Clinical Dilemma Involving Treatments for Very Low-Birth-Weight Infants and the Potential Risk of Necrotizing Enterocolitis: A Narrative Literature Review
Abstract
1. Introduction
2. Literature Research Methods
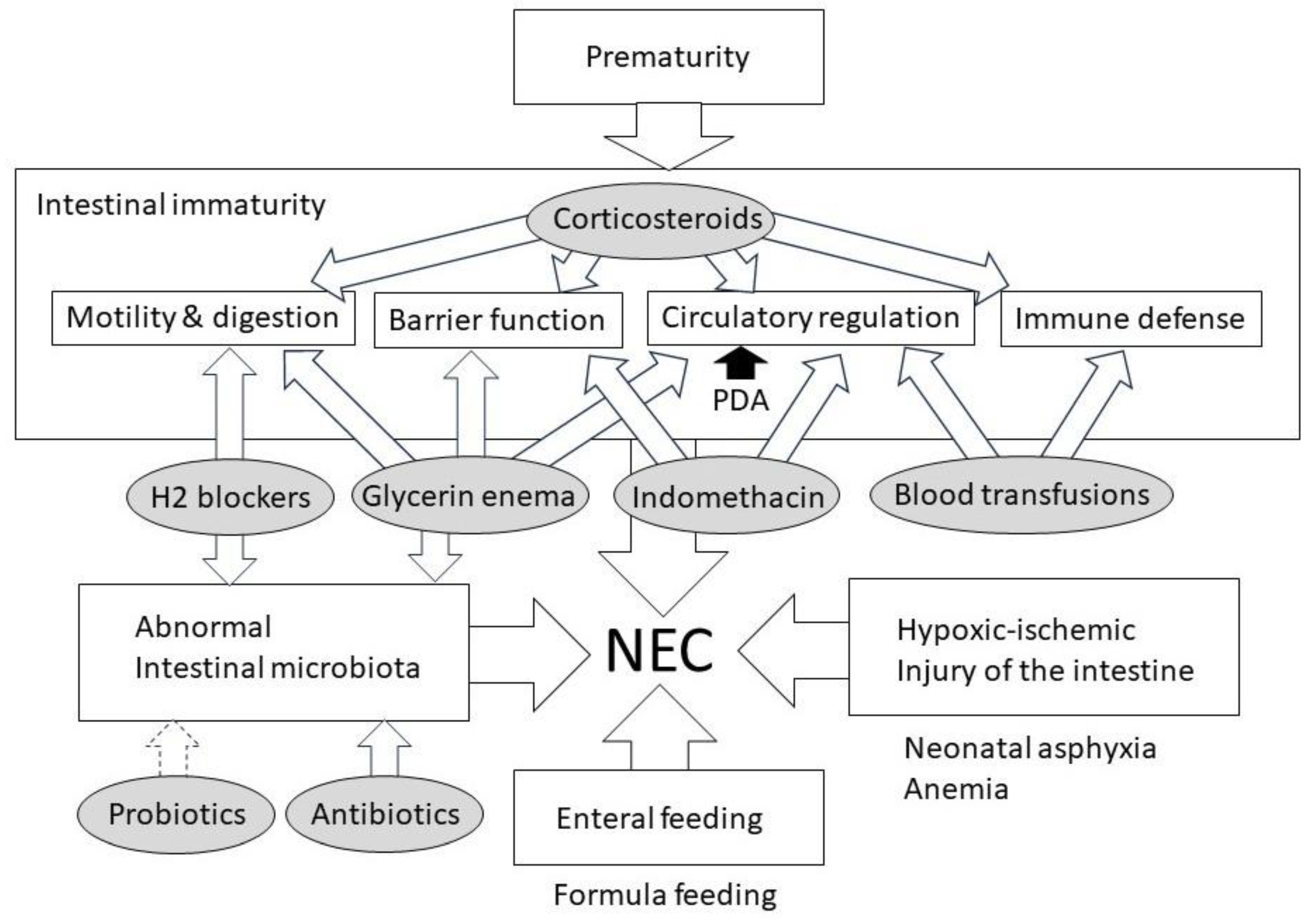
3. Pathophysiology of NEC
4. Clinical Dilemma Involving Medications and the Risk of NEC
4.1. Indomethacin
4.1.1. Mechanisms Underlying Indomethacin and NEC Development
4.1.2. Clinical Insights Regarding Indomethacin and NEC
4.1.3. Strategies for Risk Mitigation and Exploration of Safer Alternatives
4.2. Corticosteroids
4.2.1. Mechanisms Underlying Corticosteroids and NEC Development
4.2.2. Clinical Insights Regarding Corticosteroids and NEC
4.2.3. Strategies for Risk Mitigation and Exploration of Safer Alternatives
4.3. H2 Blockers
4.3.1. Mechanisms Underlying H2 Blockers and NEC Development
4.3.2. Clinical Insights Regarding H2 Blockers and NEC
4.3.3. Strategies for Risk Mitigation and Exploration of Safer Alternatives
4.4. Doxapram
4.4.1. Mechanisms Underlying Doxapram and NEC Development
4.4.2. Clinical Insights Regarding Doxapram and NEC
4.4.3. Strategies for Risk Mitigation
4.5. Glycerin Enemas
4.5.1. Mechanisms Underlying Glycerin Enema Use and NEC Development
4.5.2. Clinical Insights Regarding Glycerin Enema Use and NEC
4.5.3. Strategies for Risk Mitigation and Exploration of Safer Alternatives
4.6. Antibiotics
4.6.1. Mechanisms Underlying Antibiotics and NEC Development
4.6.2. Clinical Insights Regarding Antibiotics and NEC
4.6.3. Strategies for Risk Mitigation
5. Clinical Dilemma Involving Other Treatments and the Risk of NEC
5.1. Blood Transfusions
5.1.1. Mechanisms Underlying Blood Transfusions and NEC
5.1.2. Clinical Evidence of Blood Transfusions and NEC
5.1.3. Strategies for Risk Mitigation
5.2. Probiotics
5.2.1. Mechanisms Underlying the Actions of Probiotics and NEC Prevention
5.2.2. Clinical Insights Regarding Probiotics and NEC Prevention
5.2.3. Probiotics and Adverse Effects
5.2.4. Balancing the Risks and Benefits
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.A.; Neu, J. Disorders of the gastrointestinal tract. In Avery & MacDonald’s Neonatology, 8th ed.; Boardman, J.P., Groves, A.M., Ramasethu, J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 546–563. [Google Scholar]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, W.; Xu, F.; Jinfeng Liao, J.; Li, J.; Mai, M.; Xie, H.; He, X.; Li, N. Application of abdominal ultrasonography in surgical necrotizing enterocolitis: A retrospective study. Front. Microbiol. 2023, 14, 1211846. [Google Scholar] [CrossRef] [PubMed]
- Alganabi, M.; Lee, C.; Bindi, E.; Li, B.; Pierro, A. Recent advances in understanding necrotizing enterocolitis. F1000Research 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging biomarkers for prediction and early diagnosis of necrotizing enterocolitis in the era of metabolomics and proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef] [PubMed]
- Gephart, S.M.; McGrath, J.M.; Effken, J.A.; Halpern, M.D. Necrotizing enterocolitis risk: State of the science. Adv. Neonatal Care 2012, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; McNamara, P.J. The ductus arteriosus: A refined approach! Semin. Perinatol. 2012, 36, 105–113. [Google Scholar] [CrossRef]
- Coombs, R.C.; Morgan, M.E.I.; Durbin, G.M.; Booth, I.W.; McNeish, A.S. Gut blood flow velocities in the newborn: Effects of patent ductus arteriosus and parenteral indomethacin. Arch. Dis. Child. 1990, 65, 1067–1071. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Grosfeld, J.L.; Chaet, M.; Molinari, F.; Engle, W.; Engum, S.A.; West, K.W.; Rescorla, F.J.; Scherer, L.R., 3rd. Increased risk of necrotizing enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann. Surg. 1996, 224, 350–355; discussion 355. [Google Scholar] [CrossRef]
