The Utility of Three-Dimensional Printing in Physician-Modified Stent Grafts for Aortic Lesions Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Considering Studies for This Review
2.1.1. Types of Studies
2.1.2. Types of Participants
2.1.3. Types of Interventions
2.1.4. Types of Outcome Measures
2.2. Search Methods for Identification of Studies
2.2.1. Electronic Searches
2.2.2. Searching Other Resources
2.3. Data Collection and Management
2.3.1. Assessment of Risk of Bias in Included Studies
2.3.2. Measures of Treatment Effect
2.3.3. Dealing with Missing Data
2.3.4. Assessment of Heterogeneity
2.3.5. Assessment of Reporting Biases
3. Results
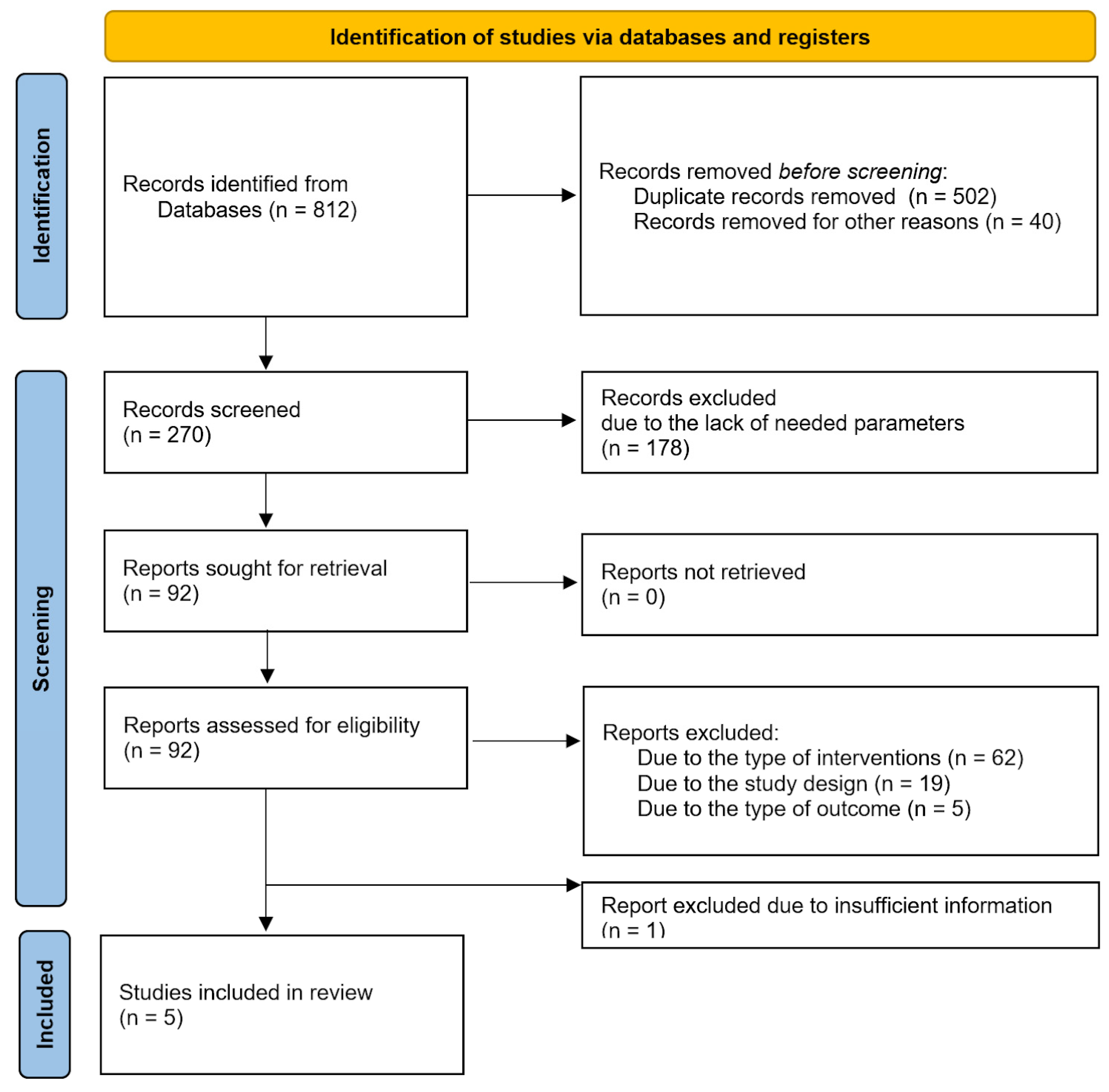
3.1. Description of Studies
3.2. Statistical Analysis
3.3. Bias Assessment
4. Discussion
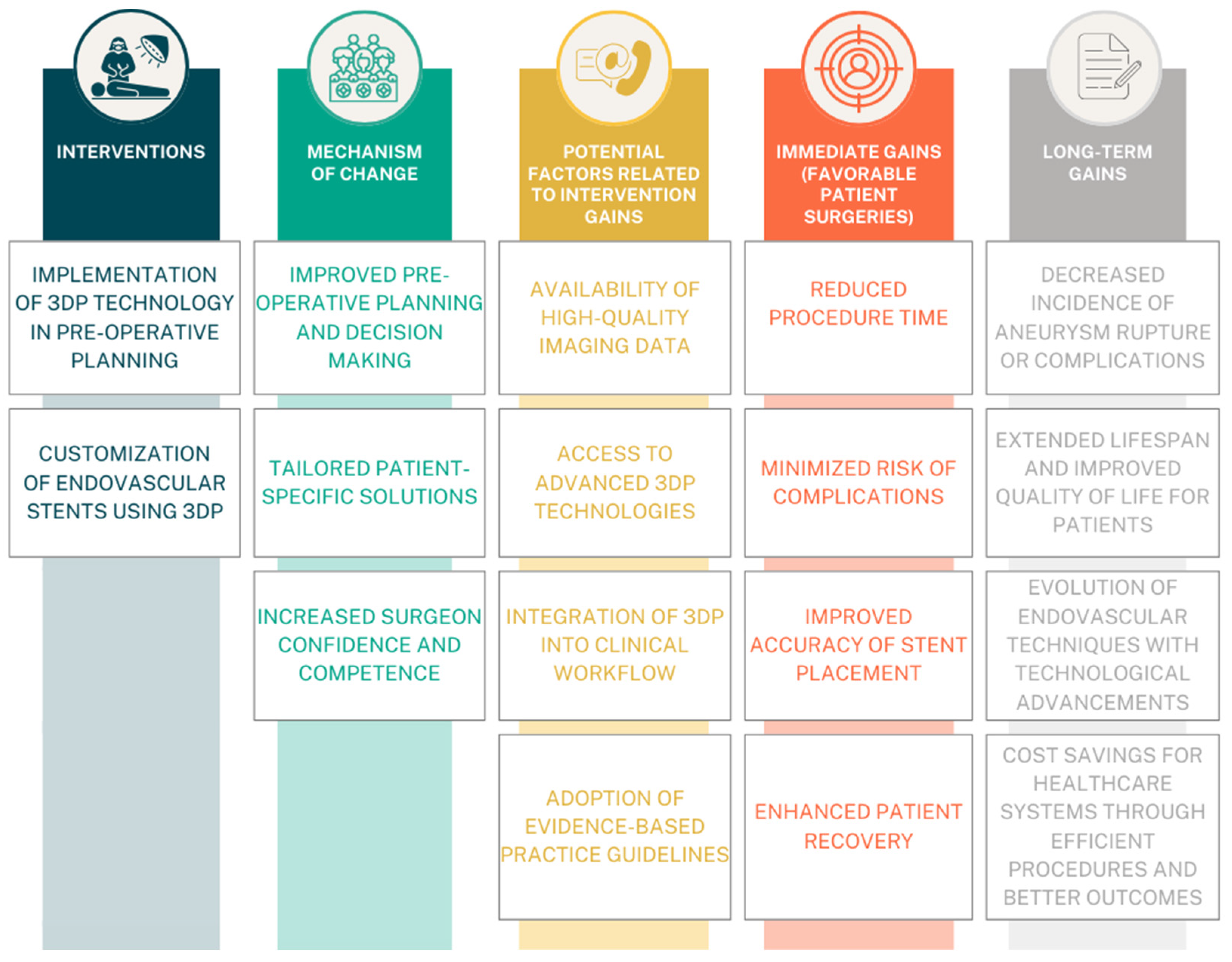
4.1. The Utility of 3D Printing in Urgent Surgeries
4.2. The Reference to Custom-Made Stent Grafts and Conventional PMSG
4.3. Utilization in Training
4.4. Stent-Graft Technique and Material
4.5. Standardization and Optimization of the Procedures
4.6. Enhancing Patient Outcomes and Risk Assessment
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, M.; Radacsi, N.; Robert, C.; McCarthy, E.D.; Callanan, A.; Conlisk, N.; Hoskins, P.R.; Koutsos, V. On the Optimization of Low-Cost FDM 3D Printers for Accurate Replication of Patient-Specific Abdominal Aortic Aneurysm Geometry. 3D Print. Med. 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2018, 15, 2805. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent Advances on the Role of Cytokines in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochem. Biokhimiia 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Shrivastava, S.; Avula, S.N.; Anusheel; Thondamala, V.; Onuchukwu, C.V.; Mohammed, L. Emphasis on Early Identification of Risk Factors to Curtail High Mortality Involved with Ischemic Colitis (IC) After Abdominal Aortic Aneurysm (AAA) Repair. Cureus 2022, 14, e23492. [Google Scholar] [CrossRef]
- Martínez-López, D.; Cedó, L.; Metso, J.; Burillo, E.; García-León, A.; Canyelles, M.; Lindholt, J.S.; Torres-Fonseca, M.; Blanco-Colio, L.M.; Vázquez, J.; et al. Impaired HDL (High-Density Lipoprotein)-Mediated Macrophage Cholesterol Efflux in Patients With Abdominal Aortic Aneurysm-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Long, C.; Hong, Y.; Gu, X.; Weng, R.; Zhong, Z. Prevalence of Risk Factors Associated with Rupture of Abdominal Aortic Aneurysm (AAA): A Single Center Retrospective Study. PeerJ 2023, 11, e15752. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery Practice Guidelines on the Care of Patients with an Abdominal Aortic Aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed]
