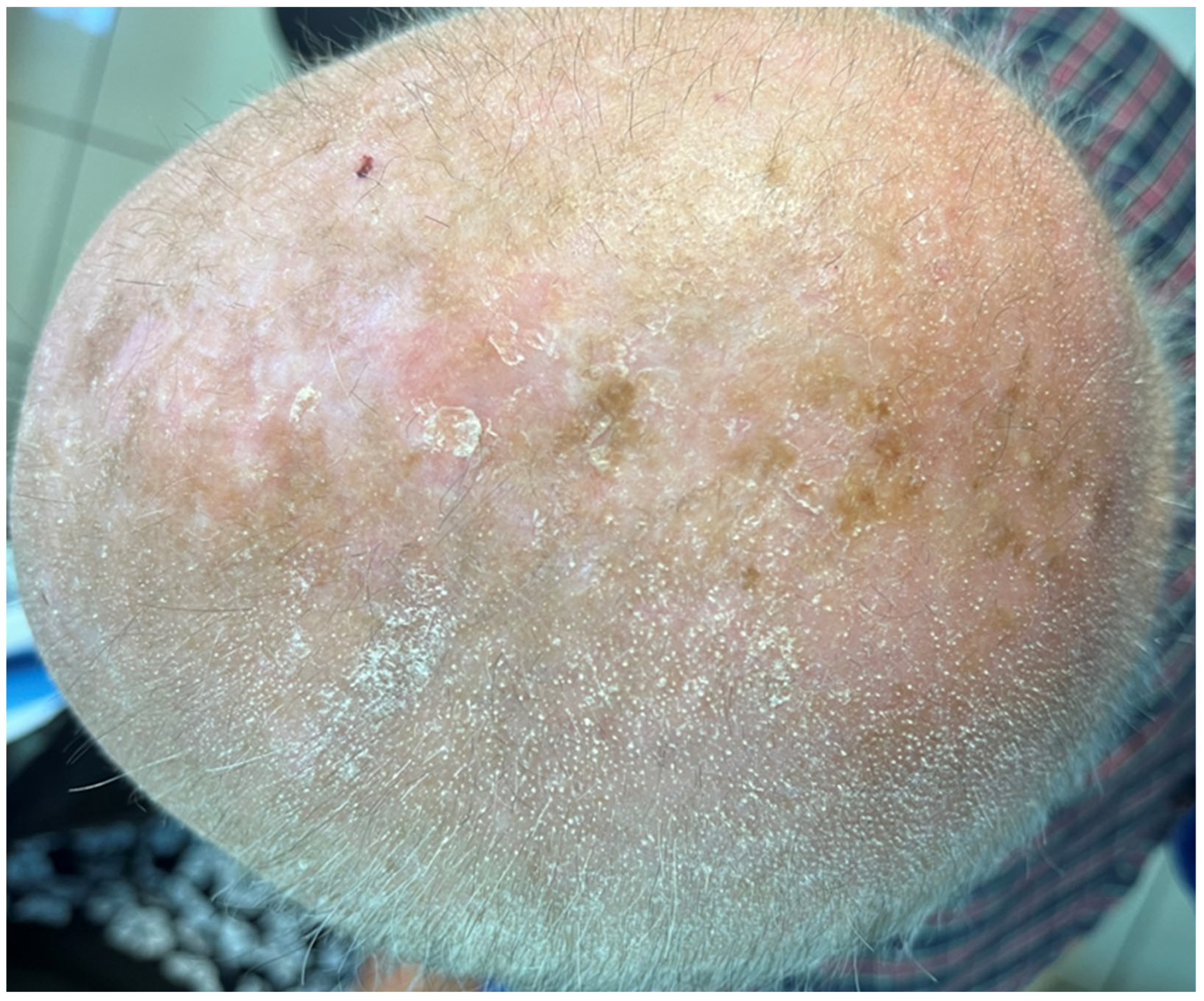
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochi, Y. Genetic landscape of chronic myeloid leukemia. Int. J. Hematol. 2023, 117, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2018, 93, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.E.G.; Deininger, M.W. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Basso, I.N.; Kim, D.D.H. Target spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia. Int. J. Hematol. 2021, 113, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Cortés, J.E.; Kantarjian, H. Optimizing treatment with Bcr-Abl tyrosine kinase inhibitors in Philadelphia chromosome-positive chronic myeloid leukemia: Focus on dosing schedules. Clin. Lymphoma Myeloma 2008, 8 (Suppl. S3), S75–S81. [Google Scholar] [CrossRef] [PubMed]
- Rix, U.; Hantschel, O.; Dürnberger, G.; Remsing Rix, L.L.; Planyavsky, M.; Fernbach, N.V.; Kaupe, I.; Bennett, K.L.; Valent, P.; Colinge, J.; et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007, 110, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.; Manley, P. What do kinase inhibition profiles tell us about tyrosine kinase inhibitors used for the treatment of CML? Leuk. Res. 2012, 36, 253–261. [Google Scholar] [CrossRef]
- Steegmann, J.L.; Cervantes, F.; le Coutre, P.; Porkka, K.; Saglio, G. Off-target effects of BCR-ABL1 inhibitors and their potential long-term implications in patients with chronic myeloid leukemia. Leuk. Lymphoma 2012, 53, 2351–2361. [Google Scholar] [CrossRef]
- Giles, F.J.; O’Dwyer, M.; Swords, R. Class effects of tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia 2009, 23, 1698–1707. [Google Scholar] [CrossRef]
- Hantschel, O.; Rix, U.; Superti-Furga, G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk. Lymphoma 2008, 49, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Park, N.S.; Park, Y.K.; Yadav, A.K.; Shin, Y.M.; Bishop-Bailey, D.; Choi, J.S.; Park, J.W.; Jang, B.C. Anti-growth and pro-apoptotic effects of dasatinib on human oral cancer cells through multi-targeted mechanisms. J. Cell. Mol. Med. 2021, 25, 8300–8311. [Google Scholar] [CrossRef] [PubMed]
- Nekoukar, Z.; Moghimi, M.; Salehifar, E. A narrative review on adverse effects of dasatinib with a focus on pharmacotherapy of dasatinib-induced pulmonary toxicities. Blood Res. 2021, 56, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Binotto, G. Bosutinib for Chronic Myeloid Leukemia. Rare Cancers Ther. 2015, 3, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Targeting BCR-Abl in the treatment of Philadelphia-chromosome positive chronic myelogenous leukemia. Pharmacol. Res. 2022, 178, 106156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Schmaier, A. Ponatinib and other CML Tyrosine Kinase Inhibitors in Thrombosis. Int. J. Mol. Sci. 2020, 21, 6556. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ding, Y.; Tai, X.R.; Zhang, C.; Wang, D. Ponatinib: An update on its drug targets, therapeutic potential and safety. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188949. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Zhong, Y.; Dai, S. Rash with different types of BCR-ABL inhibitors in chronic myelogenous leukemia: A systematic review and meta-analysis. Future Oncol. 2023, 19, 1215–1227. [Google Scholar] [CrossRef]