- Fujii, A.M.; Brown, E.; Mirochnick, M.; O’Brien, S.; Kaufman, G. Neonatal necrotizing enterocolitis with intestinal perforation in extremely premature infants receiving early indomethacin treatment for patent ductus arteriosus. J. Perinatol. 2002, 22, 535–540. [Google Scholar] [CrossRef]
- Schmidt, B.; Davis, P.; Moddemann, D.; Ohlsson, A.; Roberts, R.S.; Saigal, S.; Solimano, A.; Vincer, M.; Wright, L.L. Trial of Indomethacin Prophylaxis in Preterm Investigators. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N. Engl. J. Med. 2001, 344, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, D.J.; Baetiong, A.; Adams, K.; Chen, A.; Smith, E.O.; Adams, J.M.; Weisman, L.E. Necrotizing enterocolitis and gastrointestinal complications after indomethacin therapy and surgical ligation in premature infants with patent ductus arteriosus. J. Perinatol. 2003, 23, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Dollberg, S.; Lusky, A.; Reichman, B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: A population-based study. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Cooke, L.; Steer, P.; Woodgate, P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst. Rev. 2003, 2003, CD003745. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Florez, I.D.; Tamayo, M.E.; Mbuagbaw, L.; Vanniyasingam, T.; Veroniki, A.A.; Zea, A.M.; Zhang, Y.; Sadeghirad, B.; Thabane, L. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: A systematic review and meta-analysis. JAMA 2018, 319, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Clyman, R.I. Recommendations for the postnatal use of indomethacin: An analysis of four separate treatment strategies. J. Pediatr. 1996, 128, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Parker, G.C.; Van Overmeire, B.; Aranda, J.V. A meta-analysis of ibuprofen versus indomethacin for closure of patent ductus arteriosus. Eur. J. Pediatr. 2005, 164, 135–140. [Google Scholar] [CrossRef]
- Ohlsson, A.; Walia, R.; Shah, S.S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. 2020, 2, CD003481. [Google Scholar] [CrossRef]
- Jasani, B.; Mitra, S.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2022, 12, CD010061. [Google Scholar] [CrossRef]
- Doyle, L.W. Postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Neonatology 2021, 118, 244–251. [Google Scholar] [CrossRef]
- Ng, P.C. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Arch. Dis. Child. 1993, 68, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, S.O.; Gordon, P.V.; Thomas, V.; Thorp, J.A.; Peabody, J.; Clark, R.H. Necrotizing enterocolitis among neonates in the United States. J. Perinatol. 2003, 23, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.V.; Marshall, D.D.; Stiles, A.D.; Price, W.A. The clinical, morphologic, and molecular changes in the ileum associated with early postnatal dexamethasone administration: From the baby’s bowel to the researcher’s bench. Mol. Genet. Metab. 2001, 72, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.R.; Morrison, J.C.; Poole, W.K.; Korones, S.B.; Boehm, J.J.; Rigatto, H.; Zachman, R.D. A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 1984, 73, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Z.; Lin, X.; Li, H.; Wen, C.; Bao, J.; He, Z. Gut microbiota mediated the therapeutic efficacies and the side effects of prednisone in the treatment of MRL/lpr mice. Arthritis Res. Ther. 2021, 23, 240. [Google Scholar] [CrossRef] [PubMed]
- Rentzhog, L.; Wikström, S. Corticosteroid therapy in regional small bowel ischaemia. Upsala J. Med. Sci. 1977, 82, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.V.; Price, W.A.; Stiles, A.D. Dexamethasone administration to newborn mice alters mucosal and muscular morphology in the ileum and modulates IGF-I localization. Pediatr. Res. 2001, 49, 93–100. [Google Scholar] [CrossRef][Green Version]
- Hackam, D.J.; Sodhi, C.P. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 229–238.e1. [Google Scholar] [CrossRef]
