Development and Internal Validation of Nomograms for Survival of Advanced Epithelial Ovarian Cancer Based on Established Prognostic Factors and Hematologic Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Study Population
2.3. Definitions
2.4. Statistical Analysis and Software
2.5. Model Development
- I.
- Discrimination, i.e., the model’s ability to distinguish between patients with and without the survival outcome of interest, was assessed using the Harrell’s concordance (c)-index [40]. A value of 0.5 indicates that the model is no better than predicting an outcome than random chance. Conversely, a value of 1 indicates that the model perfectly predicts who will experience a certain outcome from those who will not.
- II.
- Calibration, i.e., the agreement between the predicted and observed rates on a (sub)group level, was assessed with calibration plots, calibration intercepts, and slopes.
- III.
- The Brier score is an overall performance measure calculated as the mean (squared) difference between the observed and the predicted outcomes. The lower the score, the better the predictions reflect the observed data. A score near 0 indicates perfect accuracy.
2.6. Model Validation
2.7. Ethical Approval
3. Results
3.1. Study Population
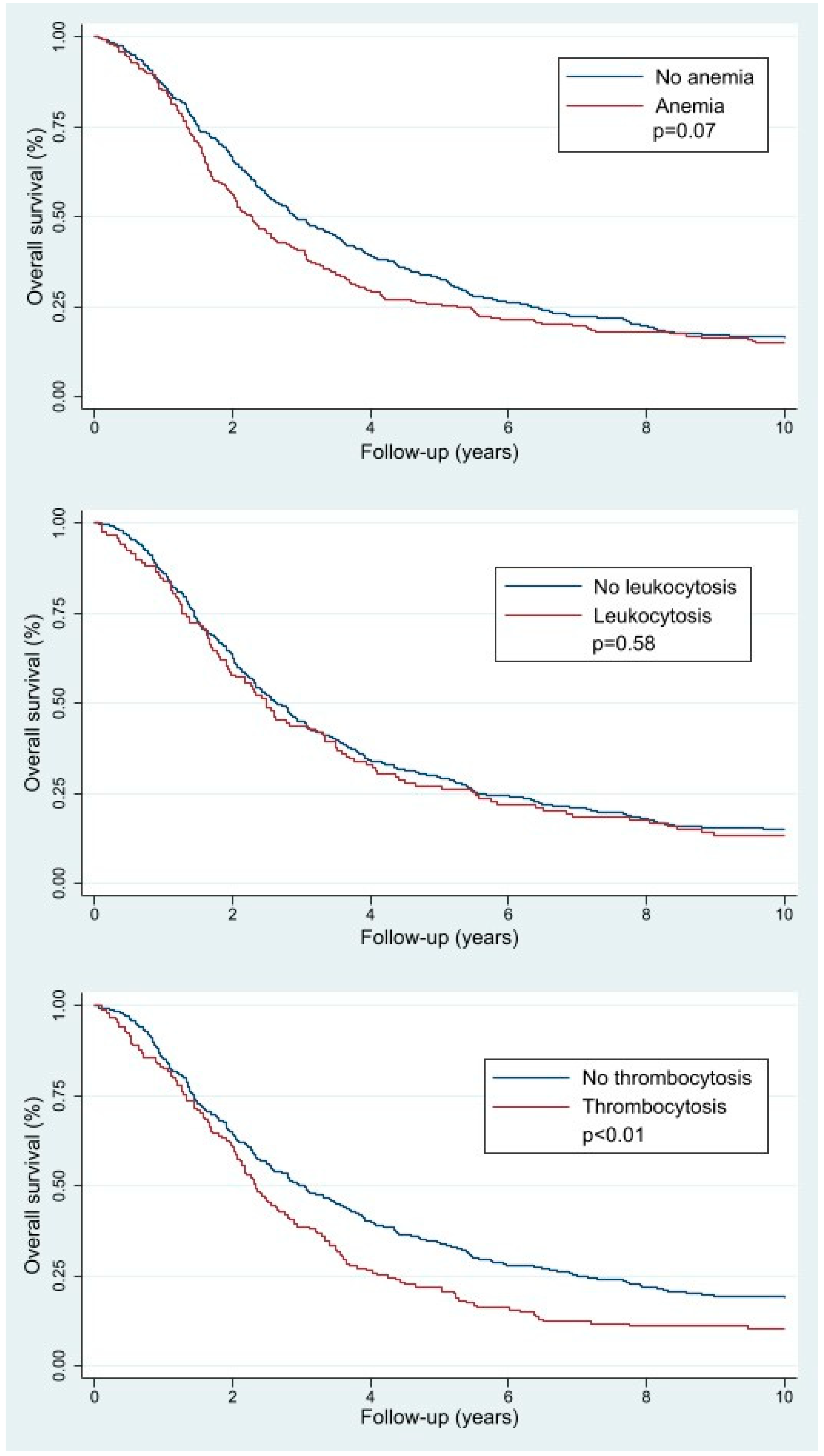
3.2. OS and Pretreatment Hematologic Parameters
3.3. Final Prediction Models and Their Parameters
3.4. Model Performance
3.5. Internal Validation
3.6. Risk Stratification
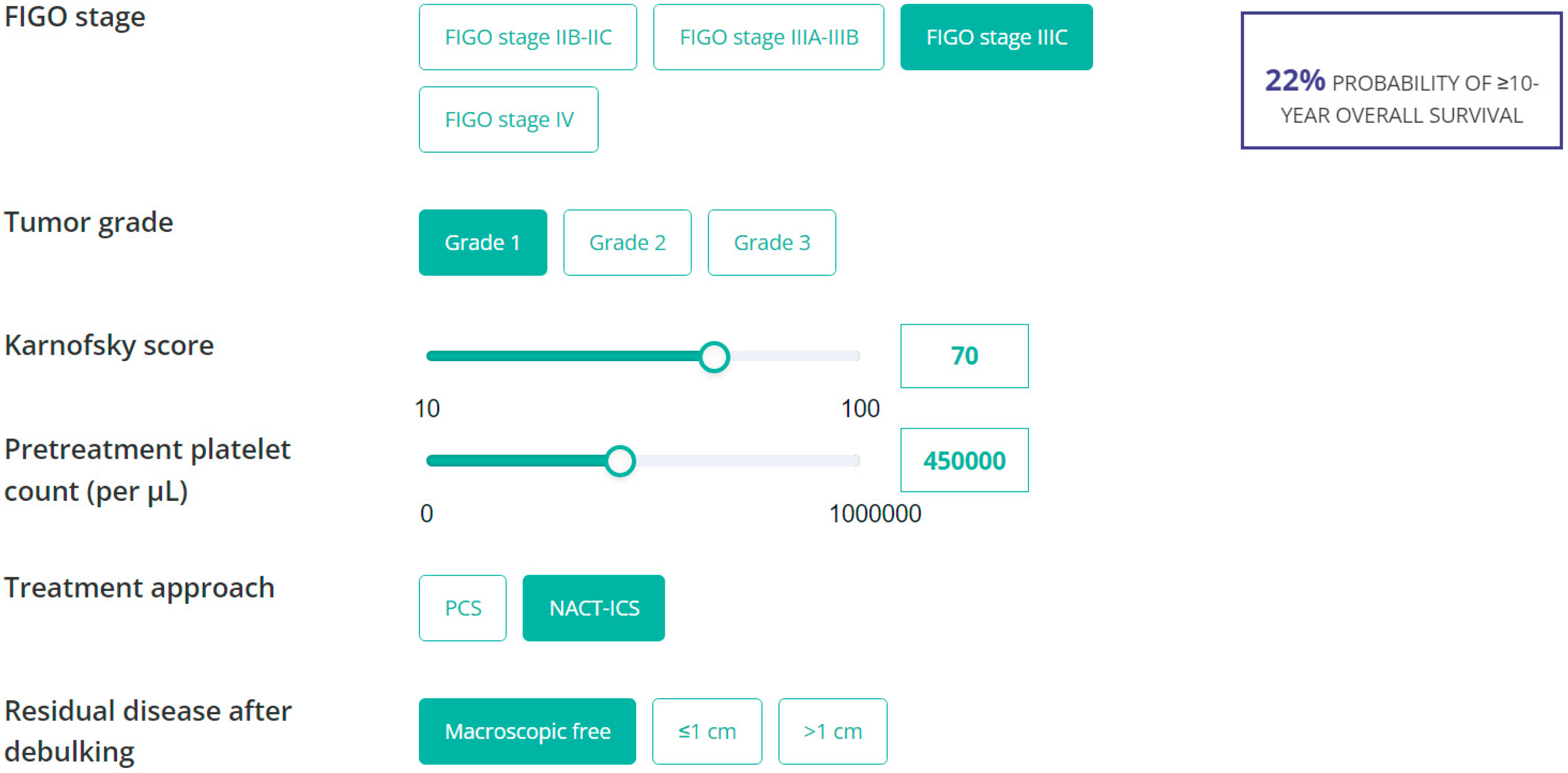
3.7. Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOC | Epithelial Ovarian Cancer |
| FIGO | International Federation of Gynecology and Obstretics |
| PCS | Primary Cytoreductive Surgery |
| NACT-ICS | Neo-Adjuvant Chemotherapy followed by Interval Cytoreductive Surgery |
| IL-6 | Interleukin 6 |
| OS | Overall Survival |
| CA-125 | Cancer Antigen 125 |
| NCR | Netherlands Cancer Registry |
| IQR | Interquartile Range |
| LR+ | Positive Likelihood Ratio |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| CI | Confidence Interval |
| HIPEC | Hyperthermic Intraperitoneal Chemotherapy |
