Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets
Abstract
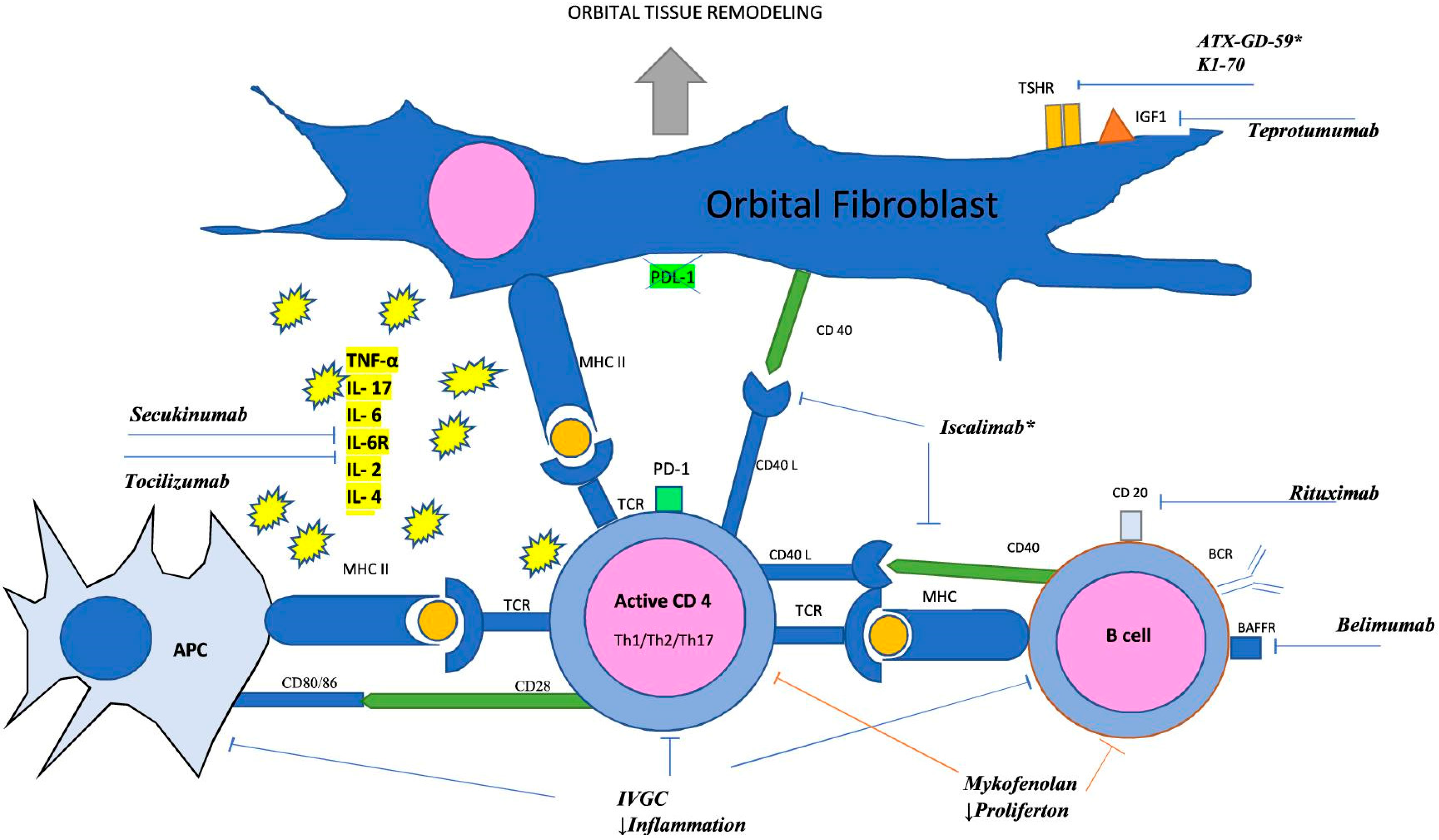
:1. Introduction
2. Pathogenesis
2.1. T Lymphocytes
2.2. Cytokines and Chemokines
- IL-17A
- RANTES and IL-16
2.3. Fibroblasts
2.4. TSHR
2.5. Insulin-like Growth Factor 1 Receptor (IGF-1R)
2.6. B Lymphocytes
2.7. CD40/CD154
2.8. BAFF
3. Potential and Current Drug Targets
3.1. Glucocorticoids (GCs)
3.2. Mycophenolate Mofetil (MMF)
3.3. Therapies Targeting Immune Checkpoints (ICPs)
3.4. Rituximab (RTX)
3.5. Belimumab
3.6. Iscalimab
3.7. Secukinumab
3.8. Tocilizumab
3.9. K1-70 Targeting of the Pathophysiology of Major GD and TED Antigens
3.10. Teprotumumab (TPT)
3.11. ATX-GD-59 Restoration of Tolerance
| Drug | Mechanism of Action | Study Population | Design | Intervention | Main Findings |
|---|---|---|---|---|---|
| Mycophenolate mofetil (MMF) [88] | Inhibition of IMPDH2 | 164 patients with active moderate-to-severe Graves’ orbitopathy | Multicenter RCT | MMF with ivMP vs. ivMP alone | No significant difference in the rate of response at 12 weeks or rate of relapse at 24 and 36 weeks. The combination of MMF with ivMP improved the response rate by 24 weeks in patients with active and moderate-to-severe Graves’ orbitopathy. |
| Rituximab (RTX) [97] | Anti-CD20 MAb | 32 patients with active moderate-to-severe TED | Single-center RCT | RTX vs. ivMP | RTX was more effective after 16, 20, and 24 weeks. Patients receiving RTX achieved improved motility after 52 weeks. There was no disease reactivation in the RTX group. |
| Rituximab (RTX) [98] | Anti-CD20 MAb | 25 patients with active moderate-to-severe TED | Single-center RCT | RTX vs. placebo | There were no differences between the RTX and placebo groups in the proportion of patients showing CAS improvement after 24 weeks or in the decrease in CAS from baseline to 24 or 52 weeks. |
| Belimumab (BMB) [100] | Anti-BAFF MAb | 27 patients with active, moderate-to-severe TED | Single-center RCT | BMB vs. ivMP | After 12 weeks, patients treated with ivMP had significantly lower CAS than patients treated with BMB. CAS decreased significantly in both groups after 24 weeks. Proptosis improved in all patients (p < 0.05), but improvement was greater in ivMP group than in patients treated with BMB (p < 0.04) |
| Secukinumab [NCT04737330] | Anti-IL-17A MAb | 28 patients with active, moderate-to-severe TED | Multicenter RCT | Secukinumab vs. placebo | Analysis of blinded patient data showed a very low probability of the study meeting the primary efficacy endpoints. |
| Tocilizumab (TCZ) [109] | Anti-IL-6R MAB | 32 patients with active, moderate-to-severe corticosteroid-resistant TED | Multicenter RCT | TCZ vs. placebo | 93.3% of the patients receiving tocilizumab had a change of at least 2 in the CAS from baseline to week 16 of vs. 58.8% receiving a placebo. Tocilizumab was more effective in improving the EUGOGO-proposed composite ophthalmic outcome at week 16 (p = 0.03) and in reducing the size of exophthalmos from baseline to week 16 (p = 0.01). |
| Tocilizumab (TCZ) [113] | Anti-IL-6R MAB | A total of 12 trials with 448 patients were included | Meta-analysis | TCZ was most likely to be the best treatment in terms of response and proptosis reduction, followed by TMB and RTX. TMB was most likely to be the best treatment in terms of improving diplopia, followed by TCZ and RTX. TCZ had the best safety profile, followed by RTX and TMB. | |
| Teprotumumab (TPT) [116,117] | Anti-IGF-1R MAb | Phase II: 88 patients with active moderate-to-severe TED Phase III: 83 patients with active moderate-to-severe TED | Two multicenter RCTs | TPT vs. placebo | Taking both phases into account, 73% of patients in the TPT groups (vs. 14% in the placebo groups) responded, with improvements both in CAS and exophthalmos. Improvements in proptosis of ≥2 mm occurred in 77% of patients in the TPT-treated groups and in 15% of patients in the placebo group at week 24. |