- Nakano, R. Combination therapy with indomethacin and dexamethasone increases toll-like receptor 4 expression in the intestinal tract in a platelet-activating factor-induced neonatal rat necrotizing enterocolitis model. J. Jpn. Soc. Premature Newborn Med. 2008, 20, 533. (In Japanese) [Google Scholar]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Early (<8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001146. [Google Scholar] [CrossRef]
- Doyle, L.W.; Cheong, J.L.; Ehrenkranz, R.A.; Halliday, H.L. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017, 10, CD001145. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, J.; Yu, Y.; Claud, E. Necrotizing enterocolitis intestinal barrier function protection by antenatal dexamethasone and surfactant-D in a rat model. Pediatr. Res. 2021, 90, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, M.; Chang, E.; Hansen, C.; Hunter, K.; Milcarek, B. Betamethasone dosing interval: 12 or 24 hours apart? A randomized, noninferiority open trial. Am. J. Obs. Gynecol. 2012, 206, 201.e1–201.e11. [Google Scholar] [CrossRef]
- Kamitsuka, M.D.; Horton, M.K.; Williams, M.A. The incidence of necrotizing enterocolitis after introducing standardized feeding schedules for infants between 1250 and 2500 grams and less than 35 weeks of gestation. Pediatrics 2000, 105, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Maury, L.; Lebail, F.; Ramful, D.; El Moussawi, F.; Nicaise, C.; Zupan-Simunek, V.; Coursol, A.; Beuchée, A.; Bolot, P.; et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet 2016, 387, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, A.L.; Ruuska, T.; Karikoski, R.; Laippala, P.; Ikonen, R.S.; Janas, M.; Mäki, M. A randomized, controlled study of prophylactic ranitidine in preventing stress-induced gastric mucosal lesions in neonatal intensive care unit patients. Crit. Care Med. 1997, 25, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Paquette, L.; Friedlich, P.; Ramanathan, R.; Seri, I. Concurrent use of indomethacin and dexamethasone increases the risk of spontaneous intestinal perforation in very low birth weight neonates. J. Perinatol. 2006, 26, 486–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guillet, R.; Stoll, B.J.; Cotton, C.M.; Gantz, M.; McDonald, S.; Poole, W.K.; Phelps, D.L.; National Institute of Child Health and Human Development Neonatal Research Network. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2006, 117, e137–e142. [Google Scholar] [CrossRef]
- Giannella, R.A.; Broitman, S.A.; Zamcheck, N. Gastric acid barrier to ingested microorganisms in man: Studies in vivo and in vitro. Gut 1972, 13, 251–256. [Google Scholar] [CrossRef]
- Malcolm, W.F.; Cotten, C.M. Metoclopramide, H2 blockers, and proton pump inhibitors: Pharmacotherapy for gastroesophageal reflux in neonates. Clin. Perinatol. 2012, 39, 99–109. [Google Scholar] [CrossRef]
- Parkman, H.P.; Urbain, J.L.; Knight, L.C.; Brown, K.L.; Trate, D.M.; Miller, M.A.; Maurer, A.H.; Fisher, R.S. Effect of gastric acid suppressants on human gastric motility. Gut 1998, 42, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Passariello, A.; De Curtis, M.; Manguso, F.; Salvia, G.; Lega, L.; Messina, F.; Paludetto, R.; Canani, R.B. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 2012, 129, e40–e45. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.; Freire, M.S.; Ruth, N.S.; Santana, R.N.S.; Martins-Filho, P.R.S.; Cuevas, L.E.; Gurgel, R.Q. Association between histamine-2 receptor antagonists and adverse outcomes in neonates: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0214135. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dhayade, A.; Mohamed, A.L.; Chaudhari, T.V. Morbidity and mortality in preterm infants following antacid use: A retrospective audit. Int. J. Pediatr. 2016, 2016, 9649162. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.N.S.; Santos, V.S.; Ribeiro-Júnior, R.F.; Freire, M.S.; Menezes, M.A.S.; Cipolotti, R.; Gurgel, R.Q. Use of ranitidine is associated with infections in newborns hospitalized in a neonatal intensive care unit: A cohort study. BMC Infect. Dis. 2017, 17, 375. [Google Scholar] [CrossRef][Green Version]