- Gawinecka, J.; Schönrath, F.; von Eckardstein, A. Acute Aortic Dissection: Pathogenesis, Risk Factors and Diagnosis. Swiss Med. Wkly. 2017, 147, w14489. [Google Scholar] [CrossRef]
- Akbulut, M.; Aksoy, E.; Kara, İ.; Cekmecelioglu, D.; Koksal, C. Quality of Life After Open Surgical versus Endovascular Repair of Abdominal Aortic Aneurysms. Braz. J. Cardiovasc. Surg. 2018, 33, 265–270. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kuno, T.; Takagi, H. Meta-Analysis of Phase-Specific Survival after Elective Endovascular versus Surgical Repair of Abdominal Aortic Aneurysm from Randomized Controlled Trials and Propensity Score-Matched Studies. J. Vasc. Surg. 2020, 72, 1464–1472.e6. [Google Scholar] [CrossRef] [PubMed]
- Nation, D.A.; Wang, G.J. TEVAR: Endovascular Repair of the Thoracic Aorta. Semin. Interv. Radiol. 2015, 32, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Acharya, Y.; Soliman, O.; Parodi, J.C.; Hynes, N. TEVAR and EVAR, the Unknown Knowns of the Cardiovascular Hemodynamics; and the Immediate and Long-Term Consequences of Fabric Material on Major Adverse Clinical Outcome. Front. Surg. 2022, 9, 940304. [Google Scholar] [CrossRef] [PubMed]
- Khashan, A.; Talib, S.; Hamouda, M.; Kovacs, J.; Ibrar, A.; Saini, G.; Haq, Z.U.; Anantharamakrishnan, B.; Alam, M. Coronary Artery Stent Dislodgement and Loss in the Bloodstream: A Case Report and Management Options. Am. J. Case Rep. 2022, 23, e937598. [Google Scholar] [CrossRef] [PubMed]
- Antonuccio, M.N.; Gasparotti, E.; Bardi, F.; Monteleone, A.; This, A.; Rouet, L.; Avril, S.; Celi, S. Fabrication of Deformable Patient-Specific AAA Models by Material Casting Techniques. Front. Cardiovasc. Med. 2023, 10, 1141623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Keyoumu, R.; Shi, Y.-H.; Wang, Z.; Sun, L.-L.; Liu, Z. 3D Parametric Surface Planar Topological Guide Plate to Create a PMSG for the Emergency Endovascular Repair of an Aortic Arch Aneurysm. J. Card. Surg. 2022, 37, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Jayet, J.; Canonge, J.; Heim, F.; Coggia, M.; Chakfé, N.; Coscas, R. Mechanical Comparison between Fenestrated Endograft and Physician-Made Fenestrations. J. Clin. Med. 2023, 12, 4911. [Google Scholar] [CrossRef] [PubMed]