| PARP | Poly(ADP-ribose) Polymerase |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer. J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Gonzalez-Martin, A.; Harter, P.; Lorusso, D.; Moore, K.N.; Oaknin, A.; Ray-Coquard, I. First-line PARP inhibitors in ovarian cancer: Summary of an ESMO Open—Cancer Horizons round-table discussion. ESMO Open 2020, 5, e001110. [Google Scholar] [CrossRef] [PubMed]
- Wefers, C.; Lambert, L.J.; Torensma, R.; Hato, S.V. Cellular immunotherapy in ovarian cancer: Targeting the stem of recurrence. Gynecol. Oncol. 2015, 137, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Omura, G.A.; Brady, M.F.; Homesley, H.D.; Yordan, E.; Major, F.J.; Buchsbaum, H.J.; Park, R.C. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: The Gynecologic Oncology Group experience. J. Clin. Oncol. 1991, 9, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef]
- Dao, F.; Schlappe, B.A.; Tseng, J.; Lester, J.; Nick, A.M.; Lutgendorf, S.K.; McMeekin, S.; Coleman, R.L.; Moore, K.N.; Karlan, B.Y.; et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2016, 141, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Cress, R.D.; Chen, Y.S.; Morris, C.R.; Petersen, M.; Leiserowitz, G.S. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet. Gynecol. 2015, 126, 491–497. [Google Scholar] [CrossRef]
- Son, J.H.; Kong, T.W.; Paek, J.; Song, K.H.; Chang, S.J.; Ryu, H.S. Clinical characteristics and prognostic inflection points among long-term survivors of advanced epithelial ovarian cancer. Int. J. Gynaecol. Obstet. 2017, 139, 352–357. [Google Scholar] [CrossRef]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P. Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef]
- Hoppenot, C.; Eckert, M.A.; Tienda, S.M.; Lengyel, E. Who are the long-term survivors of high grade serous ovarian cancer? Gynecol. Oncol. 2018, 148, 204–212. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Miller, A.; Casablanca, Y.; Horowitz, N.S.; Rungruang, B.; Krivak, T.C.; Richard, S.D.; Rodriguez, N.; Birrer, M.J.; Backes, F.J.; et al. Clinicopathologic characteristics associated with long-term survival in advanced epithelial ovarian cancer: An NRG Oncology/Gynecologic Oncology Group ancillary data study. Gynecol. Oncol. 