Contemporary Management of the Failing Fontan
Abstract
:1. Introduction
2. Single-Ventricle Patients Have the Highest Mortality
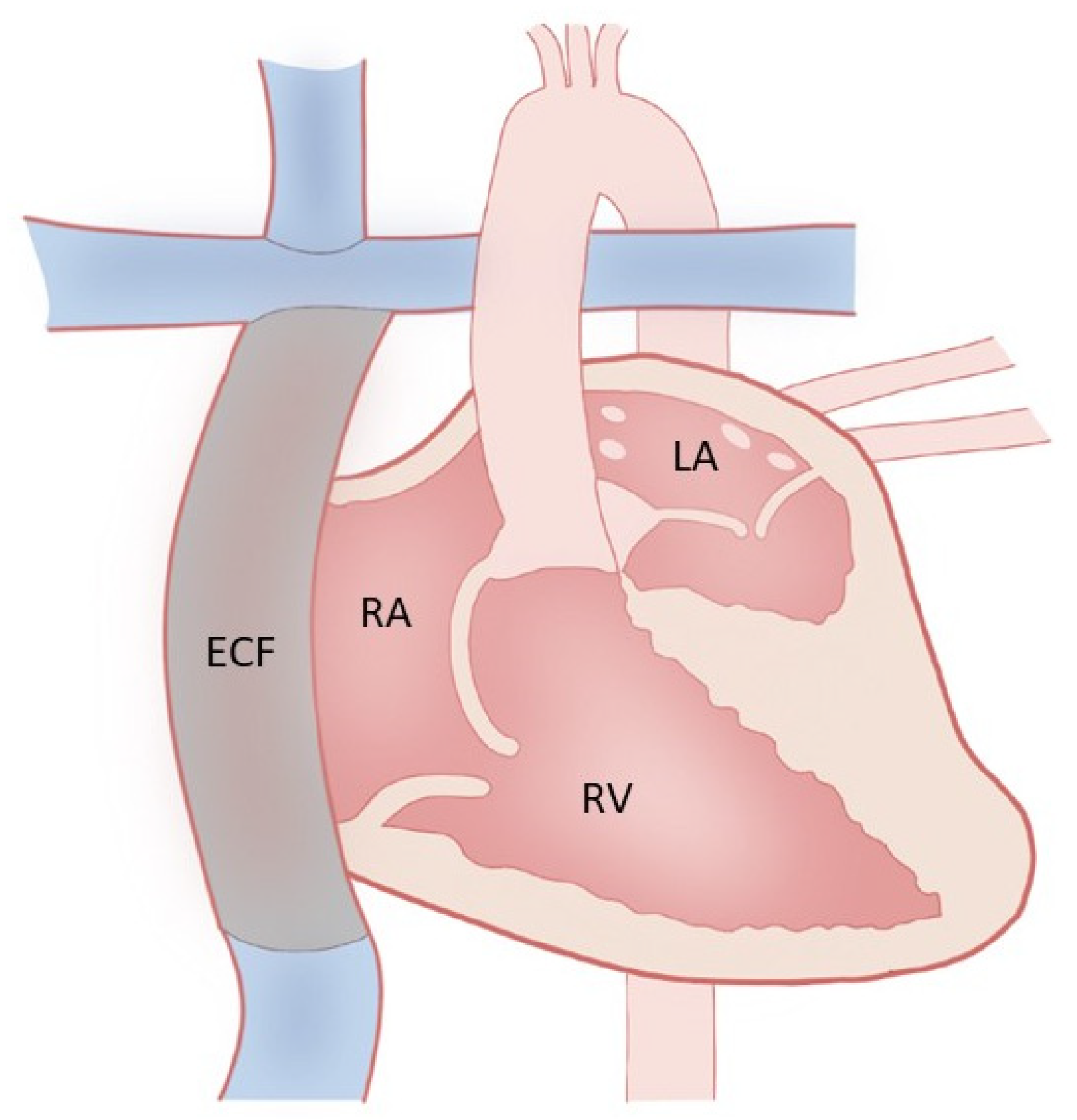
3. Fontan Palliation and Why
4. Fontan Physiology and Failure
5. Systolic Dysfunction
6. Diagnosis
7. Diastolic Dysfunction
8. Diagnosis of Diastolic Dysfunction
9. Atrioventricular Valve Regurgitation (AVVR)
10. Diagnosis of AVVR
11. Lymphatic Insufficiency
12. Diagnosis of Protein-Losing Enteropathy and Plastic Bronchitis
13. Arrhythmia
14. Diagnosis
15. Medical Treatment (Table 2)
16. Treatment of Increased Pulmonary Vascular Resistance (PVR)
17. Treatment of Arrhythmias
18. Advanced Therapies for Heart Failure
Use of Inotropes
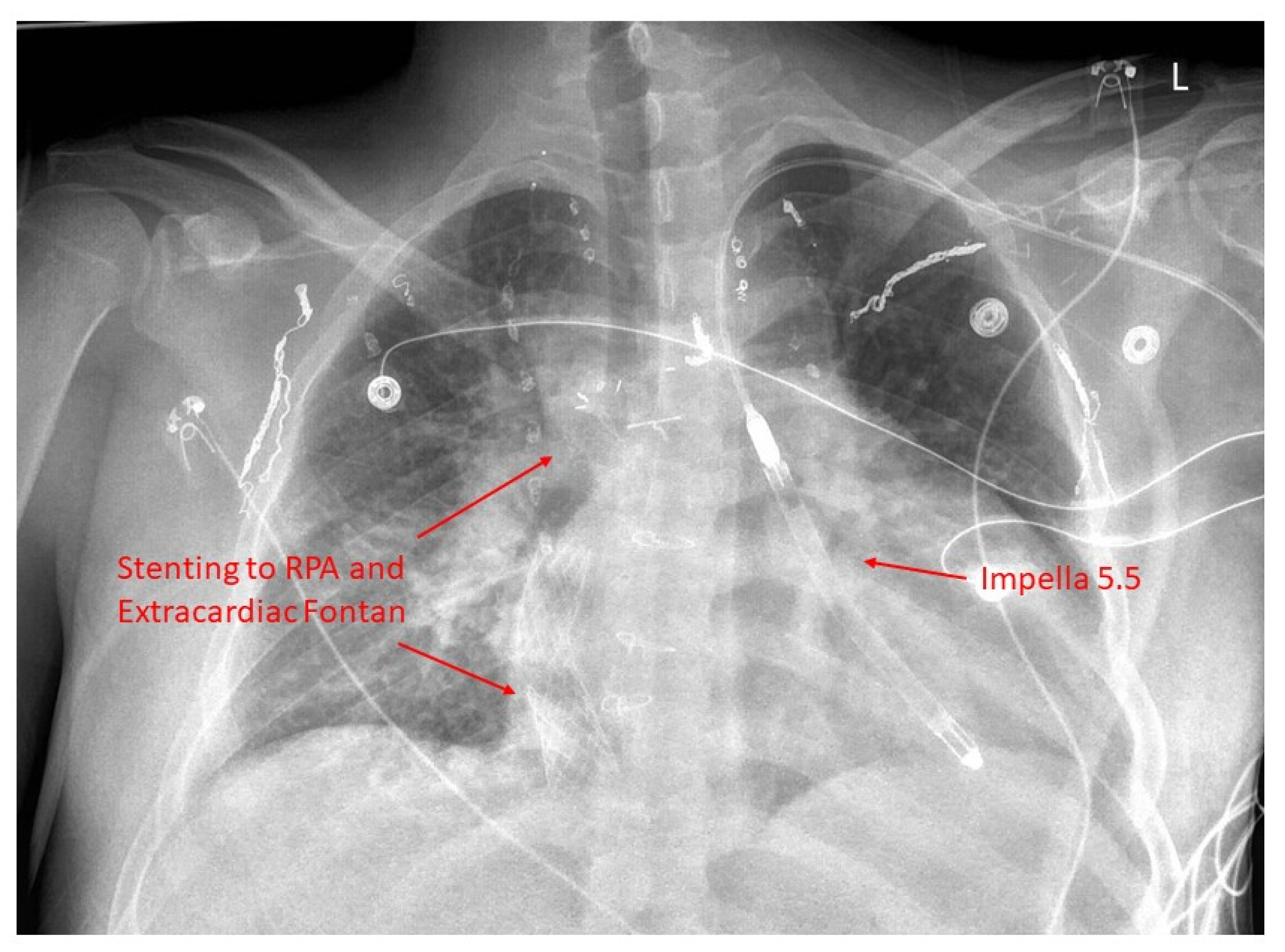
19. Mechanical Circulatory Support
20. Transplantation
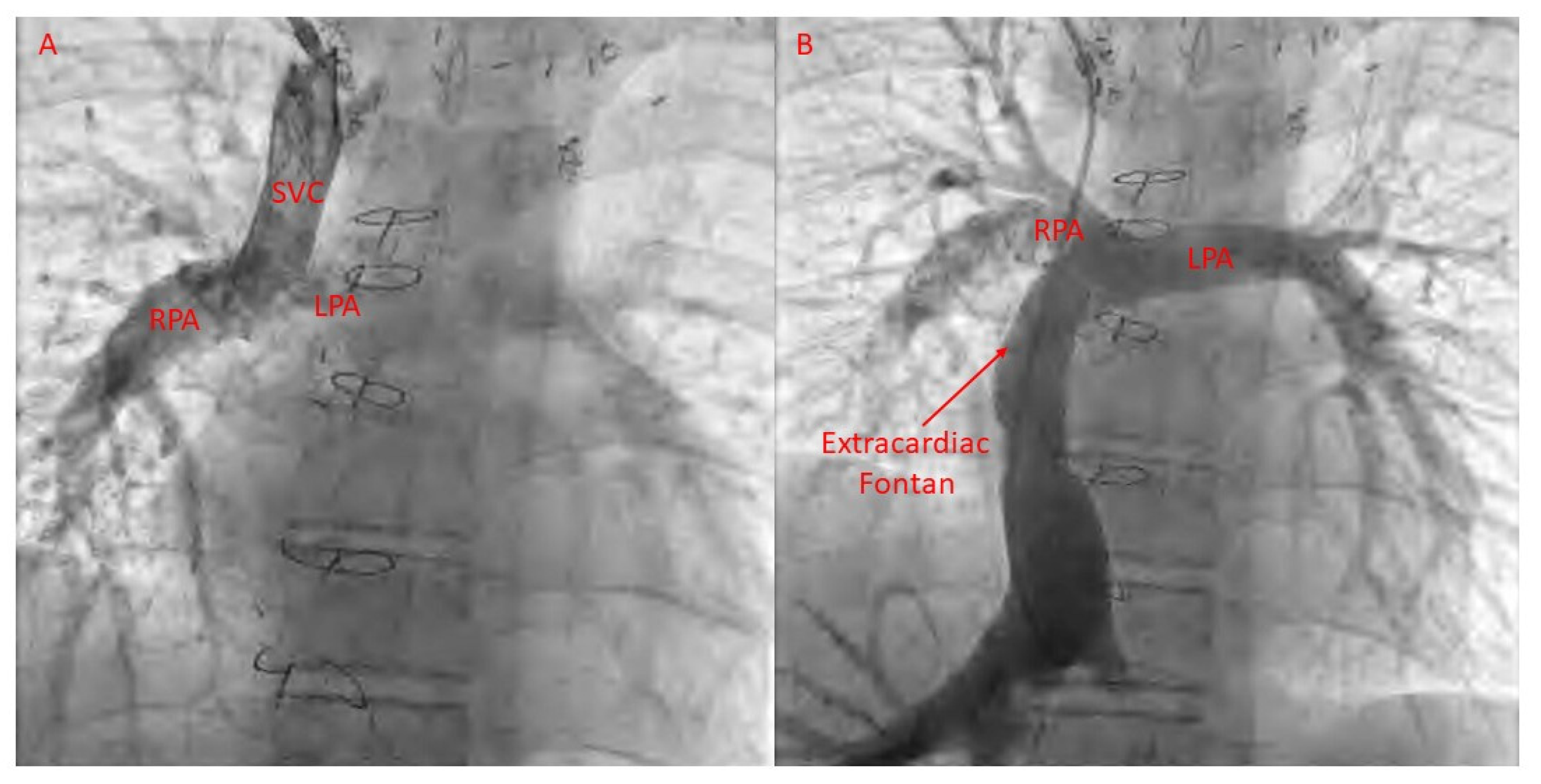
21. Catheter-Based Therapies for Fontan Failure
Arrhythmias
22. Fontan Pathway Obstruction
23. Lymphatic Disease
24. Lymphatic Embolization with Ethiodized Oil
25. Thoracic Duct Embolization
26. Selective Lymphatic Duct Embolization plus Covered Stent Placement
27. Liver Lymphatic Embolization (LLE)
28. Percutaneous Thoracic Duct (TD) Decompression
29. Percutaneous Intervention for AV Valve Regurgitation
30. Collateral Vessels
31. Summary
Funding
Conflicts of Interest
References
- Van Der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Skoglund, K.; Lappas, G.; Eriksson, P.; Dellborg, M. Survivorship in children and young adults with congenital heart disease in Sweden. JAMA Intern. Med. 2017, 177, 224–230. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Giang, K.W.; Eriksson, P.; Liden, H.; Synnergren, M.; Wahlander, H.; Fedchenko, M.; Rosengren, A.; Dellborg, M. Survival in children with congenital heart disease: Have we reached a peak at 97%? J. Am. Heart Assoc. 2020, 9, e017704. [Google Scholar] [CrossRef]
- Fedchenko, M.; Mandalenakis, Z.; Giang, K.W.; Rosengren, A.; Eriksson, P.; Dellborg, M. Long-term outcomes after myocardial infarction in middle-aged and older patients with congenital heart disease-a nationwide study. Eur. Heart J. 2021, 42, 2577–2586. [Google Scholar] [CrossRef]
- Björk, A.; Mandalenakis, Z.; Giang, K.W.; Rosengren, A.; Eriksson, P.; Dellborg, M. Incidence of Type 1 diabetes mellitus and effect on mortality in young patients with congenital heart defect—A nationwide cohort study. Int. J. Cardiol. 2020, 310, 58–63. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Karazisi, C.; Skoglund, K.; Rosengren, A.; Lappas, G.; Eriksson, P.; Dellborg, M. Risk of cancer among children and young adults with congenital heart disease compared with healthy controls. JAMA Netw. Open 2019, 2, e196762. [Google Scholar] [CrossRef]
- Bergh, N.; Skoglund, K.; Fedchenko, M.; Bollano, E.; Eriksson, P.; Dellborg, M.; Giang, K.W.; Mandalenakis, Z. Risk of heart failure in congenital heart disease: A nationwide register-based cohort study. Circulation 2023, 147, 982–984. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Gilljam, T.; Hansson, P.-O.; Skoglund, K.; Fedchenko, M.; Dellborg, M. Atrial fibrillation burden in young patients with congenital heart disease. Circulation 2018, 137, 928–937. [Google Scholar] [CrossRef]
- Jain, P.N.; Salciccioli, K.B.; Guffey, D.; Byun, J.; Cotts, T.B.; Ermis, P.; Gaies, M.; Ghanayem, N.; Kim, F.; Lasa, J.J.; et al. Risk factors for perioperative morbidity in adults undergoing cardiac surgery at children’s hospitals. Ann. Thorac. Surg. 2022, 113, 2062–2070. [Google Scholar] [CrossRef]