| Teprotumumab (TPT) [NCT04583735] | Anti-IGF-1R MAb | Phase IV: 62 patients who lived with TED from 2 to 10 years prior to the study and had low CAS | Multicenter RCT | TPT vs. placebo | TPT treatment was associated with a reduction in proptosis of 2.41 mm from baseline compared with 0.92 mm in those receiving placebo (p = 0.0004). In addition, 62% of teprotumumab-treated patients experienced clinically significant improvement at week 24 compared with 25% of placebo-treated patients (p = 0.0134). |
| K1-70 [114] | The TSHR antagonist | Phase I: 18 patients with GD, stable on anti-thyroid drug | Open label study | Single intramuscular or intravenous dose of K1-70 | There were clinically significant improvements in symptoms of both GD (reduced tremor, improved sleep, improved mental focus, reduced toilet urgency) and TED (reduced exophthalmos measurements, reduced photosensitivity). |
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahaly, G.J.; Bartalena, L.; Hegedüs, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid. J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.F.; Andersen, S.; Latif, R.; Nagayama, Y.; Barbesino, G.; Brito, M.; Eckstein, A.K.; Stagnaro-Green, A.; Kahaly, G.J. Graves’ disease. Nat. Rev. Dis. Primers. 2020, 6, 52. [Google Scholar] [CrossRef]
- Lacheta, D.; Miskiewicz, P.; Gluszko, A.; Nowicka, G.; Struga, M.; Kantor, I.; Poslednik, K.B.; Mirza, S. SzczepanskizczepanskiImmunological Aspects of Graves’ Ophthalmopathy. Biomed. Res. Int. 2019, 2019, 7453260. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; Ayvaz, G.; et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.H.; Kahaly, G.J. Pathophysiology of thyroid-associated orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y. Immunohistochemistry Study in Human Orbital Tissue of Thyroid Eye Disease. Dissertation zur Erlangung des Grades. Ph.D. Thesis, Im Promotionsfach Pharmazie am Fachbereich Chemie, Pharmazie, Geographie und Geowissenschaften der Johannes Gutenberg-Universität Mainz, Mainz, Germany, 2022. [Google Scholar]
- Kahaly, G.J. Management of Graves Thyroidal and Extrathyroidal Disease: An Update. J. Clin. Endocrinol. Metab. 2020, 105, 3704–3720. [Google Scholar] [CrossRef]
- Hai, Y.P.; Lee, A.C.H.; Frommer, L.; Diana, T.; Kahaly, G.J. Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J. Endocrinol. Investig. 2020, 43, 123–137. [Google Scholar] [CrossRef]
- Cui, X.; Wang, F.; Liu, C. A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves’ orbitopathy. Front. Immunol. 2023, 14, 1062045. [Google Scholar] [CrossRef]
- Taylor, P.N.; Zhang, L.; Lee, R.W.J.; Muller, I.; Ezra, D.G.; Dayan, C.M.; Kahaly, G.J.; Ludgate, M. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 2020, 16, 104–116. [Google Scholar] [CrossRef]
- Salvi, M.; Berchner-Pfannschmidt, U.; Ludgate, M. Pathogenesis; Wiersinga, W.M., Kahaly, G.J., Eds.; Graves’ Orbitopathy: A Multidisciplinary Approach–Questions and Answers; Karger: Basel, Switzerland, 2017; pp. 41–60. [Google Scholar]
- Huang, Y.; Fang, S.; Li, D.; Zhou, H.; Li, B.; Fan, X. The involvement of T cell pathogenesis in thyroid-associated ophthalmopathy. Eye 2019, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Duan, H.; You, S.; Liang, B.; Chen, Y.; Huang, H. Research progress on the pathogenesis of Graves’ ophthalmopathy: Based on immunity, noncoding RNA and exosomes. Front. Immunol. 2022, 13, 952954. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Gallo, D.M.; Tanda, M.L.; Kahaly, G.J. Thyroid Eye Disease: Epidemiology, Natural History, and Risk Factors. Ophthalmic Plast. Reconstr. Surg. 2023, 39, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Lu, Y.; Huang, Y.; Zhou, H.; Fan, X. Mechanisms That Underly T Cell Immunity in Graves’ Orbitopathy. Front. Endocrinol. 2021, 12, 648732. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Z.; Li, W.; Ge, Y.; Xie, M.; Lv, M.; Fan, Y.; Chen, Z.; Zhao, D.; Han, Y. Th1, Th2, and Th17 Cytokine Involvement in Thyroid Associated Ophthalmopathy. Dis. Markers. 2015, 2015, 609593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jeremiah Bell, J.; Bhandoola, A. T-cell lineage determination. Immunol. Rev. 2010, 238, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4–CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef]