2018, 148, 275–280. [Google Scholar] [CrossRef]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. Int. J. Mol. Sci. 2020, 21, 8169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Liu, W.X.; Liu, X.Y. Prognostic significance of preoperative anemia, leukocytosis and thrombocytosis in chinese women with epithelial ovarian cancer. Asian Pac. J. Cancer Prev. 2015, 16, 933–939. [Google Scholar] [CrossRef]
- Allensworth, S.K.; Langstraat, C.L.; Martin, J.R.; Lemens, M.A.; McGree, M.E.; Weaver, A.L.; Dowdy, S.C.; Podratz, K.C.; Bakkum-Gamez, J.N. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol. Oncol. 2013, 130, 499–504. [Google Scholar] [CrossRef]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Gastl, G.; Plante, M.; Finstad, C.L.; Wong, G.Y.; Federici, M.G.; Bander, N.H.; Rubin, S.C. High IL-6 levels in ascitic fluid correlate with reactive thrombocytosis in patients with epithelial ovarian cancer. Br. J. Haematol. 1993, 83, 433–441. [Google Scholar] [CrossRef]
- Crasta, J.A.; Premlatha, T.S.; Krishnan, S.M.; Vallikad, E.; Rameshkumar, K. Significance of preoperative thrombocytosis in epithelial ovarian cancer. Indian J. Pathol. Microbiol. 2010, 53, 54–56. [Google Scholar] [CrossRef]
- Barber, E.L.; Boggess, J.F.; Van Le, L.; Kim, K.H.; Bae-Jump, V.L.; Brewster, W.R.; Soper, J.T.; Gehrig, P.A. Association of Preoperative Thrombocytosis and Leukocytosis With Postoperative Morbidity and Mortality Among Patients With Ovarian Cancer. Obstet. Gynecol. 2015, 126, 1191–1197. [Google Scholar] [CrossRef]
- So, K.A.; Hong, J.H.; Jin, H.M.; Kim, J.W.; Song, J.Y.; Lee, J.K.; Lee, N.W. The prognostic significance of preoperative leukocytosis in epithelial ovarian carcinoma: A retrospective cohort study. Gynecol. Oncol. 2014, 132, 551–555. [Google Scholar] [CrossRef]