- Rynio, P.; Jedrzejczak, T.; Rybicka, A.; Milner, R.; Gutowski, P.; Kazimierczak, A. Initial Experience with Fenestrated Physician-Modified Stent Grafts Using 3D Aortic Templates. J. Clin. Med. 2022, 11, 2180. [Google Scholar] [CrossRef] [PubMed]
- Branzan, D.; Geisler, A.; Grunert, R.; Steiner, S.; Bausback, Y.; Gockel, I.; Scheinert, D.; Schmidt, A. The Influence of 3D Printed Aortic Models on the Evolution of Physician Modified Stent Grafts for the Urgent Treatment of Thoraco-Abdominal and Pararenal Aortic Pathologies. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2021, 61, 407–412. [Google Scholar] [CrossRef]
- Mafeld, S.; Logue, J.A.; Masson, S.; Thakkar, R.; Amer, A.; Wilson, C.; Sen, G.; Manas, D.; White, S.; Williams, R. Treatment of Visceral Transplant Pseudoaneurysms Using Physician-Modified Fenestrated Stent Grafts: Initial Experience. Cardiovasc. Interv. Radiol. 2019, 42, 920–926. [Google Scholar] [CrossRef]
- Asciutto, G.; Usai, M.V.; Ibrahim, A.; Oberhuber, A. Early Experience with the Bolton Relay Pro/Plus for Physician-Modified Fenestrated TEVAR. Int. Angiol. J. Int. Union Angiol. 2022, 41, 105–109. [Google Scholar] [CrossRef]
- Budge, J.; Carrell, T.; Yaqub, M.; Wafa, H.; Waltham, M.; Pilecka, I.; Kelly, J.; Murphy, C.; Palmer, S.; Wang, Y.; et al. The ARIA Trial Protocol: A Randomised Controlled Trial to Assess the Clinical, Technical, and Cost-Effectiveness of a Cloud-Based, ARtificially Intelligent Image Fusion System in Comparison to Standard Treatment to Guide Endovascular Aortic Aneurysm Repair. Trials 2024, 25, 214. [Google Scholar] [CrossRef] [PubMed]
- Nargesi, S.; Abutorabi, A.; Alipour, V.; Tajdini, M.; Salimi, J. Cost-Effectiveness of Endovascular Versus Open Repair of Abdominal Aortic Aneurysm: A Systematic Review. Cardiovasc. Drugs Ther. 2021, 35, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 6.4 (updated August 2023). Cochrane, 2023. Available online: www.training.cochrane.org/handbook (accessed on 7 April 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.-S.; Jin, Y.; Zhao, Z.-H.; Wang, C.; Shi, Y.-H.; Zhou, M.-J.; Zhao, J.-X.; Liu, C.; Qiao, T.; Liu, C.