- Rachmilewitz, D. The role of H2-receptor antagonists in the prevention of NSAID-induced gastrointestinal damage. Aliment. Pharmacol. Ther. 1988, 2 (Suppl. 1), 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.J.; Chatfield, S.L.; Brownlee, K.G.; Ng, C.; Newell, S.J.; Dear, P.R.; Primrose, J.N. The effect of intravenous ranitidine on the intragastric pH of preterm infants receiving dexamethasone. Arch. Dis. Child. 1993, 69, 37–39. [Google Scholar] [CrossRef]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 2015, 314, 595–603. [Google Scholar] [CrossRef]
- Yost, C.S. A new look at the respiratory stimulant doxapram. CNS Drug Rev. 2006, 12, 236–249. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef]
- Kumita, H.; Shinohara, M.; Yamazaki, T.; Ariyoshi, C. Doxapram therapy in idiopathic apnea of prematurity. Part 1. Its effectiveness and side effects. Acta Neonat. Jpn. 1987, 23, 458–463. (In Japanese) [Google Scholar]
- Maruyama, K.; Koizumi, T.; Miyazaki, M.; Harigaya, A.; Takei, K. Doxapram and necrotizing enterocolitis. Acta Neonat. Jpn. 1992, 28, 434–438. (In Japanese) [Google Scholar]
- Kuga, T.; Naito, J. Pharmacological studies of doxapram. Folia Pharmacol. Japón 1974, 70, 165–174. (In Japanese) [Google Scholar] [CrossRef]
- Ogawa, R.; Sato, T.; Imai, T.; Fujita, T. Effects of doxapram hydrochloride on hemodynamics—An experimental study. Jpn. J. Anesthesiol. 1974, 23, 317–320. (In Japanese) [Google Scholar] [CrossRef]
- Beaudry, M.A.; Bradley, J.M.; Gramlich, L.M.; Legatt, D. Pharmaco-kinetics of doxapram in idiopathic apnea of prematurity. Dev. Pharmacol. Ther. 1988, 11, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Kajiwara, M.; Itahashi, K.; Fujimura, M. Low-dose doxapram therapy for idiopathic apnea of prematurity. Pediatr. Int. 2001, 43, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakajima, T.; Terashi, E.; Yasaka, T.; Kitajima, J.; Matsumoto, N.; Hidaka, Y.; Nakamura, A.; Hirose, R.; Arima, T.; et al. A case of necrotizing enterocolitis with suspected low-dose doxapram involvement. J. Jpn. Pediatr. Soc. 2010, 114, 1104. (In Japanese) [Google Scholar]
- Barbeé, F.; Hansen, C.; Badonnel, Y.; Legagneur, H.; Vert, P.; Boutroy, M.J. Severe side effects and drug plasma concentrations in preterm infants treated with doxapram. Ther. Drug Monit. 1999, 21, 547–552. [Google Scholar] [CrossRef]
- Kumita, H.; Mizuno, S.; Shinohara, M.; Ichikawa, T.; Yamazaki, T. Low-dose doxapram therapy in premature infants and its CSF and serum concentrations. Acta Paediatr. Scand. 1991, 80, 786–791. [Google Scholar] [CrossRef]
- Barrington, K.J.; Finer, N.N.; Torok-Both, G.; Jamali, F.; Coutts, R.T. Dose-response relationship of doxapram in the therapy for refractory idiopathic apnea of prematurity. Pediatrics 1987, 80, 22–27. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kim, H.S.; Kim, D.H.; Kim, E.K.; Son, D.W.; Kim, B.I.; Choi, J.H. Induction of early meconium evacuation promotes feeding tolerance in very low birth weight infants. Neonatology 2007, 92, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.H.; Shawyer, A.C.; Rosenbaum, P.L.; Williams, C.; Jones, S.A.; Walton, J.M. Glycerin enemas and suppositories in premature infants: A meta-analysis. Pediatrics 2015, 135, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Anabrees, J.; Shah, V.S.; AlOsaimi, A.; AlFaleh, K. Glycerin laxatives for prevention or treatment of feeding intolerance in very low birth weight infants. Cochrane Database Syst. Rev. 2015, 2015, CD010464. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Hummler, H.; Haase, B.; Quante, M.; Wiechers, C.; Poets, C.F. Interventions for promoting meconium passage in very preterm infants—A survey of current practice at tertiary neonatal centers in Germany. Children 2022, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Burchard, P.R.; Lay, R.; Ruffolo, L.I.; Ramazani, S.N.; Walton, J.M.; Livingston, M.H. Glycerin suppositories and enemas in premature infants: A meta-analysis. Pediatrics 2022, 149, e2021053413. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Balasubramanian, H.; Patole, S. Meconium evacuation for facilitating feed tolerance in preterm neonates: A systematic review and meta-analysis. Neonatology 2016, 110, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sáenz de Pipaón Marcos, M.; Teresa Montes Bueno, M.; Sanjosé, B.; Gil, M.; Parada, I.; Amo, P. Randomized controlled trial of prophylactic rectal stimulation and enemas on stooling patterns in extremely low birth weight infants. J. Perinatol. 2013, 33, 858–860. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, T.; Li Wei, C.; Bautista, D.; Sriram, B.; Xiangzhen Fay, L.; Rajadurai, V.S. Saline enemas versus glycerin suppositories to promote enteral feeding in premature infants: A pilot randomized controlled trial. Neonatology 2017, 112, 347–353. [Google Scholar] [CrossRef]
- Shi, J.; Hu, Y.; Gong, X.; Qiu, G.; Li, N.; Chen, Y. Warm saline enema and probiotics to promote feeding tolerance in preterm infants—A preliminary study. Int. J. Clin. Exp. Med. 2019, 12, 4266–4272. [Google Scholar]
- Mukhopadhyay, S.; Sengupta, S.; Puopolo, K.M. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F327–F332. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Fuglsang, E.; Jiang, P.; Birck, M.M.; Pan, X.; Kamal, S.B.S.; Pors, S.E.; Gammelgaard, P.L.; Nielsen, D.S.; Thymann, T.; et al. Oral antibiotics increase blood neutrophil maturation and reduce bacteremia and necrotizing enterocolitis in the immediate postnatal period of preterm pigs. Innate Immun. 2016, 22, 51–62. [Google Scholar] [CrossRef]
- Jiang, P.; Jensen, M.L.; Cilieborg, M.S.; Thymann, T.; Wan, J.M.; Sit, W.H.; Tipoe, G.L.; Sangild, P.T. Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates. PLoS ONE 2012, 7, e44929. [Google Scholar] [CrossRef]
- Li, Y.; Shen, R.L.; Ayede, A.I.; Berrington, J.; Bloomfield, F.H.; Busari, O.O.; Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; Greisen, G.; et al. Early use of antibiotics is associated with a lower incidence of necrotizing enterocolitis in preterm, very low birth weight infants: The NEOMUNE-NeoNutriNet Cohort Study. J. Pediatr. 2020, 227, 128–134. [Google Scholar] [CrossRef]
- Raba, A.A.; O’Sullivan, A.; Miletin, J. Pathogenesis of necrotising enterocolitis: The impact of the altered gut microbiota and antibiotic exposure in preterm infants. Acta Paediatr. 2021, 110, 433–440. [Google Scholar] [CrossRef]
- Dierikx, T.H.; Deianova, N.; Groen, J.; Vijlbrief, D.C.; Hulzebos, C.; de Boode, W.P.; D’haens, E.J.; Cossey, V.; Kramer, B.W.; van Weissenbruch, M.M.; et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: A multicenter cohort study. Eur. J. Pediatr. 2022, 181, 3715–3724. [Google Scholar] [CrossRef]
- Fjalstad, J.W.; Esaiassen, E.; Juvet, L.K.; van den Anker, J.N.; Klingenberg, C. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: A systematic review. J. Antimicrob. Chemother. 2018, 73, 569–580. [Google Scholar] [CrossRef]
- Klingenberg, C.; Kornelisse, R.F.; Buonocore, G.; Maier, R.F.; Stocker, M. Culture-negative early-onset neonatal sepsis—At the crossroad between efficient sepsis care and antimicrobial stewardship. Front. Pediatr. 2018, 6, 285. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, H.; Yuan, L.; Wang, L.; Deng, F. Prolonged antibiotic therapy increased necrotizing enterocolitis in very low birth weight infants without culture-proven sepsis. Front. Pediatr. 2022, 10, 949830. [Google Scholar] [CrossRef]
- Esmaeilizand, R.; Shah, P.S.; Seshia, M.; Yee, W.; Yoon, E.W.; Dow, K.; Canadian Neonatal Network Investigators. Antibiotic exposure and development of necrotizing enterocolitis in very preterm neonates. Paediatr. Child. Health 2018, 23, e56–e61. [Google Scholar] [CrossRef]