- Amitay-Laish, I.; Stemmer, S.M.; Lacouture, M.E. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol. Ther. 2011, 24, 386–395. [Google Scholar] [CrossRef]
- Brazzelli, V.; Grasso, V.; Borroni, G. Imatinib, dasatinib and nilotinib: A review of adverse cutaneous reactions with emphasis on our clinical experience. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1471–1480. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Kwong, B.Y. Cutaneous Adverse Events of Targeted Therapies for Hematolymphoid Malignancies. Clin. Lymphoma Myeloma Leuk. 2017, 17, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Wu, S.; Busam, K.J.; Berman, E.; Amitay-Laish, I.; Lacouture, M.E. Rash with the multitargeted kinase inhibitors nilotinib and dasatinib: Meta-analysis and clinical characterization. Eur. J. Haematol 2013, 90, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.; Giraudier, S.; Ortonne, N.; Zehou, O.; Cordonnier, C.; Hulin, A.; Chosidow, O.; Tulliez, M.; Valeyrie-Allanore, L. Adverse cutaneous reactions to the new second-generation tyrosine kinase inhibitors (dasatinib, nilotinib) in chronic myeloid leukemia. J. Am. Acad. Dermatol. 2013, 69, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Solomon, A.R.; Mauro, M.J.; Ehst, B.D. Unique Cutaneous Reaction to Second- and Third-Generation Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia. Dermatology 2016, 232, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Alloo, A.; Sheu, J.; Butrynski, J.E.; DeAngelo, D.J.; George, S.; Murphy, G.F.; LeBoeuf, N.R. Ponatinib-induced pityriasiform, folliculocentric and ichthyosiform cutaneous toxicities. Br. J. Dermatol. 2015, 173, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Vastarella, M.; Fabbrocini, G.; Sibaud, V. Hyperkeratotic Skin Adverse Events Induced by Anticancer Treatments: A Comprehensive Review. Drug Saf. 2020, 43, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of chronic myeloid leukemia in 2023—Common ground and common sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef]
- Curman, P.; Näsman, A.; Brauner, H. Trichodysplasia spinulosa: A comprehensive review of the disease and its treatment. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1067–1076. [Google Scholar] [CrossRef]
- Tomasini, C.; Michelerio, A.; Quaglino, P. Spiky/keratosis-pilaris-like early follicular mycosis fungoides: A clinicopathologic study of 20 cases with extended follow-up. J. Cutan. Pathol. 2021, 48, 1124–1132. [Google Scholar] [CrossRef]
- Llamas-Velasco, M.; Steegmann, J.L.; Carrascosa, R.; Fraga, J.; García Diez, A.; Requena, L. Perforating folliculitis in a patient treated with nilotinib: A further evidence of C-kit involvement. Am. J. Dermatopathol. 2014, 36, 592–593. [Google Scholar] [CrossRef]
- Oak, A.S.; Jaleel, T.; Fening, K.; Pavlidakey, P.G.; Sami, N. A case of scurvy associated with nilotinib. J. Cutan. Pathol. 2016, 43, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Manabat-Hidalgo, C. Follicular spicules of multiple myeloma. Dermatol. Online J. 2019, 25, 11. [Google Scholar] [CrossRef]