-J.; et al. Three-Dimensional Printing to Guide Fenestrated/Branched TEVAR in Triple Aortic Arch Branch Reconstruction with a Curative Effect Analysis. J. Endovasc. Ther. 2023, 15266028231161244. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhu, F.; Cheng, C.; Huang, W.; Zhang, H.; He, X.; Lu, Q.; Xi, H.; Shen, K.; Yu, H. 3D Printing-Assisted versus Conventional Extracorporeal Fenestration Tevar for Stanford Type B Arteries Dissection with Undesirable Proximal Anchoring Zone: Efficacy Analysis. Heart Surg. Forum 2023, 26, E363–E371. [Google Scholar] [CrossRef]
- Tong, Y.-H.; Yu, T.; Zhou, M.-J.; Liu, C.; Zhou, M.; Jiang, Q.; Liu, C.-J.; Li, X.-Q.; Liu, Z. Use of 3D Printing to Guide Creation of Fenestrations in Physician-Modified Stent-Grafts for Treatment of Thoracoabdominal Aortic Disease. J. Endovasc. Ther. 2020, 27, 385–393. [Google Scholar] [CrossRef]
- Lucatelli, P.; Cini, M.; Benvenuti, A.; Saba, L.; Tommasino, G.; Guaccio, G.; Munneke, G.; Neri, E.; Ricci, C. Custom-Made Endograft for Endovascular Repair of Thoraco-Abdominal Aneurysm and Type B Dissection: Single-Centre Experience. Cardiovasc. Interv. Radiol. 2018, 41, 1174–1183. [Google Scholar] [CrossRef]
- Canonge, J.; Jayet, J.; Heim, F.; Chakfé, N.; Coggia, M.; Coscas, R.; Cochennec, F. Comprehensive Review of Physician Modified Aortic Stent Grafts: Technical and Clinical Outcomes. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 560–569. [Google Scholar] [CrossRef]
- Mafeld, S.; Nesbitt, C.; McCaslin, J.; Bagnall, A.; Davey, P.; Bose, P.; Williams, R. Three-Dimensional (3D) Printed Endovascular Simulation Models: A Feasibility Study. Ann. Transl. Med. 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Kärkkäinen, J.M.; Sandri, G.; Tenorio, E.R.; Alexander, A.; Bjellum, K.; Matsumoto, J.; Morris, J.; Mendes, B.C.; DeMartino, R.R.; Oderich, G.S. Simulation of Endovascular Aortic Repair Using 3D Printed Abdominal Aortic Aneurysm Model and Fluid Pump. Cardiovasc. Interv. Radiol. 2019, 42, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Zech, C.J.; Takes, M.; Brantner, P.; Thieringer, F.; Deutschmann, M.; Hergan, K.; Scharinger, B.; Hecht, S.; Rezar, R.; et al. Vascular 3D Printing with a Novel Biological Tissue Mimicking Resin for Patient-Specific Procedure Simulations in Interventional Radiology: A Feasibility Study. J. Digit. Imaging 2022, 35, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Discher, P.; Kreibich, M.; Berger, T.; Kondov, S.; Eschenhagen, M.; Schibilsky, D.; Bork, M.; Walter, T.; Chikvatia, S.; Gottardi, R.; et al. Training in Aortic Arch Surgery as a Blueprint for a Structured Educational Team Approach: A Review. Medicina 2023, 59, 1391. [Google Scholar] [CrossRef]