- van Altena, A.M.; van den Akker, P.A.; de Hullu, J.A.; Ottevanger, P.B.; Aalders, A.L.; Gerritse, R.; Happel, M.; Hoekstra, M.P.; Janssen, M.J.; Samlal, R.A.; et al. Efficacy of a regional network for ovarian cancer care. Obstet. Gynecol. 2013, 122, 668–675. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Alomar, O.; Abuzaid, M.; Baradwan, S.; Salem, H.; Al-Badawi, I.A. Preoperative anemia predicts poor prognosis in patients with endometrial cancer: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, C.M.; Park, J.M.; Jung, S.Y.; Lee, K.B. An association between preoperative anemia and poor prognostic factors and decreased survival in early stage cervical cancer patients. Obstet. Gynecol. Sci. 2014, 57, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Said, S.A.; van der Aa, M.A.; Veldmate, G.; de Hullu, J.A.; van Altena, A.M. Oncologic outcomes after splenectomy during initial cytoreductive surgery in advanced epithelial ovarian cancer: A nationwide population-based cohort study. Acta Obstet. Gynecol. Scand. 2022, 101, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Yu, C.; Hill, E.K.; Zivanovic, O.; Kolev, V.; Levine, D.A.; Sonoda, Y.; Abu-Rustum, N.R.; Huh, J.; Barakat, R.R.; et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol. Oncol. 2012, 125, 25–30. [Google Scholar] [CrossRef]
- Chi, D.S.; Palayekar, M.J.; Sonoda, Y.; Abu-Rustum, N.R.; Awtrey, C.S.; Huh, J.; Eisenhauer, E.L.; Barakat, R.R.; Kattan, M.W. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol. Oncol. 2008, 108, 191–194. [Google Scholar] [CrossRef]
- Gerestein, C.G.; Eijkemans, M.J.; de Jong, D.; van der Burg, M.E.; Dykgraaf, R.H.; Kooi, G.S.; Baalbergen, A.; Burger, C.W.; Ansink, A.C. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG 2009, 116, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.J.; Boldingh, J.H.; Schuit, E.; Trum, H.; van Driel, W.; Mol, B.W.; Kenter, G.G.; Buist, M.R. Development and internal validation of a prognostic model for survival after debulking surgery for epithelial ovarian cancer. Gynecol. Oncol. 2014, 135, 13–18. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software, Release 17; StataCorp LLC: College Station, TX, USA, 2021.
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. Available online: http://www.rstudio.com/ (accessed on 1 January 2023).
- Harrell, F.; Dupont, C., Jr. Hmisc: R Package, version 4.7-0; RStudio, PBC: Boston, MA, USA, 2021.
