Therapeutic Potential of Targeting the JAK/STAT Pathway in Psoriasis: Focus on TYK2 Inhibition
Abstract
1. Introduction
2. Overview of Psoriasis Pathogenesis
3. Role of JAK/STAT and TYK2 Signaling Pathways in Psoriasis
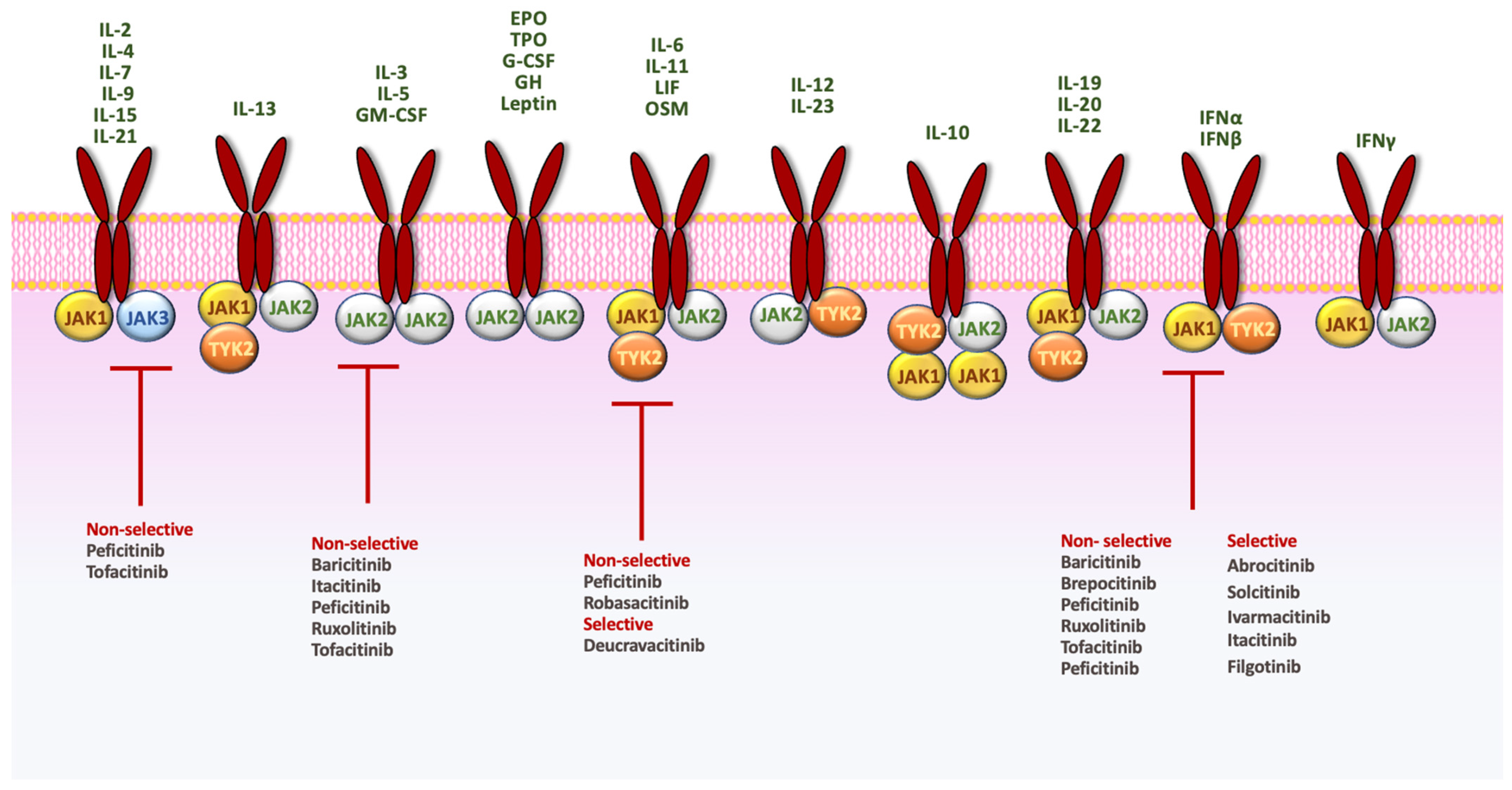
4. JAK Inhibitors in Psoriasis
5. TYK2 Inhibitors in Psoriasis: A New Promising Class of Therapeutic Agents
5.1. Deucravacitinib
5.2. Brepocitinib
5.3. Ropsacitinb
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dragotto, M.; Capalbo, E.; Cartocci, A.; Margiotta, F.M.; Michelucci, A.; Rosi, E.; Ricceri, F.; Simoni, B.; Savarese, I.; Milanesi, N.; et al. Real-life effectiveness of Risankizumab according to body mass index: Results of an Italian multicentre retrospective study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, E1075–E1077. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Mehta, M.D.; Schupp, C.W.; Gondo, G.C.; Bell, S.J.; Griffiths, C.E.M. Psoriasis Prevalence in Adults in the United States. JAMA Dermatol. 2021, 157, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, G. Prevalence of skin diseases in a population; a census study from the faroe islands. Dan Med. Bull. 1964, 11, 1–7. [Google Scholar] [PubMed]
- Langley, R.G.B.; Krueger, G.G.; Griffiths, C.E.M. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii18–ii23; discussion ii24–ii25. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Glaeske, G.; Radtke, M.A.; Christophers, E.; Reich, K.; Schäfer, I. Epidemiology and comorbidity of psoriasis in children. Br. J. Dermatol. 2010, 162, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Christophers, E.; Barker, J.; Chalmers, R.; Chimenti, S.; Krueger, G.; Leonardi, C.; Menter, A.; Ortonne, J.-P.; Fry, L. A classification of psoriasis vulgaris according to phenotype. Br. J. Dermatol. 2007, 156, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Baker, H. Psoriasis—Clinical features. Br. Med. J. 1971, 3, 231–233. [Google Scholar] [CrossRef]
- Gisondi, P.; Bellinato, F.; Girolomoni, G. Topographic Differential Diagnosis of Chronic Plaque Psoriasis: Challenges and Tricks. J. Clin. Med. 2020, 9, 3594. [Google Scholar] [CrossRef]
- Picciani, B.L.S.; Domingos, T.A.; Teixeira-Souza, T.; Santos, V.D.C.B.D.; Gonzaga, H.F.D.S.; Cardoso-Oliveira, J.; Gripp, A.C.; Dias, E.P.; Carneiro, S. Geographic tongue and psoriasis: Clinical, histopathological, immunohistochemical and genetic correlation—A literature review. An. Bras. Dermatol. 2016, 91, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Veale, D.J.; Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 2018, 391, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- D’onghia, M.; Ursini, F.; Cinotti, E.; Calabrese, L.; Tognetti, L.; Cartocci, A.; Lazzeri, L.; Frediani, B.; Rubegni, P.; Trovato, E. Psoriasis and Fibromyalgia: A Systematic Review. J. Pers. Med. 2024, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Trovato, E.; Rubegni, P.; Prignano, F. Place in therapy of anti-IL-17 and 23 in psoriasis according to the severity of comorbidities: A focus on cardiovascular disease and metabolic syndrome. Expert Opin. Biol. Ther. 2022, 22, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Krueger, G.G. Psoriasis assessment tools in clinical trials. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii65–ii68; discussion ii69–ii73. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Del Giglio, M.; Girolomoni, G. Treatment Approaches to Moderate to Severe Psoriasis. Int. J. Mol. Sci. 2017, 18, 2427. [Google Scholar] [CrossRef] [PubMed]