- Özcan, C.; Kocatürk, Ö.; Işlak, C.; Öztürk, C. Integrated Particle Image Velocimetry and Fluid-Structure Interaction Analysis for Patient-Specific Abdominal Aortic Aneurysm Studies. Biomed. Eng. Online 2023, 22, 113. [Google Scholar] [CrossRef]
- Yuan, D.; Luo, H.; Yang, H.; Huang, B.; Zhu, J.; Zhao, J. Precise Treatment of Aortic Aneurysm by Three-Dimensional Printing and Simulation before Endovascular Intervention. Sci. Rep. 2017, 7, 795. [Google Scholar] [CrossRef]
- Mitsuoka, H.; Terai, Y.; Miyano, Y.; Naitou, T.; Tanai, J.; Kawaguchi, S.; Goto, S.; Miura, Y.; Nakai, M.; Yamazaki, F. Preoperative Planning for Physician-Modified Endografts Using a Three-Dimensional Printer. Ann. Vasc. Dis. 2019, 12, 334–339. [Google Scholar] [CrossRef]
- Rynio, P.; Gutowski, P.; Kazimierczak, A. Physician-Modified Stent-Grafts Created in the Three-Dimensionally Aortic Template Have Better Reliability and Greater Alignment With the Target Vessels Than Stent-Grafts Modified Based on Measurements From Computed Tomography. J. Endovasc. Ther. 2023, 30, 769–778. [Google Scholar] [CrossRef]
- Lee, J.; Chadalavada, S.C.; Ghodadra, A.; Ali, A.; Arribas, E.M.; Chepelev, L.; Ionita, C.N.; Ravi, P.; Ryan, J.R.; Santiago, L.; et al. Clinical Situations for Which 3D Printing Is Considered an Appropriate Representation or Extension of Data Contained in a Medical Imaging Examination: Vascular Conditions. 3D Print. Med. 2023, 9, 34. [Google Scholar] [CrossRef]
- Li, D.; Li, M.; Zhou, X.; An, Q. Comparison of the Fenestrated and Non-Fenestrated Fontan Procedures. Medicine 2019, 98, e16554. [Google Scholar] [CrossRef]
- Touma, J.; Verscheure, D.; Majewski, M.; Desgranges, P.; Cochennec, F. Parallel Grafts Used in Combination with Physician-Modified Fenestrated Stent Grafts for Complex Aortic Aneurysms in High-Risk Patients with Hostile Anatomies. Ann. Vasc. Surg. 2018, 46, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Heidemann, F.; Rohlffs, F.; Diener, H.; Wipper, S.; Debus, E.S.; Kölbel, T. Outcome of Surgeon-Modified Fenestrated/Branched Stent-Grafts for Symptomatic Complex Aortic Pathologies or Contained Rupture. J. Endovasc. Ther. 2017, 24, 825–832. [Google Scholar] [CrossRef]