- Perinpanayagam, M.; Larsen, S.H.; Emmertsen, K.; Møller, M.B.; Hjortdal, V.E. Nineteen years of adult congenital heart surgery in a single center. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.S.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime Prevalence of Congenital Heart Disease in the General Population from 2000 to 2010. Circulation 2014, 130, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Norozi, K.; Caroline, J.; Sedlak, N.; Bock, J.; Paul, T.; Geyer, S.; Dellas, C. Morbidity and mortality in adults with congenital heart defects in the third and fourth life decade. Clin. Res. Cardiol. 2022, 111, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.-P.; Kempny, A.; Alonso-Gonzalez, R.; Swan, L.; Uebing, A.; Li, W.; Babu-Narayan, S.; Wort, S.J.; Dimopoulos, K.; Gatzoulis, M.A. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation 2015, 132, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Moore, B.M.; Kotchetkova, I.; Cordina, R.L.; Celermajer, D.S. Causes of death in a contemporary adult congenital heart disease cohort. Heart 2018, 104, 1678–1682. [Google Scholar] [CrossRef]
- Constantine, A.; Ferrero, P.; Gribaudo, E.; Mitropoulou, P.; Krishnathasan, K.; Costola, G.; Lwin, M.T.; Fitzsimmons, S.; Brida, M.; Montanaro, C.; et al. Morbidity and mortality in adults with a Fontan circulation beyond the fourth decade of life. Eur. J. Prev. Cardiol. 2024, zwae031. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Brown, S.C. The Fontan circulation after 45 years: Update in physiology. Heart 2016, 102, 1081–1086. [Google Scholar] [CrossRef]
- Kamsheh, A.M.; O’connor, M.J.; Rossano, J.W. Management of circulatory failure after Fontan surgery. Front. Pediatr. 2022, 10, 1020984. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Rathod, R.H.; Aboulhosn, J.A.; Budts, W.; Anderson, J.B.; Baumgartner, H.; Brown, D.W.; Cordina, R.; D’Udekem, Y.; Ginde, S.; et al. Reaching consensus for unified medical language in Fontan care. ESC Heart Fail. 2021, 8, 3894–3905. [Google Scholar] [CrossRef]
- Miller, J.R.; Simpson, K.E.; Epstein, D.J.; Lancaster, T.S.; Henn, M.C.; Schuessler, R.B.; Balzer, D.T.; Shahanavaz, S.; Murphy, J.J.; Canter, C.E.; et al. Improved survival after heart transplant for failed Fontan patients with preserved ventricular function. J. Heart Lung Transplant. 2016, 35, 877–883. [Google Scholar] [CrossRef]
- Anderson, P.A.; Sleeper, L.A.; Mahony, L.; Colan, S.D.; Atz, A.M.; Breitbart, R.E.; Gersony, W.M.; Gallagher, D.; Geva, T.; Margossian, R.; et al. Contemporary outcomes after the Fontan procedure: A Pediatric Heart Network multicenter study. Circulation 2008, 52, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and management of the child and adult with Fontan circulation: A scientific statement from the American Heart Association. Circulation 2019, 140, E234–E284. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.H.; Prakash, A.; Powell, A.J.; Geva, T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J. Am. Coll. Cardiol. 2010, 55, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quintana, D.; Climent, V.; Ho, S.Y.; Anderson, R.H. Myoarchitecture and connective tissue in hearts with tricuspid atresia. Heart 1999, 81, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Rösner, A.; Khalapyan, T.; Dalen, H.; McElhinney, D.B.; Friedberg, M.K.; Lui, G.K. Classic-pattern dyssynchrony in adolescents and adults with a Fontan circulation. J. Am. Soc. Echocardiogr. 2018, 31, 211–219. [Google Scholar] [CrossRef]