- Caldarola, G.; Falco, G.M.; Calabrese, L.; D’Amore, A.; Chiricozzi, A.; Mariani, M.; Palmisano, G.; De Simone, C.; Peris, K. Drug survival of biologics and non-biologics in patients affected by palmoplantar psoriasis: A «real-world», mono-center experience. Int. J. Dermatol. 2024, 63, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Malvaso, D.; Antonelli, F.; Mannino, M.; Peris, K.; Chiricozzi, A. Investigational systemic drugs for moderate to severe plaque psoriasis: What’s new? Expert. Opin. Investig. Drugs 2023, 32, 229–243. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- D’Urso, D.F.; Chiricozzi, A.; Pirro, F.; Calabrese, L.; Caldarola, G.; Fossati, B.; De Simone, C.; Peris, K. New JAK inhibitors for the treatment of psoriasis and psoriatic arthritis. G. Ital. Dermatol. Venereol. 2020, 155, 411–420. [Google Scholar] [CrossRef]
- Calabrese, L.; Malvaso, D.; Chiricozzi, A.; Tambone, S.; D’urso, D.F.; Guerriero, C.; Peris, K. Baricitinib: Therapeutic potential for moderate to severe atopic dermatitis. Expert Opin. Investig. Drugs 2020, 29, 1089–1098. [Google Scholar] [CrossRef]
- Calabrese, L.; Chiricozzi, A.; De Simone, C.; Fossati, B.; D’Amore, A.; Peris, K. Pharmacodynamics of Janus kinase inhibitors for the treatment of atopic dermatitis. Expert Opin. Drug Metab. Toxicol. 2022, 18, 347–355. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- McKay, I.A.; Leigh, I.M. Altered keratinocyte growth and differentiation in psoriasis. Clin. Dermatol. 1995, 13, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, R.; Röcken, M.; Ghoreschi, K. Angiogenesis drives psoriasis pathogenesis. Int. J. Exp. Pathol. 2009, 90, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, A.A. Innate and adaptive immunity and the pathophysiology of psoriasis. J. Am. Acad. Dermatol. 2006, 54 (Suppl. 2), S67–S80. [Google Scholar] [CrossRef]
- Girolomoni, G.; Strohal, R.; Puig, L.; Bachelez, H.; Barker, J.; Boehncke, W.H.; Prinz, J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Nair, R.P.; Duffin, K.C.; Helms, C.; Ding, J.; Stuart, P.E.; Goldgar, D.; Gudjonsson, J.E.; Li, Y.; Tejasvi, T.; Feng, B.J.; et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 2009, 41, 199–204. [Google Scholar] [CrossRef]
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.-J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143. [Google Scholar] [CrossRef]