- Iwahori, K. Cytotoxic CD8(+) Lymphocytes in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 53–62. [Google Scholar]
- Kaech, S.M.; Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012, 12, 749–761. [Google Scholar] [CrossRef]
- Hai, Y.-P.; Saeed, M.E.; Ponto, K.A.; Elflein, H.M.; Lee, A.C.H.; Fang, S.; Zhou, H.; Frommer, L.; Längericht, J.; Efferth, T.; et al. A Multicenter, Single-Blind, Case-Control, Immunohistochemical Study of Orbital Tissue in Thyroid Eye Disease. Thyroid 2022, 32, 1547–1558. [Google Scholar] [CrossRef]
- Dottore, G.R.; Torregrossa, L.; Caturegli, P.; Ionni, I.; Sframeli, A.; Sabini, E.; Menconi, F.; Piaggi, P.; Sellari-Franceschini, S.; Nardi, M.; et al. Association of T and B Cells Infiltrating Orbital Tissues with Clinical Features of Graves Orbitopathy. JAMA Ophthalmol 2018, 136, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.M.; Feldon, S.E.; Smith, T.J.; Phipps, R.P.; Han, Y.E.; Hwang, S.; Kim, J.H.; Byun, J.W.; Yoon, J.S.; Lee, E.J.; et al. Immune Mechanisms in Thyroid Eye Disease. Thyroid 2008, 18, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, K. Immunologia. 8 Immunoltolerancja. 12 Immunofarmakologia-Immunosupresja i Immunopotencjacja; Edra Urban & Partner: Wroclaw, Poland, 2017. [Google Scholar]
- Gołąb, J.; Lasek, W.; Jakóbisiak MStokłosa, T. 5 Prezentacja Antygenów Limfocytom. 6. Aktywacja Limfocytów; PWN: Warszawa, Poland, 2017. [Google Scholar]
- Read, K.A.; Powell, M.D.; Sreekumar, B.K.; Oestreich, K.J. In Vitro Differentiation of Effector CD4(+) T Helper Cell Subsets. Methods Mol. Biol. 2019, 1960, 75–84. [Google Scholar] [PubMed]
- SL, R. Development in motion: Helper T cells at work. Cell 2007, 129, 33–36. [Google Scholar]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional diversity of helper T lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef]
- Zhang, Q.; Vignali, D.A. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity 2016, 44, 1034–1051. [Google Scholar] [CrossRef]
- Park, Y.-J.; Kuen, D.-S.; Chung, Y. Future prospects of immune checkpoint blockade in cancer: From response prediction to overcoming resistance. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-Novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Ford, M.L.; Adams, A.B.; Pearson, T.C. Targeting co-stimulatory pathways: Transplantation and autoimmunity. Nat. Rev. Nephrol. 2014, 10, 14–24. [Google Scholar] [CrossRef]
- Zhai, Y.; Moosavi, R.; Chen, M. Immune Checkpoints, a Novel Class of Therapeutic Targets for Autoimmune Diseases. Front. Immunol. 2021, 12, 645699. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef] [PubMed]
- Daroszewski, J.; Pawlak, E.; Karabon, L.; Frydecka, I.; Jonkisz, A.; Slowik, M.; Bolanowski, M. Soluble CTLA-4 receptor an immunological marker of Graves’ disease and severity of ophthalmopathy is associated with CTLA-4 Jo31 and CT60 gene polymorphisms. Eur. J. Endocrinol. 2009, 161, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, P.; Wawrusiewicz-Kurylonek, N.; Eckstein, A.; Reszec, J.; Luczynski, W.; Johnson, K.; Kretowski, A.; Bakunowicz-Lazarczyk, A.; Gorska, M.; Szamatowicz, J.; et al. Disturbances of Modulating Molecules (FOXP3, CTLA-4/CD28/B7, and CD40/CD40L) mRNA Expressions in the Orbital Tissue from Patients with Severe Graves’ Ophthalmopathy. Mediat. Inflamm. 2015, 2015, 340934. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, M.; Zarganes-Tzitzikas, T.; Magiera-Mularz, K.; Holak, T.A.; Dömling, A. Immune Checkpoint PD-1/PD-L1: Is There Life Beyond Antibodies? Angew. Chem. Int. Ed. 2018, 57, 4840–4848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Liu, M.; Gong, Q.; Shi, A.; Li, X.; Bai, X.; Guan, X.; Hao, B.; Liu, F.; et al. PD-L1 Inhibits T Cell-Induced Cytokines and Hyaluronan Expression via the CD40-CD40L Pathway in Orbital Fibroblasts From Patients With Thyroid Associated Ophthalmopathy. Front. Immunol. 2022, 13, 849480. [Google Scholar] [CrossRef]
- Khamisi, S.; Karlsson, F.A.; Ljunggren, Ö.; Thulin, M.; Larsson, A. Increased plasma levels of soluble programmed death ligand 1 (sPD-L1) and fibroblast growth factor 23 (FGF-23) in patients with Graves’ ophthalmopathy in comparison to hyperthyroid patients without Graves’ ophthalmopathy. Cytokine 2023, 169, 156269. [Google Scholar] [CrossRef]