- Harrell, F., Jr. rms: Regression Modeling Strategies: R Package, version 6.3-0; RStudio, PBC: Boston, MA, USA, 2022.
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training R Package, Version 6.0-93; RStudio, PBC: Boston, MA, USA, 2022. Available online: https://CRAN.R-project.org/package=caret(accessed on 1 January 2023).
- Steyerberg, E.W.; Vergouwe, Y. Towards better clinical prediction models: Seven steps for development and an ABCD for validation. Eur. Heart J. 2014, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Eijkemans, M.J.; Harrell, F.E., Jr.; Habbema, J.D. Prognostic modeling with logistic regression analysis: In search of a sensible strategy in small data sets. Med. Decis. Making 2001, 21, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.W.; Hughes, R.A. Bootstrap inference for multiple imputation under uncongeniality and misspecification. Stat. Methods Med. Res. 2020, 29, 3533–3546. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E., Jr. Regression Modeling Strategies; Springer Series in Statistics; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Davis, A.N.; Afshar-Kharghan, V.; Sood, A.K. Platelet effects on ovarian cancer. Semin. Oncol. 2014, 41, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Madden, A.C.; Cass, I.; Leuchter, R.S.; Lagasse, L.D.; Karlan, B.Y. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol. Oncol. 2004, 92, 211–214. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, A.; McCool, K.; Harrison, R.; Sampene, E.; Connor, J.; Barroilhet, L. Neoadjuvant chemotherapy is associated with more anemia and perioperative blood transfusions than primary debulking surgery in women with advanced stage ovarian cancer. Gynecol. Oncol. 2018, 150, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Van Belle, S.; Barrett-Lee, P.; Birgegård, G.; Bokemeyer, C.; Gascón, P.; Kosmidis, P.; Krzakowski, M.; Nortier, J.; Olmi, P.; et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer 2004, 40, 2293–2306. [Google Scholar] [CrossRef]
- Worley, M.J., Jr.; Nitschmann, C.C.; Shoni, M.; Vitonis, A.F.; Rauh-Hain, J.A.; Feltmate, C.M. The significance of preoperative leukocytosis in endometrial carcinoma. Gynecol. Oncol. 2012, 125, 561–565. [Google Scholar] [CrossRef]