- Kodali, N.; Patel, V.M.; Schwartz, R.A. Keratosis pilaris: An update and approach to management. Ital. J. Dermatol. Venerol. 2023, 158, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Orlow, S.J. Keratosis Pilaris and its Subtypes: Associations, New Molecular and Pharmacologic Etiologies, and Therapeutic Options. Am. J. Clin. Dermatol. 2018, 19, 733–757. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Hattori, M.; Takeuchi, Y.; Ishikawa, O. Generalized keratosis pilaris-like eruptions in a chronic myelogenous leukemia patient treated with nilotinib. J. Dermatol. 2016, 43, 1100–1101. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.M.; Aw, C.W. Nilotinib-Induced Keratosis Pilaris. Case Rep. Dermatol. 2016, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Khetarpal, S.; Sood, A.; Billings, S.D. Nilontinib induced keratosis pilaris atrophicans. Dermatol. Online J. 2016, 22, 8. [Google Scholar] [CrossRef]
- Tawil, M.H.; El Khoury, R.; Tomb, R.; Ghosn, M. Nilotinib-induced Keratosis Pilaris Associated with Alopecia Areata and Eyebrow Thinning. Int. J. Trichology. 2017, 9, 87–89. [Google Scholar]
- Oro-Ayude, M.; Feito, M.; Quintana-Castanedo, L.; Beato-Merino, M.J.; De Lucas, R. Keratosis pilaris-like eruption secondary to nilotinib in a child. Pediatr. Dermatol. 2020, 37, 968–969. [Google Scholar] [CrossRef]
- Frioui, R.; Rabhi, F.; Gargouri, F.; Jaber, K.; Dhaoui, A. Nilotinib-induced keratosis pilaris associated with cicatricial alopecia resembling frontal fibrosing alopecia. Dermatol. Ther. 2021, 34, e14579. [Google Scholar] [CrossRef]
- Kowe, P.A.; Wankhade, V.H.; Malpani, S.S.; Singh, R.P. Nilotinib-induced generalized keratosis pilaris: Report of a rare case. Indian. J. Pharmacol. 2021, 53, 330–331. [Google Scholar] [PubMed]
- Jimenez-Cauhe, J.; Fernandez-Gonzalez, P.; Ortega-Quijano, D.; Fernandez-Nieto, D.; Saceda-Corralo, D. Keratosis pilaris-like eruption induced by nilotinib. Ital. J. Dermatol. Venerol. 2021, 156 (Suppl. S1–S6), 47–48. [Google Scholar]
- Anforth, R.; Fernandez-Peñas, P.; Long, G.V. Cutaneous toxicities of RAF inhibitors. Lancet Oncol. 2013, 14, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Rinderknecht, J.D.; Goldinger, S.M.; Rozati, S.; Kamarashev, J.; Kerl, K.; French, L.E.; Dummer, R.; Belloni, B. RASopathic skin eruptions during vemurafenib therapy. PLoS ONE 2013, 8, e58721. [Google Scholar] [CrossRef]
- Packer, L.M.; Rana, S.; Hayward, R.; O’Hare, T.; Eide, C.A.; Rebocho, A.; Heidorn, S.; Zabriskie, M.S.; Niculescu-Duvaz, I.; Druker, B.J.; et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell 2011, 20, 715–727. [Google Scholar] [CrossRef]
- Okereke, U.R.; Colozza, S.; Cohen, D.E. A case of new onset keratosis pilaris after discontinuation of erlotinib. J. Drugs Dermatol. 2014, 13, 1410–1411. [Google Scholar] [PubMed]
- Kong, H.H.; Turner, M.L. Array of cutaneous adverse effects associated with sorafenib. J. Am. Acad. Dermatol. 2009, 61, 360–361. [Google Scholar] [CrossRef]