- Aniszewski, J.P.; Valyasevi, R.W.; Bahn, R.S. Relationship between Disease Duration and Predominant Orbital T Cell Subset in Graves’ Ophthalmopathy. J. Clin. Endocrinol. Metab. 2000, 85, 776–780. [Google Scholar] [CrossRef]
- Han, R.; Smith, T.J. T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1beta in orbital fibroblasts: Implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology 2006, 147, 13. [Google Scholar] [CrossRef]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.-F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The Immunology of Fibrosis. Annu. Rev. Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Liu, X.; Zhong, S.; Wang, N.; Zhao, B.; Li, Y.; Sun, J.; Wang, Y.; Zhang, S.; et al. Interaction between CCR6+ Th17 Cells and CD34+ Fibrocytes Promotes Inflammation: Implications in Graves’ Orbitopathy in Chinese Population. Investig. Opthalmol. Vis. Sci. 2018, 59, 2604–2614. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Zhong, S.; Li, Y.; Zhang, Y.; Li, Y.; Sun, J.; Liu, X.; Wang, Y.; Zhang, S.; et al. Regulation of Orbital Fibrosis and Adipogenesis by Pathogenic Th17 Cells in Graves Orbitopathy. J. Clin. Endocrinol. Metab. 2017, 102, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yoon, J.S.; Kim, K.H.; Lee, S.Y. Increased serum interleukin-17 in Graves’ ophthalmopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Guan, M.; Qin, Y.; Xie, C.; Fu, X.; Gao, F.; Xue, Y. Circulating levels of miR-146a and IL-17 are significantly correlated with the clinical activity of Graves’ ophthalmopathy. Endocr. J. 2014, 61, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Wang, S.; Zhang, Y.; Luo, X.; Liu, L.; Zhong, S.; Liu, X.; Li, D.; Liang, R.; et al. IL-17A Exacerbates Fibrosis by Promoting the Proinflammatory and Profibrotic Function of Orbital Fibroblasts in TAO. J. Clin. Endocrinol. Metab. 2016, 101, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Ujhelyi, B.; Gogolak, P.; Erdei, A.; Nagy, V.; Balazs, E.; Rajnavolgyi, E.; Berta, A.; Nagy, E.V. Graves’ orbitopathy results in profound changes in tear composition; a study of Plasminogen activator inhibitor-1 (PAI-1) and seven cytokines. Thyroid 2012, 22, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Luo, Q.; Yang, H.; Mao, Y. Changes of Lacrimal Gland and Tear Inflammatory Cytokines in Thyroid-Associated Ophthalmopathy. Investig. Opthalmol. Vis. Sci. 2014, 55, 4935–4943. [Google Scholar] [CrossRef]
- Fang, S.; Huang, Y.; Zhong, S.; Zhang, Y.; Liu, X.; Wang, Y.; Gu, P.; Zhou, H.; Fan, X. IL-17A Promotes RANTES Expression, But Not IL-16, in Orbital Fibroblasts Via CD40-CD40L Combination in Thyroid-Associated Ophthalmopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 6123–6133. [Google Scholar] [CrossRef]
- Gu, X.; Zheng, L.; Chen, X.; Ruan, L.; Zhang, H.; Ge, S.; Zhu, H.; Lin, X.; Shen, F. Elevated Serum IL-16 and RANTES Levels in Patients with Autoimmune Thyroid Diseases and Modulation by Methimazole Therapy. Horm. Metab. Res. 2012, 44, 482–487. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, Y.; Li, H.; Guan, H.; Xiao, H.; Li, Y. The Potential of Artemisinins as Novel Treatment for Thyroid Eye Disease by Inhibiting Adipogenesis in Orbital Fibroblasts. Investig. Opthalmol. Vis. Sci. 2023, 64, 28. [Google Scholar] [CrossRef] [PubMed]
- Dik, W.A.; Virakul, S.; van Steensel, L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp. Eye Res. 2016, 142, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Bahn, R. Immunopathogenesis of Graves’ ophthalmopathy: The role of the TSH receptor. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Koumas, L.; Smith, T.J.; Phipps, R.P. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur. J. Immunol. 2002, 32, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Cawood, T.J.; Moriarty, P.; O’farrelly, C.; O’shea, D. The effects of tumour necrosis factor-α and interleukin1 on an in vitro model of thyroid-associated ophthalmopathy; contrasting effects on adipogenesis. Eur. J. Endocrinol. 