- Cotten, C.M.; Taylor, S.; Stoll, B.; Goldberg, R.N.; Hansen, N.I.; Sánchez, P.J.; Ambalavanan, N.; Benjamin, D.K., Jr.; NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009, 123, 58–66. [Google Scholar] [CrossRef]
- Rina, P.; Zeng, Y.; Ying, J.; Qu, Y.; Mu, D. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur. J. Pediatr. 2020, 179, 1047–1056. [Google Scholar] [CrossRef]
- Cionci, N.B.; Lucaccioni, L.; Pietrella, E.; Ficara, M.; Spada, C.; Torelli, P.; Bedetti, L.; Lugli, L.; Di Gioia, D.; Berardi, A. Antibiotic exposure, common morbidities and main intestinal microbial groups in very preterm neonates: A pilot study. Antibiotics 2022, 11, 237. [Google Scholar] [CrossRef]
- Greenberg, R.G.; Chowdhury, D.; Hansen, N.I.; Smith, P.B.; Stoll, B.J.; Sánchez, P.J.; Das, A.; Puopolo, K.M.; Mukhopadhyay, S.; Higgins, R.D.; et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr. Res. 2019, 85, 994–1000. [Google Scholar] [CrossRef]
- Strauss, R.G. Practical issues in neonatal transfusion practice. Am. J. Clin. Pathol. 1997, 107 (Suppl. 1), S57–S63. [Google Scholar]
- Stritzke, A.I.; Smyth, J.; Synnes, A.; Lee, S.K.; Shah, P.S. Transfusion-associated necrotising enterocolitis in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F10–F14. [Google Scholar] [CrossRef]
- Khashu, M.; Dame, C.; Lavoie, P.M.; De Plaen, I.G.; Garg, P.M.; Sampath, V.; Malhotra, A.; Caplan, M.D.; Kumar, P.; Agrawal, P.B.; et al. Current understanding of transfusion-associated necrotizing enterocolitis: Review of clinical and experimental studies and a call for more definitive evidence. Newborn 2022, 1, 201–208. [Google Scholar] [CrossRef]
- Elabiad, M.T.; Harsono, M.; Talati, A.J.; Dhanireddy, R. Effect of birth weight on the association between necrotising enterocolitis and red blood cell transfusions in ≤ 1500 g infants. BMJ Open 2013, 3, e003823. [Google Scholar] [CrossRef]
- Blau, J.; Calo, J.M.; Dozor, D.; Sutton, M.; Alpan, G.; La Gamma, E.F. Transfusion-related acute gut injury: Necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J. Pediatr. 2011, 158, 403–409. [Google Scholar] [CrossRef]
- Christensen, R.D.; Lambert, D.K.; Henry, E.; Wiedmeier, S.E.; Snow, G.L.; Baer, V.L.; Gerday, E.; Ilstrup, S.; Pysher, T.J. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion 2010, 50, 1106–1112. [Google Scholar] [CrossRef]
- Patel, R.M.; Knezevic, A.; Shenvi, N.; Hinkes, M.; Keene, S.; Roback, J.D.; Easley, K.A.; Josephson, C.D. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA 2016, 315, 889–897. [Google Scholar] [CrossRef]
- Singh, R.; Visintainer, P.F.; Frantz, I.D., 3rd; Shah, B.L.; Meyer, K.M.; Favila, S.A.; Thomas, M.S.; Kent, D.M. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J. Perinatol. 2011, 31, 176–182. [Google Scholar] [CrossRef]
- Patel, R.M.; Underwood, M.A. Probiotics and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 39–46. [Google Scholar] [CrossRef]
- MohanKumar, K.; Namachivayam, K.; Song, T.; Cha, B.J.; Slate, A.; Hendrickson, J.E.; Pan, H.; Wickline, S.A.; Oh, J.Y.; Patel, R.P.; et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 2019, 10, 3494. [Google Scholar] [CrossRef]
- Gephart, S.M. Transfusion-associated necrotizing enterocolitis: Evidence and uncertainty. Adv. Neonatal Care 2012, 12, 232–236. [Google Scholar] [CrossRef]
- Mohamed, A.; Shah, P.S. Transfusion associated necrotizing enterocolitis: A meta-analysis of observational data. Pediatrics 2012, 129, 529–540. [Google Scholar] [CrossRef]
- Kirpalani, H.; Zupancic, J.A. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin. Perinatol. 2012, 36, 269–276. [Google Scholar] [CrossRef]
- Harsono, M.; Talati, A.; Dhanireddy, R.; Elabiad, M.T. Are packed red blood cell transfusions protective against late onset necrotizing enterocolitis in very low birth weight infants? E-PAS 2011, 2011, 509. [Google Scholar]