| First Author [Reference] | N. | Sex | Age (Years) | Previous TKI | Culprit TKI (Daily Dose at Rash Onset) | KP/KP-like Eruption | Other Findings | Histopathology | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Details | Localization | Time to Onset (after TKI Start) | ||||||||
| Drucker [22] | 2 | F | 52 | - | N (500 mg) | CTCAE grade 2 | Face, extremities | NR | Erythematous papules Tenderness, pain, pruritus | NA |
| M | 69 | - | D (100 mg) | CTCAE grade 2 | Trunk, extremities | NR | Pustules Pruritus | Milium | ||
| Delgado [23] | 9 | NR | NR | - | N or D (not specified) | Extensive KP | - | NR | Follicular atrophy, perifollicular fibrosis (biopsy in 4 patients) | |
| Shimizu [35] | 1 | F | 68 | - | N (600 mg) | Asymptomatic, milium-sized, skin-colored keratotic papules | Trunk, extremities | 6 months | Keratotic plug within the follicle, slight perifollicular lymphocytic infiltration | |
| Patel [24] | 9 | F | 56 | I * | N (400 mg b.i.d.) | KP-like eruption (from subtle follicular prominence to flat-topped follicular erythematous to violaceous papules with mild scale) | Face, trunk, and/or extremities (usually forehead, temples, lateral cheeks, proximal extremities; occasionally, trunk) | 1 month | Pruritus Scalp LPP-like eruption with nonscarring alopecia Follicular erythematous papules of eyebrows with nonscarring alopecia | Arm: KP-like or suppurative folliculitis Scalp: subtle LPP-like |
| F | 54 | I * | N (400 mg b.i.d.) | 2 months | Pruritus Scalp LPP-like eruption LP-like lesions on trunk Oral erosive LP | Arm: KP-like Scalp: subtle LPP-like Trunk: LP | ||||
| F | 55 | I *, D | P (30 mg; initially 4 mg) | weeks after dose escalation | Scalp LPP-like eruption Follicular erythematous papules of eyebrows with nonscarring alopecia | NA | ||||
| M | 53 | I *, D, N | P (45 mg) | 2 months | Pruritus Scalp LPP-like eruption | NA | ||||
| F | 41 | - | D (100 mg) | 2–3 months | Pruritus Scalp LPP-like eruption Facial acneiform eruption | Thigh: KP-like with fibrosis | ||||
| F | 51 | - | N (300 mg b.i.d.) | 2 months | Pruritus | NA | ||||
| M | 49 | - | N (300 mg b.i.d.) | 1.5 months | Pruritus | NA | ||||
| F | 56 | I ^ | N (400 mg) | days after restart | Pruritus Scalp LPP-like eruption with scarring alopecia Follicular erythematous papules of eyebrows with nonscarring alopecia | |||||
| D (100 mg) | continued | Back: KP-like with vacuolar changes Scalp: LPP-like | ||||||||
| M | 61 | I * | D (180 mg; initially 140 mg) | weeks after dose escalation | Pruritus | |||||
| N (400 mg b.i.d.) | continued | Pruritus Scalp LPP-like eruption with scarring alopecia Follicular erythematous papules of eyebrows with nonscarring alopecia | Trunk: perifolliculitis Scalp: LPP-like | |||||||
| Leong [36] | 1 | M | 27 | I * | N (400 mg b.i.d.) | Non-pruritic, rough, brown, follicular papules | Trunk, limbs (especially the extensor portion of upper limbs) | 3 days | No alopecia | NA |
| Khetarpal [37] | 1 | M | 46 | I * | N (400 mg b.i.d.) | Prominent perifollicular pink papules | Arms, chest, back, shoulders, legs | 2 months | Complete or partial hair loss in affected areas; lateral thinning of eyebrows; follicular accentuation on the forehead; skin dryness and roughness | Prominent perifollicular fibrosis extending to the dermis (KP atrophicans) |