2006, 155, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Baglole, C.J.; O’Loughlin, C.W.; Feldon, S.E.; Phipps, R.P. Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: Implications for thyroid eye disease. J. Biol. Chem. 2010, 285, 15794–15804. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Koumas, L.; Gagnon, A.; Bell, A.; Sempowski, G.D.; Phipps, R.P.; Sorisky, A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2002, 87, 385–392. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Feldon, S.E.; Park, D.J.J.; O’loughlin, C.W.; Nguyen, V.T.; Landskroner-Eiger, S.; Chang, D.; Thatcher, T.H.; Phipps, R.P. Autologous T-Lymphocytes Stimulate Proliferation of Orbital Fibroblasts Derived from Patients with Graves’ Ophthalmopathy. Investig. Opthalmol. Vis. Sci. 2005, 46, 3913–3921. [Google Scholar] [CrossRef]
- Wang, Z.-M. The role of cell mediated immunopathogenesis in thyroid-associated ophthalmopathy. Int. J. Ophthalmol. 2019, 12, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.J.; Afifiyan, N.; Sand, D.; Naik, V.; Said, J.; Pollock, S.J.; Chen, B.; Phipps, R.P.; Goldberg, R.A.; Smith, T.J.; et al. Orbital fibroblasts from patients with References thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Feliciello, A.; Ciullo, I.; Fenzi, G.; Bonavolontà, G.; Porcellini, A.; Avvedimento, E. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet 1993, 342, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat. Rev. Endocrinol. 2015, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tsui, S.; Naik, V.; Hoa, N.; Hwang, C.J.; Afifiyan, N.F.; Hikim, A.S.; Gianoukakis, A.G.; Douglas, R.S.; Smith, T.J. Evidence for an Association between Thyroid-Stimulating Hormone and Insulin-Like Growth Factor 1 Receptors: A Tale of Two Antigens Implicated in Graves’ Disease. J. Immunol. 2008, 181, 4397–4405. [Google Scholar] [CrossRef] [PubMed]
- Weightman, D.R.; Perros, P.; Sherif, I.H.; Kendall-Taylor, P. Autoantibodies to Igf-1 Binding Sites in Thyroid Associated Ophthalmopathy. Autoimmunity 1993, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, P.; Poplawska, I.; Mysliwiec, J.; Dik, W.A.; Eckstein, A.; Berchner-Pfannschmidt, U.; Milewski, R.; Lawicki, S.; Dzieciol-Anikiej, Z.; Rejdak, R.; et al. Search of reference biomarkers reflecting orbital tissue remodeling in the course of Graves’ orbitopathy. Folia Histochem. Cytobiol. 2020, 58, 37–45. [Google Scholar] [CrossRef]
- Krieger, C.C.; Place, R.F.; Bevilacqua, C.; Marcus-Samuels, B.; Abel, B.S.; Skarulis, M.C.; Kahaly, G.J.; Neumann, S.; Gershengorn, M.C. TSH/IGF-1 Receptor Cross Talk in Graves’ Ophthalmopathy Pathogenesis. J. Clin. Endocrinol. Metab. 2016, 101, 2340–2347. [Google Scholar] [CrossRef]
- Wang, F.-M.; Chen, X.-M.; Hao, C.; Tang, H.-B.; Shen, Q.; Xu, X.-Y.; Xu, X.-G.; Qian, H.; Li, Q.; Yao, P.; et al. Costimulatory molecule CD40 expression in thyroid tissue of Graves’ disease patients and its immune pathogenetic significance. Zhonghua Yi Xue Za Zhi 2013, 93, 764–767. (In Chinese) [Google Scholar]
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Zhang, A.; Fang, C.; Ma, Q.; Jiang, P. Altered expression profile of BAFF receptors on peripheral blood B lymphocytes in Graves’ disease. BMC Endocr. Disord. 2021, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Chen, X.; Mao, Y.; Wan, S.; Ai, S.; Yang, H.; Liu, G.; Zou, Y.; Lin, M.; Dan, L. Orbital fibroblasts of Graves’ orbitopathy stimulated with proinflammatory cytokines promote B cell survival by secreting BAFF. Mol. Cell. Endocrinol. 2017, 446, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, G.; Covelli, D.; Currò, N.; Dazzi, D.; Maffini, A.; Campi, I.; Bonara, P.; Guastella, C.; Pignataro, L.; Ratiglia, R.; et al. Serum BAFF Concentrations in Patients with Graves’ Disease and Orbitopathy before and after Immunosuppressive Therapy. J. Clin. Endocrinol. Metab. 2012, 97, E755–E759. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Ponto, K.A.; Pitz, S.; Kahaly, G.J. Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J. Endocrinol. Investig. 2011, 34, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Ponto, K.A.; Kahaly, G.J. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: Efficacy and morbidity. J. Clin. Endocrinol. Metab. 2011, 96, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M. Advances in treatment of active, moderate-to-severe Graves’ ophthalmopathy. Lancet Diabetes Endocrinol. 2017, 5, 134–142. [Google Scholar] [CrossRef]