- Rychik, J.; A Fogel, M.; Donofrio, M.T.; Goldmuntz, E.; Cohen, M.S.; Spray, T.L.; Jacobs, M.L. Comparison of patterns of pulmonary venous blood flow in the functional single ventricle heart after operative aortopulmonary shunt versus superior cavopulmonary shunt. Am. J. Cardiol. 1997, 80, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Rathod, R.H.; Prakash, A.; Kim, Y.Y.; Germanakis, I.E.; Powell, A.J.; Gauvreau, K.; Geva, T. Cardiac magnetic resonance parameters predict transplantation-free survival in patients with fontan circulation. Circ. Cardiovasc. Imaging 2014, 7, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.L.; Clair, N.S.; Powell, A.J.; Geva, T.; Rathod, R.H. Integrated clinical and magnetic resonance imaging assessments late after Fontan operation. Circulation 2021, 77, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.A. The Fontan: “Straining” to Understand That “the Bigger They Come, the Harder They Fall”; American College of Cardiology Foundation: Washington, DC, USA, 2021; pp. 2490–2493. [Google Scholar]
- Bosch, E.v.D.; Bossers, S.S.; Robbers-Visser, D.; Boersma, E.; Roos-Hesselink, J.W.; Breur, H.M.; Blom, N.A.; Kroft, L.J.; Snoeren, M.M.; Kapusta, L.; et al. Ventricular response to dobutamine stress CMR is a predictor for outcome in Fontan patients. JACC Cardiovasc. Imaging 2019, 12, 368–370. [Google Scholar] [CrossRef]
- Budts, W.; Ravekes, W.J.; Danford, D.A.; Kutty, S. Diastolic heart failure in patients with the Fontan circulation: A review. JAMA Cardiol. 2020, 5, 590–597. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Penny, D.J.; Redington, A.N. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart 2000, 83, 420–424. [Google Scholar] [CrossRef]
- Miranda, W.R.; Warnes, C.A.; Connolly, H.M.; Taggart, N.W.; O’Leary, P.W.; Oh, J.K.; Egbe, A.C. Echo-Doppler assessment of ventricular filling pressures in adult Fontan patients. Int. J. Cardiol. 2019, 284, 28–32. [Google Scholar] [CrossRef]
- Takahashi, K.; Inage, A.; Rebeyka, I.; Ross, D.; Thompson, R.; Mackie, A.; Smallhorn, J. Real-time 3-dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation 2009, 120, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Ayer, J.; Celermajer, D.; Zentner, D.; Justo, R.; Disney, P.; Zannino, D.; D’udekem, Y. Atrioventricular valve failure in Fontan palliation. J. Am. Coll. Cardiol. 2019, 73, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Siddiqui, S.; Di Maria, M.V.; Hill, G.D.; Lubert, A.M.; Kutty, S.; Opotowsky, A.R.; Possner, M.; Morales, D.L.S.; Quintessenza, J.A.; et al. Atrioventricular Valve Regurgitation in Single Ventricle Heart Disease: A Common Problem Associated with Progressive Deterioration and Mortality. J. Am. Heart Assoc. 2020, 9, e015737. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.; Hagler, D.J.; Sauer, U.; Somerville, J.; Gewillig, M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. J. Thorac. Cardiovasc. Surg. 1998, 115, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Feldt, R.H.; Driscoll, D.J.; Offord, K.P.; Cha, R.H.; Perrault, J.; Schaff, H.V.; Puga, F.J.; Danielson, G.K. Protein-losing enteropathy after the Fontan operation. J. Thorac. Cardiovasc. Surg. 1996, 112, 672–680. [Google Scholar] [CrossRef]
- Caruthers, R.L.; Kempa, M.; Loo, A.; Gulbransen, E.; Kelly, E.; Erickson, S.R.; Hirsch, J.C.; Schumacher, K.R.; Stringer, K.A. Demographic characteristics and estimated prevalence of Fontan-associated plastic bronchitis. Pediatr. Cardiol. 2013, 34, 256–261. [Google Scholar] [CrossRef]