- Coscarella, G.; Malvaso, D.; Mannino, M.; Caldarola, G.; Fossati, B.; De Simone, C.; Chiricozzi, A.; Peris, K. The preclinical discovery and development of deucravacitinib for the treatment of psoriasis. Expert Opin. Drug Discov. 2023, 18, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Liang, Y.; Xie, H.; Dai, Z.; Zheng, G. Transcription Factor Retinoid-Related Orphan Receptor γt: A Promising Target for the Treatment of Psoriasis. Front. Immunol. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Balato, A.; Enerbäck, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011205. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Diop, A.; Santorelli, D.; Malagrinò, F.; Nardella, C.; Pennacchietti, V.; Pagano, L.; Marcocci, L.; Pietrangeli, P.; Gianni, S.; Toto, A. SH2 Domains: Folding, Binding and Therapeutical Approaches. Int. J. Mol. Sci. 2022, 23, 15944. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Lin, W.; Lu, L.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target Ther. 2023, 8, 437. [Google Scholar] [CrossRef]
- Krueger, J.G.; McInnes, I.B.; Blauvelt, A. Tyrosine kinase 2 and Janus kinase–signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J. Am. Acad. Dermatol. 2022, 86, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Rusiñol, L.; Puig, L. Tyk2 Targeting in Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 3391. [Google Scholar] [CrossRef] [PubMed]
- Muromoto, R.; Oritani, K.; Matsuda, T. Current understanding of the role of tyrosine kinase 2 signaling in immune responses. World J. Biol. Chem. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.J.; Barrett, K.; Van Abbema, A.; Chang, C.; Kohli, P.B.; Kanda, H.; Smith, J.; Lai, Y.; Zhou, A.; Zhang, B.; et al. A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J. Immunol. 2013, 191, 2205–2216. [Google Scholar] [CrossRef] [PubMed]
- Babon, J.J.; Lucet, I.S.; Murphy, J.M.; Nicola, N.A.; Varghese, L.N. The molecular regulation of Janus kinase (JAK) activation. Biochem. J. 2014, 462, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Karaghiosoff, M.; Neubauer, H.; Lassnig, C.; Kovarik, P.; Schindler, H.; Pircher, H.; McCoy, B.; Bogdan, C.; Decker, T.; Brem, G.; et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity 2000, 13, 549–560. [Google Scholar] [CrossRef]
- Karjalainen, A.; Shoebridge, S.; Krunic, M.; Simonović, N.; Tebb, G.; Macho-Maschler, S.; Strobl, B.; Müller, M. TYK2 in Tumor Immunosurveillance. Cancers 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Ragimbeau, J.; Dondi, E.; Alcover, A.; Eid, P.; Uzé, G.; Pellegrini, S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003, 22, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Puig, L.; Torres, T. JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- Shang, L.; Cao, J.; Zhao, S.; Zhang, J.; He, Y. TYK2 in Immune Responses and Treatment of Psoriasis. J. Inflamm. Res. 2022, 15, 5373–5385. [Google Scholar] [CrossRef]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Potestio, L.; Ruggiero, A.; Cacciapuoti, S.; Maione, F.; Tasso, M.; Caso, F.; Costa, L. JAK Inhibitors in Psoriatic Disease. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3129–3145. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Luchi, M.; Fidelus-Gort, R.; Jackson, S.; Zhang, H.; Flores, R.; Newton, R.; Scherle, P.; Yeleswaram, S.; Chen, X.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J. Dermatolog. Treat. 