| Tawil [38] | 1 | M | 45 | - | N (300 mg b.i.d.) | Asymptomatic, keratotic, red-brown follicular papules | Limbs (especially the extensor surfaces of upper limbs) | 4 months | Nonscarring eyebrow hair loss Thinning of chest hair Autoresolutive alopecia areata of the wrist | NA |
| Oro-Ayude [39] | 1 | M | 14 | I *, D | N (NR) | 1–2 mm, rough, skin-colored, folliculocentric papules on an erythematous base | Generalized, with prominent involvement of the eyebrows, ears, extensor surfaces of upper limbs | several days | Pruritus | Dilated follicular infundibulum, basket-weave orthokeratosis, follicular plug, perifollicular concentric fibrosis, mild perivascular lymphocytic inflammation |
| Frioui [40] | 1 | F | 49 | I * | N (NR) | Small, rough, skin-colored keratotic follicular papules | Scalp, face, neck, trunk, extremities | 4 months | Pruritus Nonscarring hair thinning in eyebrows, axillae, and pubic area Cicatricial alopecia resembling frontal fibrosing alopecia | Plugging of individual hair follicles. Scalp: largely unaffected epidermis, rarefaction of hair follicles with isthmic atrophy, keratotic plugs within the follicle, perivascular lymphocytic infiltrate |
| Kowe [41] | 1 | F | 40 | N §, I ** | N (300 mg b.i.d.) | Asymptomatic, small raised, keratotic follicular papules | Face, extensor aspects of upper extremities, upper back | 10 days (after restarting) | Follicular hyperkeratosis, dilated follicular infundibulum with keratotic plugging, mild perivascular lymphocytic infiltrate in upper dermis | |
| Jimenez-Cauhe [42] | 1 | M | 17 | - | N (350 mg b.i.d.) | Non-pruritic, millimetric, skin-colored, follicular-centered papules with rough surface | Lateral aspects of face, upper limbs, trunk, proximal portion of lower limbs | 6 days | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogio, F.; Poli, M.A.; Lospalluti, L.; Lettini, T.; Cassano, N.; Vena, G.A.; Ingravallo, G.; Cazzato, G.; Foti, C. Keratosis Pilaris-like Eruption during Treatment of Chronic Myeloid Leukemia with Tyrosine Kinase Inhibitors: Literature Review and Report of a Case Related to Imatinib. J. Clin. Med. 2024, 13, 32. https://doi.org/10.3390/jcm13010032
Ambrogio F, Poli MA, Lospalluti L, Lettini T, Cassano N, Vena GA, Ingravallo G, Cazzato G, Foti C. Keratosis Pilaris-like Eruption during Treatment of Chronic Myeloid Leukemia with Tyrosine Kinase Inhibitors: Literature Review and Report of a Case Related to Imatinib. Journal of Clinical Medicine. 2024; 13(1):32. https://doi.org/10.3390/jcm13010032
Chicago/Turabian StyleAmbrogio, Francesca, Melita Anna Poli, Lucia Lospalluti, Teresa Lettini, Nicoletta Cassano, Gino Antonio Vena, Giuseppe Ingravallo, Gerardo Cazzato, and Caterina Foti. 2024. "Keratosis Pilaris-like Eruption during Treatment of Chronic Myeloid Leukemia with Tyrosine Kinase Inhibitors: Literature Review and Report of a Case Related to Imatinib" Journal of Clinical Medicine 13, no. 1: 32. https://doi.org/10.3390/jcm13010032