- Rai, S.E.; Sidhu, A.K.; Krishnan, R.J. Transfusion-associated necrotizing enterocolitis re-evaluated: A systematic review and meta-analysis. J. Perinat. Med. 2018, 46, 665–676. [Google Scholar] [CrossRef]
- Jasani, B.; Rao, S.; Patole, S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: A systematic review. Adv. Nutr. 2017, 8, 764–769. [Google Scholar] [CrossRef]
- Yeo, K.T.; Kong, J.Y.; Sasi, A.; Tan, K.; Lai, N.M.; Schindler, T. Stopping enteral feeds for prevention of transfusion-associated necrotising enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2019, 2019, CD012888. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Yeo, K.T.; Bolisetty, S.; Michalowski, J.; Tan, A.H.K.; Lui, K. FEEding DURing red cell transfusion (FEEDUR RCT): A multi-arm randomised controlled trial. BMC Pediatr. 2020, 20, 346. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Stenger, M.R.; Reber, K.M.; Giannone, P.J.; Nankervis, C.A. Probiotics and prebiotics for the prevention of necrotizing enterocolitis. Curr. Infect. Dis. Rep. 2011, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Arciero, J.C.; Ermentrout, G.B.; Upperman, J.S.; Vodovotz, Y.; Rubin, J.E. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PLoS ONE 2010, 5, e10066. [Google Scholar] [CrossRef]
- Dermyshi, E.; Wang, Y.; Yan, C.; Hong, W.; Qiu, G.; Gong, X.; Zhang, T. The “golden age” of probiotics: A systematic review and meta-analysis of randomized and observational studies in preterm infants. Neonatology 2017, 112, 9–23. [Google Scholar] [CrossRef]
- Sato, R.; Malai, S.; Razmjouy, B. Necrotizing enterocolitis reduction using an exclusive human-milk diet and probiotic supplementation in infants with 1000–1499 gram birth weight. Nutr. Clin. Pract. 2020, 35, 331–334. [Google Scholar] [CrossRef]
- Sharpe, J.; Way, M.; Koorts, P.J.; Davies, M.W. The availability of probiotics and donor human milk is associated with improved survival in very preterm infants. World J. Pediatr. 2018, 14, 492–497. [Google Scholar] [CrossRef]
- Juber, B.A.; Boly, T.J.; Pitcher, G.J.; McElroy, S.J. Routine administration of a multispecies probiotic containing bifidobacterium and lactobacillus to very low birth weight infants had no significant impact on the incidence of necrotizing enterocolitis. Front. Pediatr. 2021, 9, 757299. [Google Scholar] [CrossRef]
- Li, D.; Rosito, G.; Slagle, T. Probiotics for the prevention of necrotizing enterocolitis in neonates: An 8-year retrospective cohort study. J. Clin. Pharm. Ther. 2013, 38, 445–449. [Google Scholar] [CrossRef]
- Cilieborg, M.S.; Thymann, T.; Siggers, R.; Boye, M.; Bering, S.B.; Jensen, B.B.; Sangild, P.T. The incidence of necrotizing enterocolitis is increased following probiotic administration to preterm pigs. J. Nutr. 2011, 141, 223–230. [Google Scholar] [CrossRef]
- Kunz, A.N.; Noel, J.M.; Fairchok, M.P. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 457–458. [Google Scholar] [CrossRef]
- Chiang, M.C.; Chen, C.L.; Feng, Y.; Chen, C.C.; Lien, R.; Chiu, C.H. Lactobacillus rhamnosus sepsis associated with probiotic therapy in an extremely preterm infant: Pathogenesis and a review for clinicians. J. Microbiol. Immunol. Infect. 2021, 54, 575–580. [Google Scholar] [CrossRef]
- Dani, C.; Coviello, C.C.; Corsini, I.I.; Arena, F.; Antonelli, A.; Rossolini, G.M. Lactobacillus sepsis and probiotic therapy in newborns: Two new cases and literature review. AJP Rep. 2016, 6, e25–e29. [Google Scholar] [CrossRef]
- Ohishi, A.; Takahashi, S.; Ito, Y.; Ohishi, Y.; Tsukamoto, K.; Nanba, Y.; Ito, N.; Kakiuchi, S.; Saitoh, A.; Morotomi, M.; et al. Bifidobacterium septicemia associated with postoperative probiotic therapy in a neonate with omphalocele. J. Pediatr. 2010, 156, 679–681. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fondén, R.; Mättö, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]