- Mabuchi, S.; Matsumoto, Y.; Isohashi, F.; Yoshioka, Y.; Ohashi, H.; Morii, E.; Hamasaki, T.; Aozasa, K.; Mutch, D.G.; Kimura, T. Pretreatment leukocytosis is an indicator of poor prognosis in patients with cervical cancer. Gynecol. Oncol. 2011, 122, 25–32. [Google Scholar] [CrossRef]
- Clark, T.G.; Stewart, M.E.; Altman, D.G.; Gabra, H.; Smyth, J.F. A prognostic model for ovarian cancer. Br. J. Cancer 2001, 85, 944–952. [Google Scholar] [CrossRef]
- Teramukai, S.; Ochiai, K.; Tada, H.; Fukushima, M. PIEPOC: A new prognostic index for advanced epithelial ovarian cancer--Japan Multinational Trial Organization OC01-01. J. Clin. Oncol. 2007, 25, 3302–3306. [Google Scholar] [CrossRef]
- van de Laar, R.; IntHout, J.; Van Gorp, T.; Verdonschot, S.; van Altena, A.M.; Gerestein, C.G.; Massuger, L.F.; Zusterzeel, P.L.; Kruitwagen, R.F. External validation of three prognostic models for overall survival in patients with advanced-stage epithelial ovarian cancer. Br. J. Cancer 2014, 110, 42–48. [Google Scholar] [CrossRef]
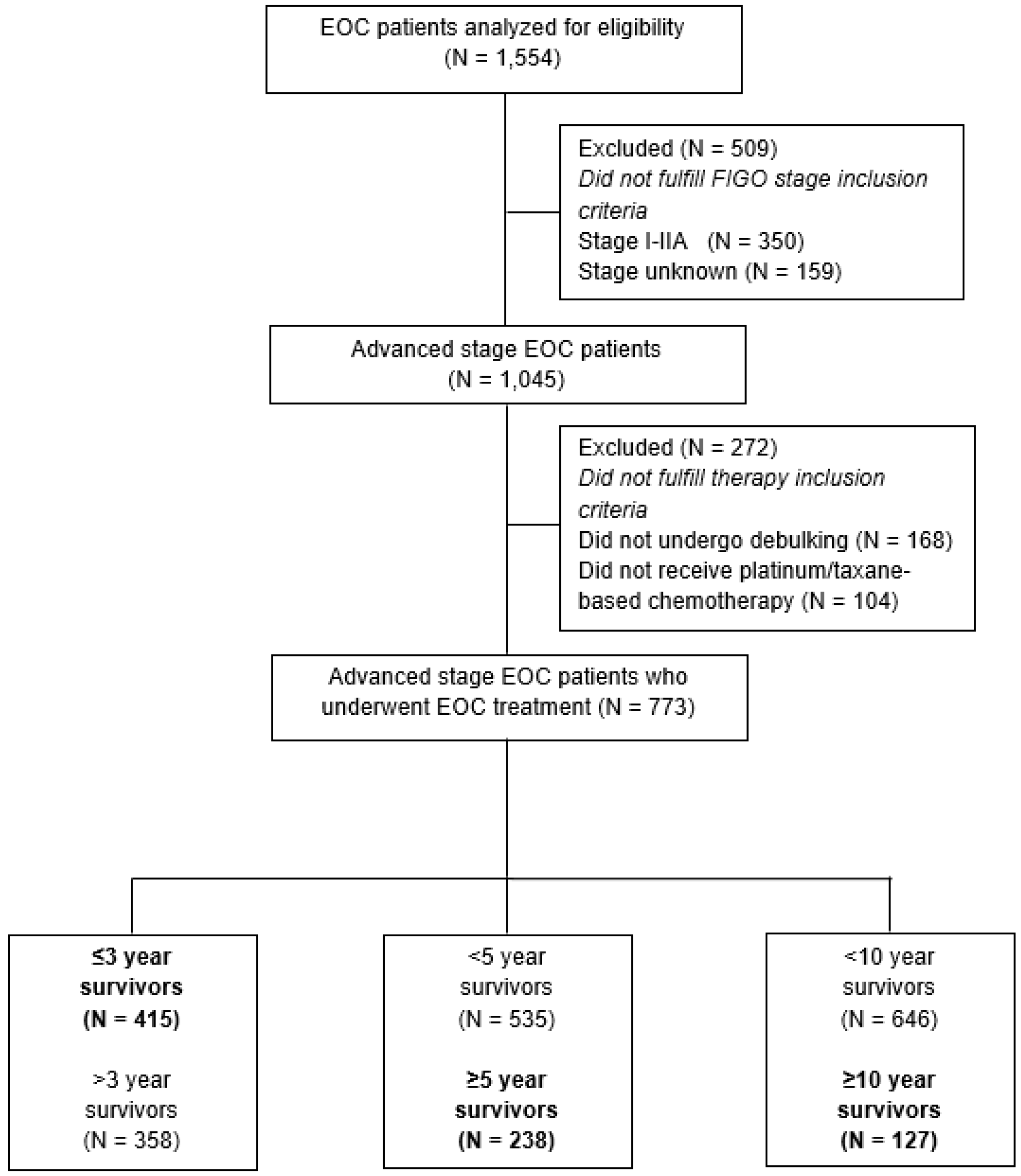
| Total N = 773 (%)/Median [IQR] | ≤3-Year OS N = 415 (%)/Median [IQR] | ≥5-Year OS N = 238 (%)/Median [IQR] | ≥10-Year OS N = 127 (%)/Median [IQR] | |
|---|---|---|---|---|
| Characteristic | ||||
| Age at diagnosis (in yrs) | ||||
| Median [IQR] | 61 [21–84] | 63 [28–84] | 60 [27–80] | 59 [38–77] |
| FIGO stage | ||||
| Stage IIB–IIC | 83 (10.7) | 16 (3.9) | 61 (25.6) | 48 (37.8) |
| Stage IIIA–IIIB | 87 (11.3) | 41 (9.9) | 31 (13.0) | 18 (14.2) |
| Stage IIIC | 506 (65.5) | 292 (70.4) | 134 (56.3) | 60 (47.2) |
| Stage IV | 97 (12.5) | 66 (15.9) | 12 (5.0) | 1 (0.8) |
| Tumor grade | ||||
| Grade 1 | 42 (5.4) | 15 (3.6) | 21 (8.8) | 19 (15.0) |
| Grade 2 | 172 (22.3) | 83 (20.0) | 63 (26.5) | 34 (26.8) |
| Grade 3 | 452 (58.5) | 259 (62.4) | 125 (52.5) | 64 (50.4) |
| Unknown | 107 (13.8) | 58 (14.0) | 29 (12.2) | 10 (7.9) |
| Histologic subtype | ||||
| Serous | 445 (57.6) | 251 (60.5) | 118 (49.6) | 54 (42.5) |
| Mucinous | 29 (3.8) | 20 (4.8) | 6 (2.5) | 4 (3.2) |
| Endometrioid | 92 (11.9) | 40 (9.7) | 41 (17.2) | 27 (21.3) |
| Clear cell | 23 (3.0) | 12 (2.9) | 9 (3.8) | 8 (6.3) |
| Adenocarcinoma NOS * | 146 (18.9) | 72 (17.4) | 51 (21.4) | 28 (22.1) |
| Other | 35 (4.5) | 19 (4.6) | 12 (5.0) | 6 (4.7) |
| Unknown | 3 (0.4) | 1 (0.2) | 1 (0.4) | 0 (0) |
| Karnofsky score | ||||