- Stahn, S.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Beleslin, B.N.; Ciric, J.; Stojkovic, M.; Savic, S.; Lalic, T.; Stojanovic, M.; Miletic, M.; Knezevic, M.; Stankovic, B.; Zarkovic, M. Comparison of efficacy and safety of parenteral versus parenteral and oral glucocorticoid therapy in Graves’ orbitopathy. Int. J. Clin. Pract. 2020, 74, e13608. [Google Scholar] [CrossRef]
- Bartalena, L.; Tanda, M.L. Current concepts regarding Graves’ orbitopathy. J. Intern. Med. 2022, 292, 692–716. [Google Scholar] [CrossRef]
- Douglas, R.S.; Dailey, R.; Subramanian, P.S.; Barbesino, G.; Ugradar, S.; Batten, R.; Qadeer, R.A.; Cameron, C. Proptosis and Diplopia Response with Teprotumumab and Placebo vs. the Recommended Treatment Regimen with Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison. JAMA Ophthalmol. 2022, 140, 328–335. [Google Scholar] [CrossRef]
- Burch, H.B.; Perros, P.; Bednarczuk, T.; Cooper, D.S.; Dolman, P.J.; Leung, A.M.; Mombaerts, I.; Salvi, M.; Stan, M.N. Management of Thyroid Eye Disease: A Consensus Statement by the American Thyroid Association and the European Thyroid Association. Thyroid 2022, 32, 1439–1470. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Pharmacology and toxicology of mycophenolate in organ transplant recipients: An update. Arch. Toxicol. 2014, 88, 1351–1389. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hu, Y.; Zhang, C.; Shi, H.; Zhang, P.; Yang, Y.; Chen, S.; Cui, W.; Cui, D. Efficacy and safety of mycophenolate mofetil in the treatment of moderate to severe Graves’ orbitopathy: A meta-analysis. Bioengineered 2022, 13, 14719–14729. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Riedl, M.; König, J.; Pitz, S.; Ponto, K.; Diana, T.; Kampmann, E.; Kolbe, E.; Eckstein, A.; Moeller, L.C.; et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): A randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018, 6, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F.; Xue, J.-L.; Guan, L.; Su, F.-F.; Wang, H.; Zhang, D.-F. Therapeutic outcomes of mycophenolate mofetil and glucocorticoid in thyroid-associated ophthalmopathy patients. Front. Endocrinol. 2023, 14, 1140196. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Hesari, M.; Chalabi, M.; Salari, F.; Khademi, F. An overview of immune checkpoint therapy in autoimmune diseases. Int. Immunopharmacol. 2022, 107, 108647. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.Y.C.; Shih, K.C.; Chow, L.L.W.; Lee, V.H.F. Considerations for Use of Immune Checkpoint Inhibitors in Cancer Therapy for Patients with Co-Existing Thyroid Eye Disease. Ophthalmol. Ther. 2021, 10, 5–12. [Google Scholar] [CrossRef]
- Sagiv, O.; Kandl, T.J.; Thakar, S.D.; Thuro, B.A.; Busaidy, N.L.; Cabanillas, M.; Jimenez, C.; Dadu, R.; Graham, P.H.; Debnam, J.M.; et al. Extraocular Muscle Enlargement and Thyroid Eye Disease-like Orbital Inflammation Associated with Immune Checkpoint Inhibitor Therapy in Cancer Patients. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 50–52. [Google Scholar] [CrossRef]
- McElnea, E.; Ní Mhéalóid, Á.; Moran, S.; Kelly, R.; Fulcher, T. Thyroid-Like Ophthalmopathy in a Euthyroid Patient Receiving Ipilimumab. Orbit 2014, 33, 424–427. [Google Scholar] [CrossRef]
- Min, L.; Vaidya, A.; Becker, C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur. J. Endocrinol. 2011, 164, 303–307. [Google Scholar] [CrossRef]