2016, 27, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, G.; Draelos, Z.; Pariser, D.; Banfield, C.; Cox, L.; Hodge, M.; Kieras, E.; Parsons-Rich, D.; Menon, S.; Salganik, M.; et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: Phase II, randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2018, 179, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, V.; Hicks, K.; Hanrott, K.; Patel, J.; Binks, M.; Wyres, M.; Watson, J.; Wilson, P.; Simeoni, M.; Schifano, L.; et al. Investigation of selective JAK1 inhibitor GSK2586184 for the treatment of psoriasis in a randomized placebo-controlled phase IIa study. Br. J. Dermatol. 2016, 174, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; Coates, L.C.; Helliwell, P.S.; Stanislavchuk, M.; Rychlewska-Hanczewska, A.; Dudek, A.; Abi-Saab, W.; Tasset, C.; Meuleners, L.; Harrison, P.; et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): Results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018, 392, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.A.; Menter, M.A.; Raman, M.; Disch, D.; Schlichting, D.E.; Gaich, C.; Macias, W.; Zhang, X.; Janes, J.M. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2016, 174, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.M.; Juhasz, M.; Mesinkovska, N.A. Topical Janus kinase inhibitors: A review of applications in dermatology. J. Am. Acad. Dermatol. 2018, 79, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Pariser, D.; Catlin, M.; Wierz, G.; Ball, G.; Akinlade, B.; Zeiher, B.; Krueger, J. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2015, 173, 767–776. [Google Scholar] [CrossRef]
- Gladman, D.; Rigby, W.; Azevedo, V.F.; Behrens, F.; Blanco, R.; Kaszuba, A.; Kudlacz, E.; Wang, C.; Menon, S.; Hendrikx, T.; et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N. Engl. J. Med. 2017, 377, 1525–1536. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieślak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Nash, P.; Coates, L.C.; Kivitz, A.; Mease, P.J.; Gladman, D.D.; Covarrubias-Cobos, J.A.; Fitzgerald, O.; Fleishaker, D.; Wang, C.; Wu, J.; et al. Safety and Efficacy of Tofacitinib in Patients with Active Psoriatic Arthritis: Interim Analysis of OPAL Balance, an Open-Label, Long-Term Extension Study. Rheumatol. Ther. 2020, 7, 553–580. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Menter, M.; Abe, M.; Elewski, B.; Feldman, S.; Gottlieb, A.; Langley, R.; Luger, T.; Thaci, D.; Buonanno, M.; et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Results from two randomized, placebo-controlled, phase III trials. Br. J. Dermatol. 2015, 173, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Bachelez, H.; van de Kerkhof, P.C.M.; Strohal, R.; Kubanov, A.; Valenzuela, F.; Lee, J.-H.; Yakusevich, V.; Chimenti, S.; Papacharalambous, J.; Proulx, J.; et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: A phase 3 randomised non-inferiority trial. Lancet 2015, 386, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.; Clark, J.D.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Cueto, I.; Wang, C.Q.; Tan, H.; Wolk, R.; Rottinghaus, S.T.; Whitley, M.Z.; et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: A randomized phase 2 study. J. Allergy Clin. Immunol. 