- Queiroz, A.B.; Lopes, J.B.; Santos, V.P.; Cruz, P.B.A.F.; Fidelis, R.J.R.; Filho, J.S.A.; Passos, L.C.S. Physician-Modified Endovascular Grafts for Zone-2 Thoracic Endovascular Aortic Repair. Aorta 2022, 10, 13–19. [Google Scholar] [CrossRef]
- Kubíček, L.; Staffa, R.; Vlachovský, R.; Novotný, T.; Biroš, E. Modern Diagnostic Approach to Patients with Abdominal Aortic Aneurysm. Rozhl. Chir. Mesic. Ceskoslovenske Chir. Spol. 2022, 101, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, B.; Cui, C. In Situ Fenestration Combined with in Vitro Pre-Fenestration Techniques for Treating Multiple Aortic Aneurysms. Asian J. Surg. 2023, 46, 4094–4095. [Google Scholar] [CrossRef]
- Wang, T.-H.; Zhao, J.-C.; Xiong, F.; Yang, Y. Use of Three Dimensional-Printing in the Management of Floating Aortic Thrombus Due to Occult Aortic Dissection: A Case Report. World J. Clin. Cases 2021, 9, 1755–1760. [Google Scholar] [CrossRef]
- Tinelli, G.; D’Oria, M.; Sica, S.; Mani, K.; Rancic, Z.; Resh, T.A.; Beccia, F.; Azizzadeh, A.; Da Volta Ferreira, M.M.; Gargiulo, M.; et al. The Sac Evolution Imaging Follow-up after Endovascular Aortic Repair: An International Expert Opinion-Based Delphi Consensus Study. J. Vasc. Surg. 2024, Epub ahead of print. [Google Scholar] [CrossRef]
- Tsialtas, D.; Bolognesi, M.G.; Assimopoulos, S.; Azzarone, M.; Volpi, R.; Bolognesi, R. Perioperative Complications Following Major Vascular Surgery. Correlations with Preoperative Clinical, Electrocardiographic and Echocardiographic Features. Acta Bio-Medica Atenei Parm. 2022, 93, e2022255. [Google Scholar] [CrossRef]
- Geiger, J.T.; Aquina, C.T.; Esce, A.; Zhao, P.; Glocker, R.; Fleming, F.; Iannuzzi, J.; Stoner, M.; Doyle, A. One-Year Patient Survival Correlates with Surgeon Volume after Elective Open Abdominal Aortic Surgery. J. Vasc. Surg. 2021, 73, 108–116.e1. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Cremat, D.L.; Serpa, E.; Qian, S.; Blebea, J. Applying Artificial Intelligence to Predict Complications After Endovascular Aneurysm Repair. Vasc. Endovasc. Surg. 2024, 58, 65–75. [Google Scholar] [CrossRef]
- China: Health Expenditure. Available online: https://www.statista.com/statistics/279400/health-expenditures-in-china/ (accessed on 6 May 2024).