- Borodic, G.; Hinkle, D.M.; Cia, Y. Drug-Induced Graves Disease from CTLA-4 Receptor Suppression. Ophthalmic Plast. Reconstr. Surg. 2011, 27, e87–e88. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, L. Abatacept: A novel therapy approved for the treatment of patients with rheumatoid arthritis. Adv. Ther. 2007, 24, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Vannucchi, G.; Currò, N.; Campi, I.; Covelli, D.; Dazzi, D.; Simonetta, S.; Guastella, C.; Pignataro, L.; Avignone, S.; et al. Efficacy of B-Cell Targeted Therapy with Rituximab in Patients with Active Moderate to Severe Graves’ Orbitopathy: A Randomized Controlled Study. J. Clin. Endocrinol. Metab. 2015, 100, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.N.; Garrity, J.A.; Leon, B.G.C.; Prabin, T.; Bradley, E.A.; Bahn, R.S. Randomized Controlled Trial of Rituximab in Patients With Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2015, 100, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Eid, L.; Coste-Verdier, V.; Longueville, E.; Ribeiro, E.; Nicolescu-Catargi, B.; Korobelnik, J.-F. The effects of Rituximab on Graves’orbitopathy: A retrospective study of 14 patients. Eur. J. Ophthalmol. 2020, 30, 1008–1013. [Google Scholar] [CrossRef]
- Vittoria, F.; Currò, N.; Campi, I.; Lazzaroni, E.; Covelli, D.; Vannucchi, G.; Dazzi, D.; Muller, I.; Minorini, V.; Guastella, C.; et al. Efficacy of the Anti-BAFF monoclonal antibody belimumab vs. methylprednisolone in active moderate-severe graves’ orbitopathy: Preliminary analysis of a randomized controlled trial. Endocr. Abstr. 2021, 73, YI2. [Google Scholar] [CrossRef]
- Stohl, W.; Schwarting, A.; Okada, M.; Scheinberg, M.; Doria, A.; Hammer, A.E.; Kleoudis, C.; Groark, J.; Bass, D.; Fox, N.L.; et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: A fifty-two–week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 2017, 69, 1016–1127. [Google Scholar] [CrossRef]
- Srivastava, A. Belimumab in Systemic Lupus Erythematosus. Indian J. Dermatol. 2016, 61, 550–553. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Stan, M.N.; Frommer, L.; Gergely, P.; Colin, L.; Amer, A.; Schuhmann, I.; Espie, P.; Rush, J.S.; Basson, C.; et al. A novel anti-CD40 monoclonal antibody, iscalimab, for control of Graves hyperthyroidism—A proof-of-concept trial. J. Clin. Endocrinol. Metab. 2020, 105, 105. [Google Scholar] [CrossRef]
- Topal, I.O.; Baysak, S.; Altunay, İ.K.; Polat, A.K.; Arıkan, E.E.; Özkur, E.; Aytekin, S.; Dogan, B.; Akbulut, T.Ö.; Demir, F.T.; et al. Evaluation of the efficacy, safety and side effects of secukinumab in patients with moderate to severe psoriasis: Real-world data from a retrospective multicentre study. An. Bras. De Dermatol. 2022, 97, 566–574. [Google Scholar] [CrossRef]
- Fang, S.; Huang, Y.; Wang, N.; Zhang, S.; Zhong, S.; Li, Y.; Sun, J.; Liu, X.; Wang, Y.; Gu, P.; et al. Insights into Local Orbital Immunity: Evidence for the Involvement of the Th17 Cell Pathway in Thyroid-Associated Ophthalmopathy. J. Clin. Endocrinol. Metab. 2019, 104, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, S.C.; Valyasevi, R.W.; Harteneck, D.A.; Dutton, C.M.; Bahn, R.S.; Virakul, S.; Phetsuksiri, T.; van Holten-Neelen, C.; Schrijver, B.; van Steensel, L.; et al. Interleukin-6 Stimulates Thyrotropin Receptor Expression in Human Orbital Preadipocyte Fibroblasts from Patients with Graves’ Ophthalmopathy. Thyroid 2001, 11, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Cui, Y.; Fu, D.; Sun, F. Tear inflammatory cytokines and ocular surface changes in patients with active thyroid eye disease treated with high-dose intravenous glucocorticoids. J. Endocrinol. Investig. 2020, 43, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Balazs, C. High Circulating IL-6 Level in Graves’ Ophthalmopathy. Autoimmunity 1997, 25, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreiras, J.V.; Gomez-Reino, J.J.; Maneiro, J.R.; Perez-Pampin, E.; Romo Lopez, A.; Rodriguez Alvarez, F.M.; Castillo Laguarta, J.M.; Del Estad Cabello, A.; Gessa Sorroche, M.; Espana Gregori, E.; et al. Tocilizumab in Graves Orbitopathy Study G. Efficacy of Tocilizumab in Patients with Moderate-toSevere Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 195, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Moi, L.; Hamedani, M.; Ribi, C. Long-term outcomes in corticosteroid-refractory Graves’ orbitopathy treated with tocilizumab. Clin. Endocrinol. 2022, 97, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Cortez, O.D.; Grivet, D.; Perrillat, N.; Gain, P.; Thuret, G. Treatment of corticosteroid-resistant Graves’ orbitopathy with tocilizumab: A single-centre prospective study. Orbit 2023, 42, 411–417. [Google Scholar] [CrossRef]