2016, 137, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Iversen, L.; Sofen, H.; Griffiths, C.; Foley, P.; Romiti, R.; Bachinsky, M.; Rottinghaus, S.; Tan, H.; Proulx, J.; et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: A randomized controlled trial. Br. J. Dermatol. 2015, 172, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Anderson, J.K.; Magrey, M.; Merola, J.F.; Liu, Y.; Kishimoto, M.; Jeka, S.; Pacheco-Tena, C.; Wang, X.; Chen, L.; et al. Trial of Upadacitinib and Adalimumab for Psoriatic Arthritis. N. Engl. J. Med. 2021, 384, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Lertratanakul, A.; Anderson, J.K.; Papp, K.; Van den Bosch, F.; Tsuji, S.; Dokoupilova, E.; Keiserman, M.; Wang, X.; Zhong, S.; et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 2021, 80, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.A.; Bashyam, A.M.; Ghamrawi, R.I.; Feldman, S.R. Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin. Emerg. Drugs. 2020, 25, 89–100. [Google Scholar] [CrossRef]
- Burke, J.R.; Cheng, L.; Gillooly, K.M.; Strnad, J.; Zupa-Fernandez, A.; Catlett, I.M.; Zhang, Y.; Heimrich, E.M.; McIntyre, K.W.; Cunningham, M.D.; et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci. Transl. Med. 2019, 11, eaaw1736. [Google Scholar] [CrossRef]
- Tokarski, J.S.; Zupa-Fernandez, A.; Tredup, J.A.; Pike, K.; Chang, C.; Xie, D.; Cheng, L.; Pedicord, D.; Muckelbauer, J.; Johnson, S.R.; et al. Tyrosine Kinase 2-mediated Signal Transduction in T Lymphocytes Is Blocked by Pharmacological Stabilization of Its Pseudokinase Domain. J. Biol. Chem. 2015, 290, 11061–11074. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, S.T.; Moslin, R.; Lin, S.; Zhang, Y.; Spergel, S.; Kempson, J.; Tokarski, J.S.; Strnad, J.; Zupa-Fernandez, A.; Cheng, L.; et al. Highly Selective Inhibition of Tyrosine Kinase 2 (TYK2) for the Treatment of Autoimmune Diseases: Discovery of the Allosteric Inhibitor BMS-986165. J. Med. Chem. 2019, 62, 8973–8995. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Gordon, K.; Thaci, D.; Morita, A.; Gooderham, M.; Foley, P.; Girgis, I.G.; Kundu, S.; Banerjee, S. Phase 2 Trial of Selective Tyrosine Kinase 2 Inhibition in Psoriasis. N. Engl. J. Med. 2018, 379, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Gooderham, M.; Warren, R.B.; Papp, K.A.; Strober, B.; Thaçi, D.; Morita, A.; Szepietowski, J.C.; Imafuku, S.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 2023, 88, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Strober, B.; Thaçi, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Warren, R.B.; Sofen, H.; Imafuku, S.; Paul, C.; Szepietowski, J.C.; Spelman, L.; Passeron, T.; Vritzali, E.; Napoli, A.; et al. Deucravacitinib in plaque psoriasis: 2-year safety and efficacy results from the phase 3 poetyk trials. Br. J. Dermatol. 2024, 190, 668–679. [Google Scholar] [CrossRef]