- Biko, D.M.; DeWitt, A.G.; Pinto, E.M.; Morrison, R.E.; Johnstone, J.A.; Griffis, H.; O’byrne, M.L.; Fogel, M.A.; Harris, M.A.; Partington, S.L.; et al. MRI Evaluation of lymphatic abnormalities in the neck and thorax after Fontan surgery: Relationship with outcome. Radiology 2019, 291, 774–780. [Google Scholar] [CrossRef]
- John, A.S.; Johnson, J.A.; Khan, M.; Driscoll, D.J.; Warnes, C.A.; Cetta, F. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J. Am. Coll. Cardiol. 2014, 64, 54–62. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yagihara, T.; Kagisaki, K.; Hagino, I.; Kobayashi, J. Ventricular performance in long-term survivors after Fontan operation. Ann. Thorac. Surg. 2011, 91, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.J.; Batra, A.S.; Burch, G.H.; Shen, I.; Ungerleider, R.M.; Brown, J.W.; Turrentine, M.W.; Mori, M.; Hsieh, Y.-C.; Balaji, S. Acute hemodynamic effects of pacing in patients with Fontan physiology: A prospective study. J. Am. Coll. Cardiol. 2005, 46, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.F.; Redington, A.N. Pathophysiology and management of heart failure in repaired congenital heart disease. Heart Fail. Clin. 2010, 6, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.E.; Boucek, M.M.; Hsu, D.T.; Boucek, R.J.; Canter, C.E.; Mahony, L.; Ross, R.D.; Pahl, E.; Blume, E.D.; Dodd, D.A.; et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 2007, 298, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Nakazawa, M.; Tomimatsu, H.; Momma, K. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: A controlled pilot study. J. Am. Coll. Cardiol. 2000, 36, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Ringel, R.E.; Peddy, S.B. Effect of high-dose spironolactone on protein-losing enteropathy in patients with Fontan palliation of complex congenital heart disease. Am. J. Cardiol. 2003, 91, 1031–1032. [Google Scholar] [CrossRef] [PubMed]
- Okano, S.; Sugimoto, M.; Takase, M.; Iseki, K.; Kajihama, A.; Azuma, H. Effectiveness of high-dose spironolactone therapy in a patient with recurrent protein-losing enteropathy after the Fontan procedure. Intern. Med. 2016, 55, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Giardini, A.; Balducci, A.; Specchia, S.; Gargiulo, G.; Bonvicini, M.; Picchio, F.M. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur. Heart J. 2008, 29, 1681–1687. [Google Scholar] [CrossRef]
- Herbert, A.; Mikkelsen, U.R.; Thilen, U.; Idorn, L.; Jensen, A.S.; Nagy, E.; Hanseus, K.; Sørensen, K.E.; Søndergaard, L. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: The TEMPO (treatment with endothelin receptor antagonist in Fontan patients, a randomized placebo-controlled, double-blind study measuring peak oxygen consumption) study. Circulation 2014, 130, 2021–2030. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Zak, V.; Goldstein, B.H.; Schumacher, K.R.; Rhodes, J.; Penny, D.J.; Petit, C.J.; Ginde, S.; Menon, S.C.; Kim, S.-H.; et al. Results of the FUEL Trial. Circulation 2020, 141, 641–651. [Google Scholar] [CrossRef]
- Weingarten, A.J.; Menachem, J.N.; Smith, C.A.; Frischhertz, B.P.; Book, W.M. Usefulness of midodrine in protein-losing enteropathy. J. Heart Lung Transplant. 2019, 38, 784–787. [Google Scholar] [CrossRef]