- Boutzios, G.; Chatzi, S.; Goules, A.V.; Mina, A.; Charonis, G.C.; Vlachoyiannopoulos, P.G.; Tzioufas, A.G. Tocilizumab improves clinical outcome in patients with active corticosteroid-resistant moderate-to-severe Graves’ orbitopathy: An observational study. Front. Endocrinol. 2023, 14, 1186105. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Lin, K.; Yu, X. Efficacy and Safety of intravenous monoclonal antibodies in patients with moderate-to-severe active Graves’ ophthalmopathy: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1160936. [Google Scholar] [CrossRef]
- Furmaniak, J.; Sanders, J.; Sanders, P.; Li, Y.; Smith, B.R. TSH receptor specific monoclonal autoantibody K1-70TM targeting of the TSH receptor in subjects with Graves’ disease and Graves’ orbitopathy—Results from a phase I clinical trial. Clin. Endocrinol. 2022, 96, 878–887. [Google Scholar] [CrossRef]
- Smith, T.J.; Janssen, J.A.M.J.L. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocr. Rev. 2019, 40, 236–267. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Kahaly, G.J.; Ezra, D.G.; Fleming, J.C.; Dailey, R.A.; Tang, R.A.; Harris, G.J.; Antonelli, A.; Salvi, M.; Goldberg, R.A.; et al. Teprotumumab for Thyroid-Associated Ophthalmopathy. N. Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.D.; Nguyen, N.T.; Chou, E.; Shakir, M.K. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Rep. 2021, 14, e242153. [Google Scholar] [CrossRef] [PubMed]
- Safo, M.B.; Silkiss, R.Z. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am. J. Ophthalmol. Case Rep. 2021, 22, 101069. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, D.C.; Jankovic, I.; El-Nachef, N.; Winn, B.J.; Kim, G.E.; Kersten, R.C. New-Onset of Inflammatory Bowel Disease in a Patient Treated with Teprotumumab for Thyroid Associated Ophthalmopathy. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e160–e164. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Marinò, M.; Marcocci, C.; Tanda, M.L. Teprotumumab for Graves’ orbitopathy and ototoxicity: Moving problems from eyes to ears? J. Endocrinol. Investig. 2022, 45, 1455–1457. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Kahaly, G.J.; Ugradar, S.; Elflein, H.; Ponto, K.A.; Fowler, B.T.; Dailey, R.; Harris, G.J.; Schiffman, J.; Tang, R.; et al. Teprotumumab Efficacy, Safety, and Durability in Longer-Duration Thyroid Eye Disease and Re-treatment: OPTIC-X Study. Ophthalmology 2022, 129, 438–449. [Google Scholar] [CrossRef]
- Ugradar, S.; Kang, J.; Kossler, A.L.; Zimmerman, E.; Braun, J.; Harrison, A.R.; Bose, S.; Cockerham, K.; Douglas, R.S. Teprotumumab for the treatment of chronic thyroid eye disease. Eye 2022, 36, 1553–1559. [Google Scholar] [CrossRef]
- Ozzello, D.J.; Dallalzadeh, L.O.; Liu, C.Y. Teprotumumab for chronic thyroid eye disease. Orbit 2022, 41, 539–546. [Google Scholar] [CrossRef]
- Moote, W.; Kim, H.; Ellis, A.K. Allergen-specific immunotherapy. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 53. [Google Scholar] [CrossRef] [PubMed]
- A Akdis, C.; Akdis, M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.H.; Dayan, C.; Wraith, D.C.; Barrell, K.; Olive, N.; Jansson, L.; Walker-Smith, T.; Carnegie, C.; Martin, K.F.; Boelaert, K.; et al. Antigen-Specific Immunotherapy with Thyrotropin Receptor Peptides in Graves’ Hyperthyroidism: A Phase I Study. Thyroid 2019, 29, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieplińska, K.; Niedziela, E.; Kowalska, A. Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets. J. Clin. Med. 2024, 13, 72. https://doi.org/10.3390/jcm13010072
Cieplińska K, Niedziela E, Kowalska A. Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets. Journal of Clinical Medicine. 2024; 13(1):72. https://doi.org/10.3390/jcm13010072
Chicago/Turabian StyleCieplińska, Katarzyna, Emilia Niedziela, and Aldona Kowalska. 2024. "Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets" Journal of Clinical Medicine 13, no. 1: 72. https://doi.org/10.3390/jcm13010072