- Poland: Healthcare Expenditure as a Share of GDP 2022. Available online: https://www.statista.com/statistics/429697/healthcare-expenditure-as-a-share-of-gdp-in-poland/ (accessed on 6 May 2024).
- Germany: Health Expenditure as a Share of GDP 1980–2022. Statista. Available online: https://www.statista.com/statistics/429202/healthcare-expenditure-as-a-share-of-gdp-in-germany/ (accessed on 6 May 2024).
- South Korea: National Health Spending. 2022. Available online: https://www.statista.com/statistics/647320/health-spending-south-korea/ (accessed on 6 May 2024).
- Bachrati, P.Z.; La Torre, G.; Chowdhury, M.M.; Healy, S.J.; Singh, A.A.; Boyle, J.R. A State-of-the-Art Review of Intra-Operative Imaging Modalities Used to Quality Assure Endovascular Aneurysm Repair. J. Clin. Med. 2023, 12, 3167. [Google Scholar] [CrossRef]
- European Society of Radiology (ESR). Medical Imaging in Personalised Medicine: A White Paper of the Research Committee of the European Society of Radiology (ESR). Insights Imaging 2015, 6, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, J.K.; Kim, H.R.; Kim, T.; Lee, S.; Kim, G.B.; Yang, D.H.; Kim, J.B. The Result of Prospective Evaluation of 3-Dimensional Printing-Aided Extensive Thoracoabdominal Aorta Repair. JTCVS Tech. 2023, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Forero, D.A.; Lopez-Leon, S.; González-Giraldo, Y.; Bagos, P.G. Ten Simple Rules for Carrying out and Writing Meta-Analyses. PLoS Comput. Biol. 2019, 15, e1006922. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R. Randomized Controlled Trials: Still the Backbone of Vascular Surgery? Gefasschirurgie 2016, 21, 25–30. [Google Scholar] [CrossRef]
- Wawak, M.; Tekieli, Ł.; Badacz, R.; Pieniążek, P.; Maciejewski, D.; Trystuła, M.; Przewłocki, T.; Kabłak-Ziembicka, A. Clinical Characteristics and Outcomes of Aortic Arch Emergencies: Takayasu Disease, Fibromuscular Dysplasia, and Aortic Arch Pathologies: A Retrospective Study and Review of the Literature. Biomedicines 2023, 11, 2207. [Google Scholar] [CrossRef]


| Author | Year of Publication | Patients (n) | Type of Surgery | Mean Modification Time [Minutes] | Mean Procedure Time [Minutes] | Optimal Angiographic Result Obtained (%) | 30-Day Survival Rate (%) | Mean Follow-Up [Months] |
|---|---|---|---|---|---|---|---|---|
| Fu et al. [27] | 2023 | 44 | FEVAR, BEVAR | 44.05 ± 7.72 | 298.2 ± 84 | 100 | 100 | 6 (42 patients,) 12 (35 Patients) |
| Zheng et al. [28] | 2023 | 32 | TEVAR | 37.63 ± 2.99 | 147.84 ± 33.94 | 100 | 100 | 16.14 ± 3.76 |
| Rynio et al. [18] | 2022 | 43 | FEVAR, BEVAR | 86 ± 12 | 247 ± 70 | 86.05 | 88 | 14 ± 12 |
| Branzan et al. [19] | 2021 | 19 | FEVAR | 109.6 ± 10.7 | 161 ± 95 | 100 | 100 | 14.4 |
| Tong et al. [29] | 2020 | 34 | TEVAR | 75.6 ± 21 | 336 ± 72 | 100 | 100 | 8.5 |
| Author | Software ** | Model of the 3D Printer *** | Name of the Endograft Modified * | Sterilization Technique |
|---|---|---|---|---|