- Imafuku, S.; Tada, Y.; Hippeli, L.; Banerjee, S.; Morita, A.; Ohtsuki, M. Efficacy and safety of the selective TYK2 inhibitor, deucravacitinib, in Japanese patients with moderate to severe plaque psoriasis: Subgroup analysis of a randomized, double-blind, placebo-controlled, global phase 3 trial. J. Dermatol. 2023, 50, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, S.; Okubo, Y.; Tada, Y.; Ohtsuki, M.; Colston, E.; Napoli, A.; Shao, Y.; Banerjee, S.; Morita, A. Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in Japanese patients with moderate to severe plaque, erythrodermic, or generalized pustular psoriasis: Efficacy and safety results from an open-label, phase 3 trial. J. Dermatol. 2024, 51, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Maleki, F.; Clark, E.; Banfield, C.; Byon, W.; Nicholas, T. Population pharmacokinetic modeling of oral brepocitinib in healthy volunteers and patients with immuno-inflammatory diseases. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 551–562. [Google Scholar] [CrossRef]
- King, B.; Guttman-Yassky, E.; Peeva, E.; Banerjee, A.; Sinclair, R.; Pavel, A.B.; Zhu, L.; Cox, L.A.; Craiglow, B.; Chen, L.; et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J. Am. Acad. Dermatol. 2021, 85, 379–387. [Google Scholar] [CrossRef]
- Kimball, A.B.; Peeva, E.; Forman, S.; Moiin, A.; Khattri, S.; Porter, M.L.; Mangold, A.R.; Ghosh, P.; Banfield, C.; Oemar, B. Brepocitinib, Zimlovisertib, and Ropsacitinib in Hidradenitis Suppurativa. NEJM Evid. 2024, 3, EVIDoa2300155. [Google Scholar] [CrossRef] [PubMed]
- Page, K.M.; Suarez-Farinas, M.; Suprun, M.; Zhang, W.; Garcet, S.; Fuentes-Duculan, J.; Li, X.; Scaramozza, M.; Kieras, E.; Banfield, C.; et al. Molecular and Cellular Responses to the TYK2/JAK1 Inhibitor PF-06700841 Reveal Reduction of Skin Inflammation in Plaque Psoriasis. J. Investig. Dermatol. 2020, 140, 1546–1555.e4. [Google Scholar] [CrossRef] [PubMed]
- Banfield, C.; Scaramozza, M.; Zhang, W.; Kieras, E.; Page, K.M.; Fensome, A.; Vincent, M.; Dowty, M.E.; Goteti, K.; Winkle, P.J.; et al. The Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a TYK2/JAK1 Inhibitor (PF-06700841) in Healthy Subjects and Patients With Plaque Psoriasis. J. Clin. Pharmacol. 2018, 58, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Forman, S.B.; Pariser, D.M.; Poulin, Y.; Vincent, M.S.; Gilbert, S.A.; Kieras, E.M.; Qiu, R.; Yu, D.; Papacharalambous, J.; Tehlirian, C.; et al. TYK2/JAK1 Inhibitor PF-06700841 in Patients with Plaque Psoriasis: Phase IIa, Randomized, Double-Blind, Placebo-Controlled Trial. J. Investig. Dermatol. 2020, 140, 2359–2370.e5. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; Helliwell, P.; Silwinska-Stanczyk, P.; Miakisz, M.; Ostor, A.; Peeva, E.; Vincent, M.S.; Sun, Q.; Sikirica, V.; Winnette, R.; et al. Efficacy and Safety of the TYK2/JAK1 Inhibitor Brepocitinib for Active Psoriatic Arthritis: A Phase IIb Randomized Controlled Trial. Arthritis Rheumatol. 2023, 75, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Landis, M.N.; Smith, S.R.; Berstein, G.; Fetterly, G.; Ghosh, P.; Feng, G.; Pradhan, V.; Aggarwal, S.; Banfield, C.; Peeva, E.; et al. Efficacy and safety of topical brepocitinib cream for mild-to-moderate chronic plaque psoriasis: A phase IIb randomized double-blind vehicle-controlled parallel-group study. Br. J. Dermatol. 2023, 189, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Tehlirian, C.; Peeva, E.; Kieras, E.; Scaramozza, M.; Roberts, E.S.; Singh, R.S.P.; Pradhan, V.; Banerjee, A.; Garcet, S.; Xi, L.; et al. Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: A phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatol. 2021, 3, e204–e213. [Google Scholar] [CrossRef] [PubMed]