- Schumacher, K.R.; Cools, M.; Goldstein, B.H.; Ioffe-Dahan, V.; King, K.; Gaffney, D.; Russell, M.W. Oral budesonide treatment for protein-losing enteropathy in Fontan-palliated patients. Pediatr. Cardiol. 2011, 32, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Connolly, H.M.; Khan, A.R.; Niaz, T.; Said, S.S.; Dearani, J.A.; Warnes, C.A.; Deshmukh, A.J.; Kapa, S.; McLeod, C.J. Outcomes in adult Fontan patients with atrial tachyarrhythmias. Am. Heart J. 2017, 186, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.; O’Leary, E.T.; Mah, D.Y.; Harrild, D.M.; Rhodes, J. Cardiac resynchronization therapy improves the ventricular function of patients with Fontan physiology. Am. Heart J. 2020, 230, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, L.E.; Berg, L.E.M.V.; Ismailova, G.; Dulfer, K.; Takkenberg, J.J.M.; A Helbing, W. Physical exercise training in patients with a Fontan circulation: A systematic review. Eur. J. Prev. Cardiol. 2021, 28, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Shannon, K.M. Transpulmonary atrial pacing: An approach transvenous pacemaker implantation after extracardiac conduit Fontan surgery. J. Cardiovasc. Electrophysiol. 2014, 25, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, L.M.M.; Alexander, P.M.A.M.; Butt, W.W.F.; Shann, F.A.M.; Penny, D.J.M.; Shekerdemian, L.S.M. Rotating inotrope therapy in a pediatric population with decompensated heart failure. Pediatr. Crit. Care Med. 2011, 12, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.M.; Dunbar-Masterson, C.; Allan, C.K.; Gauvreau, K.; Newburger, J.W.; McGowan, F.X.; Wessel, D.L.; Mayer, J.E.; Salvin, J.W.; Dionne, R.E.; et al. Impact of empiric nesiritide or milrinone infusion on early postoperative recovery after Fontan surgery: A randomized, double-blind, placebocontrolled trial. Circ. Heart Fail. 2014, 7, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, G.K.; Ramamoorthy, C.; Lynn, A.M.; French, J.; Stevenson, J.G. Hemodynamic effects of amrinone in children after Fontan surgery. Anesth. Analg. 1996, 82, 241–246. [Google Scholar]
- Elkayam, U.; Tasissa, G.; Binanay, C.; Stevenson, L.W.; Gheorghiade, M.; Warnica, J.W.; Young, J.B.; Rayburn, B.K.; Rogers, J.G.; DeMarco, T.; et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am. Heart J. 2007, 153, 98–104. [Google Scholar] [CrossRef]
- Friedland-Little, J.M.; Gajarski, R.J.; Schumacher, K.R. Dopamine as a potential rescue therapy for refractory protein-losing enteropathy in Fontan-palliated patients. Pediatr. Transplant. 2017, 21, e12925. [Google Scholar] [CrossRef]
- Miller, J.R.; Lancaster, T.S.; Callahan, C.; Abarbanell, A.M.; Eghtesady, P. An overview of mechanical circulatory support in single-ventricle patients. Transl. Pediatr. 2018, 7, 151–161. [Google Scholar] [CrossRef]
- Cedars, A.; Kutty, S.; Danford, D.; Schumacher, K.; Auerbach, S.; Bearl, D.; Chen, S.; Conway, J.; Dykes, J.; Jaworski, N.; et al. Systemic ventricular assist device support in Fontan patients: A report by ACTION. J. Heart Lung Transplant. 2021, 40, 368–376. [Google Scholar] [CrossRef]
- Poh, C.L.; Chiletti, R.; Zannino, D.; Brizard, C.; Konstantinov, I.E.; Horton, S.; Millar, J.; D’udekem, Y. Ventricular assist device support in patients with single ventricles: The Melbourne experience. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 310–316. [Google Scholar] [CrossRef]
- Chen, S.; Rosenthal, D.N.; Murray, J.; Dykes, J.C.; Almond, C.S.; Yarlagadda, V.V.; Wright, G.; Navaratnam, M.; Reinhartz, O.; Maeda, K. Bridge to transplant with ventricular assist device support in pediatric patients with single ventricle heart disease. ASAIO J. 2020, 66, 205–211. [Google Scholar] [CrossRef]