- Poindexter, B. Use of probiotics in preterm infants. Pediatrics 2021, 147, e2021051485. [Google Scholar] [CrossRef]
| Main Risk Factors |
| Prematurity |
| Low birth weight |
| Formula feeding |
| Intestinal dysbiosis |
| Maternal Factors |
| Increased body mass index |
| Intrahepatic cholestasis |
| Smoking |
| Cocaine use |
| Chorioamnionitis |
| Placenta abruption |
| Preeclampsia |
| Antenatal antibiotic use |
| Prolonged rupture of membranes |
| Cesarean delivery |
| Fetal factors |
| Genetic predisposition |
| Intrauterine growth restriction |
| Non-reassuring fetal state |
| Lack of antenatal steroids |
| Neonatal factors |
| Hypoxia |
| Congenital heart disease |
| Gastrointestinal anomaly |
| Patent ductus arteriosus |
| Anemia |
| Polycythemia |
| Treatment administered to very low-birth-weight infants |
| Medications |
| Indomethacin |
| Corticosteroids |
| Histamine-2 receptor blockers |
| Doxapram |
| Glycerin enema |
| Antibiotics |
| Blood transfusions including exchange transfusion |
| Umbilical catheterization |
| Treatment | Indication | Clinical Benefits | Potential Risks of NEC |
|---|---|---|---|
| Indomethacin | To prevent or treat symptomatic PDA | Closes the PDA | Harmful effects on blood flow to the intestines and reduces intestinal perfusion |
| Corticosteroids | To treat respiratory distress in BPD | Improves lung function and reduces BPD severity | Harmful effects on intestinal immune defense, motility, circulation, and barrier function |
| Histamine-2 receptor blockers | Gastric bleeding (e.g., bloody nasogastric tube aspirates) | Protects the delicate gastrointestinal mucosa by decreasing gastric acid secretion | Harmful effects on gastrointestinal tract host defense caused by increased gastric pH |
| Doxapram | Persistent apnea unresponsive to methylxanthines | Stimulates chemoreceptors, enhances ventilation and oxygenation | Gastrointestinal disturbance caused by gastric acid hypersecretion, intestinal smooth muscle contraction, and intestinal blood flow change |
| Glycerin enema | To promote meconium evacuation and accelerate stool passage | Reduces the risk of meconium-related complications and facilitates bowel movements | Damages bowel epithelial cells, influences the composition of the gut microbiota, and changes gut motility and intestinal blood flow |
| Antibiotics | To prevent and treat bacterial infections | Controls bacterial infections | Overuse or misuse can lead to drug resistance and disrupt the gut microbiome |
| Blood transfusion | Anemia or other blood-related conditions | Corrects anemia and improves the oxygen-carrying capacity | Changes gut perfusion and the immune response |
| Probiotics | To improve the gut microbiome | Reduces the risk of NEC by promoting healthy gut flora and enhancing the gut barrier function | Cause infections, particularly in critically ill or immunocompromised infants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iijima, S. Clinical Dilemma Involving Treatments for Very Low-Birth-Weight Infants and the Potential Risk of Necrotizing Enterocolitis: A Narrative Literature Review. J. Clin. Med. 2024, 13, 62. https://doi.org/10.3390/jcm13010062
Iijima S. Clinical Dilemma Involving Treatments for Very Low-Birth-Weight Infants and the Potential Risk of Necrotizing Enterocolitis: A Narrative Literature Review. Journal of Clinical Medicine. 2024; 13(1):62. https://doi.org/10.3390/jcm13010062
Chicago/Turabian StyleIijima, Shigeo. 2024. "Clinical Dilemma Involving Treatments for Very Low-Birth-Weight Infants and the Potential Risk of Necrotizing Enterocolitis: A Narrative Literature Review" Journal of Clinical Medicine 13, no. 1: 62. https://doi.org/10.3390/jcm13010062
APA StyleIijima, S. (2024). Clinical Dilemma Involving Treatments for Very Low-Birth-Weight Infants and the Potential Risk of Necrotizing Enterocolitis: A Narrative Literature Review. Journal of Clinical Medicine, 13(1), 62. https://doi.org/10.3390/jcm13010062