- Tehlirian, C.; Singh, R.S.P.; Pradhan, V.; Roberts, E.S.; Tarabar, S.; Peeva, E.; Vincent, M.S.; Gale, J.D. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 2022, 87, 333–342. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, K.; Shin, K.; Kim, H.; Kim, B.; Kim, M.; Ko, H.; Kim, Y.H. The safety of systemic Janus kinase inhibitors in atopic dermatitis: A systematic review and meta-analysis of randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 52–61. [Google Scholar] [CrossRef]
- Estevinho, T.; Lé, A.M.; Torres, T. Deucravacitinib in the treatment of psoriasis. J. Dermatolog. Treat. 2023, 34, 2154122. [Google Scholar] [CrossRef]
| Drug, Trial | MoA | Clinical Trial No | Phase of Clinical Trials | Status | Approved for PsO |
|---|---|---|---|---|---|
| Itacitinib, (INCB039110) | JAK1 and JAK2 inhibition | NCT01634087 | Phase II | Completed | None |
| Abrocitinib (PF-04965842) | JAK1 inhibition | NCT02201524 | Phase II | Completed | None |
| Solcitinib (GSK2586184) | JAK1 inhibition | NCT01782664 | Phase II | Completed | None |
| Baricitinib | JAK1 and JAK2 inhibition | NCT01490632 | Phase II | Completed | None |
| Ruxolitinib | JAK1 and JAK2 inhibition | NCT00617994 | Phase II | Completed | None |
| NCT00820950 | Phase II | Completed | |||
| NCT00778700 | Phase II | Completed | |||
| Tofacitinib | JAK 1 and JAK 3 inhibition | NCT02193815 | Phase I | Completed | |
| NCT01736696 | Phase I | Completed | |||
| NCT00678561 | Phase II | Completed | |||
| NCT01246583 | Phase II | Completed | |||
| NCT01831466 | Phase II | Completed | |||
| NCT01710046 | Phase II | Completed | |||
| NCT00678210 | Phase II | Completed | |||
| NCT01163253 | Phase III | Terminated | |||
| NCT01815424 | Phase III | Completed | |||
| NCT01276639 | Phase III | Completed | |||
| NCT01309737 | Phase III | Completed | |||
| NCT01519089 | Phase III | Completed | |||
| NCT01186744 | Phase III | Completed | |||
| NCT01241591 | Phase III | Completed | |||
| Peficitinib (ASP015K) | JAK1, JAK2 and JAK3 inhibition | NCT01096862 | Phase II | Completed | None |
| Drug Name | MoA | Clinical Trial No. | Study | Status | Primary Endpoint(s) |
|---|---|---|---|---|---|
| Deucravacitinib (BMS-986165) | Selective TYK2 inhibitor | NCT02931838 | Phase II, randomized, double-blind, placebo controlled | Completed | Proportion of participants achieving PASI75 by week 12; proportion of patients with AEs |
| NCT03624127 | Phase III, randomized, double-blind, placebo-controlled (POETYK PSO-1) | Completed | Proportion of participants receiving deucravacitinib with a sPGA score of 0 or 1 compared to placebo by week 16; proportion of participants achieving PASI75 by week 16 | ||