- O’Connor, M.J.; Lorts, A.; Davies, R.R.; Fynn-Thompson, F.; Joong, A.; Maeda, K.; Mascio, C.E.; McConnell, P.; Monge, M.C.; Nandi, D.; et al. Early experience with the HeartMate 3 continuous-flow ventricular assist device in pediatric patients and patients with congenital heart disease: A multicenter registry analysis. J. Heart Lung Transplant. 2020, 39, 573–579. [Google Scholar] [CrossRef]
- Rossano, J.W.; VanderPluym, C.J.; Peng, D.M.; Hollander, S.A.; Maeda, K.; Adachi, I.; Davies, R.R.; Simpson, K.E.; Fynn-Thompson, F.; Conway, J.; et al. Fifth annual pediatric interagency registry for mechanical circulatory support (Pedimacs) report. Ann. Thorac. Surg. 2021, 112, 1763–1774. [Google Scholar] [CrossRef]
- Nandi, D.; Miller, K.D.; Bober, C.M.; Rosenthal, T.M.; Montenegro, L.M.; Rossano, J.W.; Gaynor, J.W.; Mascio, C.E. Systemic atrioventricular valve excision and ventricular assist devices in pediatric patients. Ann. Thorac. Surg. 2018, 105, 170–174. [Google Scholar] [CrossRef]
- Villa, C.R.; Lorts, A.; Kasten, J.; Chin, C.; Alsaied, T.; Tiao, G.; Nathan, J.D.; Peters, A.L.; Misfeldt, A.M.; Vranicar, M.; et al. Bridge to Heart-Liver Transplantation with a Ventricular Assist Device in the Fontan Circulation. Circ. Heart Fail. 2021, 14, e008018. [Google Scholar] [CrossRef]
- Colvin, M.; Smith, J.M.; Hadley, N.; Skeans, M.A.; Uccellini, K.; Lehman, R.; Robinson, A.M.; Israni, A.K.; Snyder, J.J.; Kasiske, B.L. OPTN/SRTR 2018 annual data report: Heart. Am. J. Transplant. 2020, 20 (Suppl. S1), 340–426. [Google Scholar] [CrossRef]
- Rossano, J.W.; Goldberg, D.J.; Fuller, S.; Ravishankar, C.; Montenegro, L.M.; Gaynor, J.W. Successful use of the total artificial heart in the failing Fontan circulation. Ann. Thorac. Surg. 2014, 97, 1438–1440. [Google Scholar] [CrossRef]
- Prêtre, R.; Häussler, A.; Bettex, D.; Genoni, M. Right-sided univentricular cardiac assistance in a failing Fontan circulation. Ann. Thorac. Surg. 2008, 86, 1018–1020. [Google Scholar] [CrossRef]
- Coffman, Z.J.; Bandisode, V.M.; Kavarana, M.N.; Buckley, J.R. Utilization of an Abiomed Impella Device as a rescue therapy for acute ventricular failure in a Fontan patient. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 518–519. [Google Scholar] [CrossRef]
- Morray, B.H.; Dimas, V.V.; Lim, S.; Balzer, D.T.; Parekh, D.R.; Van Mieghem, N.M.; Ewert, P.; Kim, D.W.; Justino, H.; McElhinney, D.B.; et al. Circulatory support using the impella device in fontan patients with systemic ventricular dysfunction: A multicenter experience. Catheter. Cardiovasc. Interv. 2017, 90, 118–123. [Google Scholar] [CrossRef]
- Alshawabkeh, L.I.; Hu, N.; Carter, K.; Opotowsky, A.R.; Light-McGroary, K.; Cavanaugh, J.E.; Bartlett, H.L. Wait-list outcomes for adults with congenital heart disease listed for heart transplantation in the U.S. J. Am. Coll. Cardiol. 2016, 68, 908–917. [Google Scholar] [CrossRef]
- Simpson, K.E.; Pruitt, E.; Kirklin, J.K.; Naftel, D.C.; Singh, R.K.; Edens, R.E.; Barnes, A.P.; Canter, C.E. Fontan patient survival after pediatric heart transplantation has improved in the current era. Ann. Thorac. Surg. 2017, 103, 1315–1320. [Google Scholar] [CrossRef]
- Michielon, G.; Parisi, F.; Di Carlo, D.; Squitieri, C.; Carotti, A.; Buratta, M.; Di Donato, R.M. Orthotopic heart transplantation for failing single ventricle physiology. Eur. J. Cardio-Thoracic Surg. 2003, 24, 502–510. [Google Scholar] [CrossRef]