| Fu et al. [27] | Mimics, Geomagic Studio 2014, Geomagic Design Direct | Eden260VS | Ankura, Valiant Captivia, Endurant, Fluency, Viabahn | Ethylene Oxide |
| Zheng et al. [28] | Mimics, Geomagic Studio 2014 | Eden260VS | Ankura | Ethylene Oxide |
| Rynio et al. [18] | 3D Slicer 11.0, PreForm | Form 2 | Valiant Captiva | Hydrogen Peroxide plasma, Ethylene Oxide |
| Branzan et al. [19] | Geomagic DesignX 2019 | Form 2 | Valiant Captivia, Endurant | Steam pressure |
| Tong et al. [29] | Mimics, Geomagic Studio 2014, EndoSize, CAD | Eden260VS | Ankura, Endurant, Zenith, Viabahn | Ethylene Oxide |
| Author | Year | Country | Endoleak Type (Early and Late) | Infection | Neurological | Acute Kidney Failure | Retrograde Dissection | Post-Op Pain | All Patients | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | Unknown | Cerebral Infarction | Spinal Cord Ischemia | ||||||||
| Fu et al. [27] | 2023 | China | 2 | - | 2 | - | - | 1 | - | - | 1 | - | 44 |
| Rynio et al. [18] | 2022 | Poland | 3 | 7 | 2 | 1 | - | - | - | - | - | 43 | |
| Branzan et al. [19] | 2021 | Germany | 2 | 1 | - | - | 1 | - | 2 | 1 | - | - | 19 |
| Zheng et al. [28] | 2023 | China | 4 | 1 | - | - | 3 | 1 | - | - | - | 2 | 32 |
| Tong et al. [29] | 2020 | China | - | - | - | 5 | - | 1 | - | - | 1 | - | 34 |
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 | D11 | D12 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fu et al. [27] | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 0 | – | – | – | – | 9 |
| Zheng et al. [28] | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 19 |
| Rynio et al. [18] | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | – | – | – | – | 9 |
| Branzan et al. [19] | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | – | – | – | – | 11 |
| Tong et al. [29] | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | – | – | – | – | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasada, W.A.; Stępak, H.; Węglewska, M.; Świątek, Ł.; Kluba, J.; Krasiński, Z. The Utility of Three-Dimensional Printing in Physician-Modified Stent Grafts for Aortic Lesions Repair. J. Clin. Med. 2024, 13, 2977. https://doi.org/10.3390/jcm13102977
Zasada WA, Stępak H, Węglewska M, Świątek Ł, Kluba J, Krasiński Z. The Utility of Three-Dimensional Printing in Physician-Modified Stent Grafts for Aortic Lesions Repair. Journal of Clinical Medicine. 2024; 13(10):2977. https://doi.org/10.3390/jcm13102977
Chicago/Turabian StyleZasada, Wiktoria Antonina, Hubert Stępak, Magdalena Węglewska, Łukasz Świątek, Jerzy Kluba, and Zbigniew Krasiński. 2024. "The Utility of Three-Dimensional Printing in Physician-Modified Stent Grafts for Aortic Lesions Repair" Journal of Clinical Medicine 13, no. 10: 2977. https://doi.org/10.3390/jcm13102977
APA StyleZasada, W. A., Stępak, H., Węglewska, M., Świątek, Ł., Kluba, J., & Krasiński, Z. (2024). The Utility of Three-Dimensional Printing in Physician-Modified Stent Grafts for Aortic Lesions Repair. Journal of Clinical Medicine, 13(10), 2977. https://doi.org/10.3390/jcm13102977