| NCT03611751 | Phase III, randomized, double-blind, placebo-controlled (POETYK PSO-2) | Completed | Proportion of participants receiving deucravacitinib with a sPGA score of 0 or 1 compared to placebo by week 16; proportion of participants achieving PASI75 by week 16 | ||
| NCT04036435 | Phase III, open-label, extension (POETYK long-term extension) (LTE) | Active, not recruiting | Incidence of AEs and serious AEs | ||
| NCT03924427 | Phase III, open-label, single-arm (POETYK PSO-4) | Completed | Proportion of participants receiving deucravacitinib with a sPGA score of 0 or 1 compared to placebo by week 16; proportion of participants achieving PASI75 by week 16 | ||
| NCT04167462 | Phase III, randomized, double-blind, placebo-controlled (POETYK PSO-3) | Completed | Proportion of participants receiving deucravacitinib with a sPGA score of 0 or 1 compared to placebo by week 16; proportion of participants achieving PASI75 by week 16 | ||
| Brepocitinib (PF-06700841) | Dual TYK2/JAK1 inhibitor | NCT02310750 | Phase I, randomized, double blind, placebo-controlled, parallel group | Completed | Pharmacokinetics and pharmacodynamics outcomes |
| NCT02969018 | Phase IIa, randomized, double-blind, placebo-controlled | Completed | PASI score by week 12 | ||
| NCT03963401 | Phase IIb, randomized, double-blind, placebo-controlled | Completed | Proportion of participants achieving an ACR 20 response by week 16 | ||
| NCT03850483 | Phase IIb, randomized, double-blind, vehicle-controlled, parallel group | Completed | PASI score by week 12 | ||
| Ropsacitinib (PF- 06826647) | Dual TYK2/JAK2 inhibitor | NCT03210961 | Phase I, randomized, double-blind, placebo-controlled | Completed | Incidence of AEs and clinical laboratory abnormalities |
| NCT03895372 | Phase IIb, randomized, double-blind, placebo controlled, parallel-group | Completed | Proportion of participants achieving PASI 90 by week 16; incidence of AEs, clinically significant changes in vital signs, laboratory tests results and treatment-emergent electrocardiogram (ECG) findings by 40 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragotto, M.; D’Onghia, M.; Trovato, E.; Tognetti, L.; Rubegni, P.; Calabrese, L. Therapeutic Potential of Targeting the JAK/STAT Pathway in Psoriasis: Focus on TYK2 Inhibition. J. Clin. Med. 2024, 13, 3091. https://doi.org/10.3390/jcm13113091
Dragotto M, D’Onghia M, Trovato E, Tognetti L, Rubegni P, Calabrese L. Therapeutic Potential of Targeting the JAK/STAT Pathway in Psoriasis: Focus on TYK2 Inhibition. Journal of Clinical Medicine. 2024; 13(11):3091. https://doi.org/10.3390/jcm13113091
Chicago/Turabian StyleDragotto, Martina, Martina D’Onghia, Emanuele Trovato, Linda Tognetti, Pietro Rubegni, and Laura Calabrese. 2024. "Therapeutic Potential of Targeting the JAK/STAT Pathway in Psoriasis: Focus on TYK2 Inhibition" Journal of Clinical Medicine 13, no. 11: 3091. https://doi.org/10.3390/jcm13113091
APA StyleDragotto, M., D’Onghia, M., Trovato, E., Tognetti, L., Rubegni, P., & Calabrese, L. (2024). Therapeutic Potential of Targeting the JAK/STAT Pathway in Psoriasis: Focus on TYK2 Inhibition. Journal of Clinical Medicine, 13(11), 3091. https://doi.org/10.3390/jcm13113091