- Lamour, J.M.; Kanter, K.R.; Naftel, D.C.; Chrisant, M.R.; Morrow, W.R.; Clemson, B.S.; Kirklin, J.K. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J. Am. Coll. Cardiol. 2009, 54, 160–165. [Google Scholar] [CrossRef]
- Dipchand, A.I.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Dobbels, F.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Yusen, R.D.; Stehlik, J. The registry of the International Society for Heart and Lung Transplantation: Seventeenth official pediatric heart transplantation report—2014; focus theme: Retransplantation. J. Heart Lung Transplant. 2014, 33, 985–995. [Google Scholar] [CrossRef]
- Serfas, J.D.; Thibault, D.; Andersen, N.D.; Chiswell, K.; Jacobs, J.P.; Jacobs, M.L.; Krasuski, R.A.; Lodge, A.J.; Turek, J.W.; Hill, K.D. The evolving surgical burden of Fontan failure: An analysis of the society of thoracic surgeons congenital heart surgery database. Ann. Thorac. Surg. 2021, 112, 179–187. [Google Scholar] [CrossRef]
- Schumacher, K.R.; Yu, S.; Butts, R.; Castleberry, C.; Chen, S.; Edens, E.; Godown, J.; Johnson, J.; Kemna, M.; Lin, K.; et al. Fontan-associated protein-losing enteropathy and post-heart transplant outcomes: A multicenter study. J. Heart Lung Transplant. 2019, 38, 17–25. [Google Scholar] [CrossRef]
- Bernstein, D.; Naftel, D.; Chin, C.; Addonizio, L.; Gamberg, P.; Blume, E.; Hsu, D.; Canter, C.; Kirklin, J.; Morrow, W.; et al. Outcome of listing for cardiac transplantation for failed Fontan: A multiinstitutional study. Circulation 2006, 114, 273–280. [Google Scholar] [CrossRef]
- Sganga, D.; Hollander, S.A.; Vaikunth, S.; Haeffele, C.; Bensen, R.; Navaratnam, M.; McDonald, N.; Profita, E.; Maeda, K.; Concepcion, W.; et al. Comparison of combined heart–liver vs heart-only transplantation in pediatric and young adult Fontan recipients. J. Heart Lung Transplant. 2021, 40, 298–306. [Google Scholar] [CrossRef]
- Moore, B.M.; Anderson, R.; Nisbet, A.M.; Kalla, M.; du Plessis, K.; D’Udekem, Y.; Bullock, A.; Cordina, R.L.; Grigg, L.; Celermajer, D.S.; et al. Ablation of atrial arrhythmias after the atriopulmonary Fontan procedure: Mechanisms of arrhythmia and outcomes. JACC Clin. Electrophysiol. 2018, 4, 1338–1346. [Google Scholar] [CrossRef]
- Mets, J.M.; Bergersen, L.; Mayer, J.E.; Marshall, A.C.; McElhinney, D.B. Outcomes of stent implantation for obstruction of intracardiac lateral tunnel Fontan pathways. Circ. Cardiovasc. Interv. 2013, 6, 92–100. [Google Scholar] [CrossRef]
- Agasthi, P.; Jain, C.C.; Egbe, A.C.; Hagler, D.J.; Cabalka, A.K.; Taggart, N.W.; Anderson, J.H.; Cetta, F.; Connolly, H.M.; Burchill, L.J.; et al. Clinical Outcomes of Percutaneous Fontan Stenting in Adults. Can. J. Cardiol. 2023, 39, 1358–1365. [Google Scholar] [CrossRef]
- Kylat, R.I.; Witte, M.H.; Barber, B.J.; Dori, Y.; Ghishan, F.K. Resolution of proteinlosing enteropathy after congenital heart disease repair by selective lymphatic embolization. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 594–600. [Google Scholar] [CrossRef]
- Dori, Y.; Keller, M.S.; Rome, J.J.; Gillespie, M.J.; Glatz, A.C.; Dodds, K.; Goldberg, D.J.; Goldfarb, S.; Rychik, J.; Itkin, M.; et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 2016, 133, 1160–1170. [Google Scholar] [CrossRef]
- DePopas, E.M.; Veress, L.A.; Ahmed, F.; Rausch, C.M.; Annam, A.; Gupta, R. Percutaneous thoracic duct intervention to treat plastic bronchitis related to Fontan palliation. Pediatr. Pulmonol. 2017, 52, E97–E101. [Google Scholar] [CrossRef]
- Tomasulo, C.E.; Chen, J.M.; Smith, C.L.; Maeda, K.; Rome, J.J.; Dori, Y. Lymphatic disorders and management in patients with congenital heart disease. Ann. Thorac. Surg. 2020, 113, 1101–1111. [Google Scholar] [CrossRef]