| 10–40 | 3 (0.4) | 2 (0.5) | 0 (0) | 0 (0) |
| 50–70 | 187 (24.2) | 128 (30.8) | 35 (14.7) | 18 (14.2) |
| 80–100 | 492 (63.7) | 224 (54.0) | 184 (77.3) | 96 (75.6) |
| Unknown | 91 (11.8) | 61 (14.7) | 19 (8.0) | 13 (10.2) |
| Pretreatment CA-125 serum level (kU/L) | ||||
| Median [IQR] | 484 [9–25,784] | 666 [24–13,995] | 334 [9–9219] | 259 [10–4180] |
| Unknown | 43 (5.6) | 26 (6.3) | 8 (3.4) | 4 (3.1) |
| Pretreatment hemoglobin level (mmol/L) | ||||
| Median [IQR] | 7.9 [4.6–9.9] | 7.8 [4.6–9.6] | 8.1 [5.7–9.7] | 8.1 [5.9–9.7] |
| No anemia | 505 (65.3) | 257 (61.9) | 167 (70.2) | 82 (64.6) |
| Anemia | 225 (29.1) | 134 (32.4) | 58 (24.4) | 34 (26.8) |
| Unknown | 43 (5.6) | 24 (5.8) | 13 (5.5) | 11 (8.7) |
| Pretreatment platelet count (×103/µL) | ||||
| Median [IQR] | 370 [144–898] | 390 [158–749] | 336 [169–637] | 324 [194–590] |
| No thrombocytosis | 369 (47.7) | 185 (44.6) | 126 (52.9) | 69 (54.3) |
| Thrombocytosis | 155 (20.1) | 95 (22.9) | 34 (14.3) | 16 (12.6) |
| Unknown | 249 (32.2) | 135 (32.5) | 78 (32.8) | 42 (33.1) |
| Pretreatment leukocyte count (×109/L) | ||||
| Median [IQR] | 8.4 [3.6–20.2] | 8.6 [4.5–16.8] | 8.1 [4–17.8] | 8.3 [4.6–14.8] |
| No leukocytosis | 461 (59.6) | 255 (61.5) | 136 (57.1) | 68 (53.5) |
| Leukocytosis | 119 (15.4) | 67 (16.2) | 32 (13.5) | 16 (12.6) |
| Unknown | 193 (25.0) | 93 (22.8) | 70 (29.4) | 43 (33.9) |
| Presence of ascites | ||||
| No | 142 (18.4) | 46 (11.1) | 75 (31.5) | 45 (35.4) |
| Yes | 608 (78.7) | 355 (84.5) | 158 (66.4) | 80 (63.0) |
| Unknown | 23 (3.0) | 14 (3.4) | 5 (2.1) | 2 (1.6) |
| Ascites volume (mL) | ||||
| Median [IQR] | 700 [0–18,000] | 2000 [0–14,000] | 100 [0–7000] | 50 [0–6000] |
| Unknown | 172 (22.2) | 91 (22.0) | 53 (22.2) | 25 (19.7) |
| Treatment approach | ||||
| PCS | 523 (67.7) | 264 (63.6) | 187 (78.6) | 105 (82.7) |
| NACT-ICS | 250 (32.3) | 151 (36.4) | 51 (21.4) | 22 (17.3) |
| Residual disease after debulking | ||||
| No macroscopic disease | 285 (36.9) | 102 (24.6) | 138 (58.0) | 85 (66.9) |
| ≤1 cm | 265 (34.3) | 153 (36.9) | 70 (29.4) | 31 (24.4) |
| >1 cm | 186 (24.1) | 137 (33.0) | 22 (9.2) | 8 (6.3) |
| Unknown | 37 (4.8) | 23 (5.4) | 8 (3.4) | 3 (2.4) |
| Predicted Probabilities b | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | LR+ |
|---|---|---|---|---|---|