- Dori, Y. Novel lymphatic imaging techniques. Technol. Vasc. Interv. Radiol. 2016, 19, 255–261. [Google Scholar] [CrossRef]
- Gray, M.; Kovatis, K.Z.; Stuart, T.; Enlow, E.; Itkin, M.; Keller, M.S.; French, H.M. Treatment of congenital pulmonary lymphangiectasia using ethiodized oil lymphangiography. J. Perinatol. 2014, 34, 720–722. [Google Scholar] [CrossRef]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-losing enteropathy in patients with congenital heart disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Hoffman, T.M.; Dori, Y.; Rome, J.J. Decompression of the thoracic duct: A novel transcatheter approach. Catheter. Cardiovasc. Interv. 2019, 95, E56–E61. [Google Scholar] [CrossRef]
- Kumar, P.; Gordon, B.M.; Kheiwa, A.; Abudayyeh, I. A case report of percutaneous MitraClip implantation in an adult with a double-outlet right ventricle. Eur. Heart J. Case Rep. 2023, 7, ytad247. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Glatz, A.C.; Hanna, B.D.; Gillespie, M.J.; Harris, M.A.; Keller, M.S.; Fogel, M.A.; Rome, J.J.; Whitehead, K.K. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: A pilot study. Circ. Cardiovasc. Interv. 2013, 6, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, J.T.; Johnson, J.N.; Taggart, N.W.; Cabalka, A.K.; Hagler, D.J.; Driscoll, D.J.; Cetta, F. Embolization of veno-venous collaterals after the fontan operation is associated with decreased survival. Congenit. Heart Dis. 2015, 10, E230–E236. [Google Scholar] [CrossRef]
- Carey, J.A.; Hamilton, J.L.; Hilton, C.J.; Dark, J.H.; Forty, J.; Parry, G.; Hasan, A. Orthotopic cardiac transplantation for the failing fontan circulation. Eur. J. Cardio-Thorac. Surg. 1998, 14, 7–14. [Google Scholar] [CrossRef]
- Tan, W.; Reardon, L.; Lin, J.; Lluri, G.; Venkatesh, P.; Bravo-Jaimes, K.; Biniwale, R.; Van Arsdell, G.; Ponder, R.D.; Aboulhosn, J. Occlusion of aortopulmonary and venovenous collaterals prior to heart or combined heart-liver transplantation in Fontan patients: A single-center experience. Int. J. Cardiol. Congenit. Heart Dis. 2021, 6, 100260. [Google Scholar] [CrossRef]




| Causes of Fontan circulatory failure |
| Systolic dysfunction |
| Diastolic dysfunction |
| Anatomic obstruction |
| Lymphatic insufficiency/protein-losing enteropathy/plastic bronchitis |
| Arrhythmia |
| Atrio-ventricular valve regurgitation |
| Heart failure | Diuretics, BB, RAAS inhibitors, ACEI, MRA |
| Pulmonary vascular modulators | PDE5 inhibitors, Endothelin receptor antagonists and prostacyclin analogs |
| PLE | Midodrine, oral budenoside, octreotide |
| Arrhythmia | Anti-arrhythmics |
| Conduction abnormalities | Pacing with restortion of AV synchrony, resynchronization therapy |
| Quality of life | Exercise therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, P.; Gao, H.; Abudayyeh, I.; Pai, R.G.; Varadarajan, P. Contemporary Management of the Failing Fontan. J. Clin. Med. 2024, 13, 3049. https://doi.org/10.3390/jcm13113049
Venkatesh P, Gao H, Abudayyeh I, Pai RG, Varadarajan P. Contemporary Management of the Failing Fontan. Journal of Clinical Medicine. 2024; 13(11):3049. https://doi.org/10.3390/jcm13113049
Chicago/Turabian StyleVenkatesh, Prashanth, Hans Gao, Islam Abudayyeh, Ramdas G. Pai, and Padmini Varadarajan. 2024. "Contemporary Management of the Failing Fontan" Journal of Clinical Medicine 13, no. 11: 3049. https://doi.org/10.3390/jcm13113049