| ≥5% | 97.6 | 31.0 | 21.8 | 98.5 | 1.4 |
| ≥10% | 88.2 | 55.6 | 28.1 | 96.0 | 2.0 |
| ≥15% | 73.2 | 71.8 | 33.8 | 93.2 | 2.6 |
| ≥20% | 62.2 | 83.3 | 42.2 | 91.8 | 3.7 |
| ≥25% | 55.9 | 87.5 | 46.7 | 91.0 | 4.5 |
| ≥30% | 48.0 | 91.3 | 52.1 | 89.9 | 5.5 |
| ≥35% | 40.2 | 94.1 | 57.3 | 88.9 | 6.8 |
| ≥40% | 37.0 | 95.2 | 60.2 | 88.5 | 7.7 |
| ≥45% | 35.4 | 95.8 | 62.5 | 88.3 | 8.4 |
| ≥50% | 33.1 | 96.3 | 63.6 | 88.0 | 8.9 |
| ≥55% | 30.0 | 97.4 | 69.0 | 87.6 | 11.5 |
| ≥60% | 23.6 | 98.0 | 69.8 | 86.7 | 11.8 |
| ≥65% | 13.4 | 98.9 | 70.8 | 85.3 | 12.2 |
| ≥70% | 6.3 | 99.7 | 80.0 | 84.4 | 21 |
| ≥75% | 4.7 | 99.7 | 75.0 | 84.2 | 15.7 |
| ≥80% | 3.9 | 100 | 100 | 84.1 | - |
| ≥85% | - | - | - | - | - |
| ≥90% | - | - | - | - | - |
| ≥95% | - | - | - | - | - |
| ≥100% | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, S.A.; IntHout, J.; den Ouden, J.E.; Walraven, J.E.W.; van der Aa, M.A.; de Hullu, J.A.; van Altena, A.M. Development and Internal Validation of Nomograms for Survival of Advanced Epithelial Ovarian Cancer Based on Established Prognostic Factors and Hematologic Parameters. J. Clin. Med. 2024, 13, 2789. https://doi.org/10.3390/jcm13102789
Said SA, IntHout J, den Ouden JE, Walraven JEW, van der Aa MA, de Hullu JA, van Altena AM. Development and Internal Validation of Nomograms for Survival of Advanced Epithelial Ovarian Cancer Based on Established Prognostic Factors and Hematologic Parameters. Journal of Clinical Medicine. 2024; 13(10):2789. https://doi.org/10.3390/jcm13102789
Chicago/Turabian StyleSaid, Sherin Abdo, Joanna IntHout, Judith E. den Ouden, Janneke E. W. Walraven, Maaike A. van der Aa, Joanne A. de Hullu, and Anne M. van Altena. 2024. "Development and Internal Validation of Nomograms for Survival of Advanced Epithelial Ovarian Cancer Based on Established Prognostic Factors and Hematologic Parameters" Journal of Clinical Medicine 13, no. 10: 2789. https://doi.org/10.3390/jcm13102789
APA StyleSaid, S. A., IntHout, J., den Ouden, J. E., Walraven, J. E. W., van der Aa, M. A., de Hullu, J. A., & van Altena, A. M. (2024). Development and Internal Validation of Nomograms for Survival of Advanced Epithelial Ovarian Cancer Based on Established Prognostic Factors and Hematologic Parameters. Journal of Clinical Medicine, 13(10), 2789. https://doi.org/10.3390/jcm13102789






