Novel Therapeutic Strategies in Alzheimer’s Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond
Abstract
1. Introduction
1.1. Pathogenic Insight into the Spectrum of Neurodegenerative Diseases
1.2. The Paths to Alzheimer’s Disease Therapy
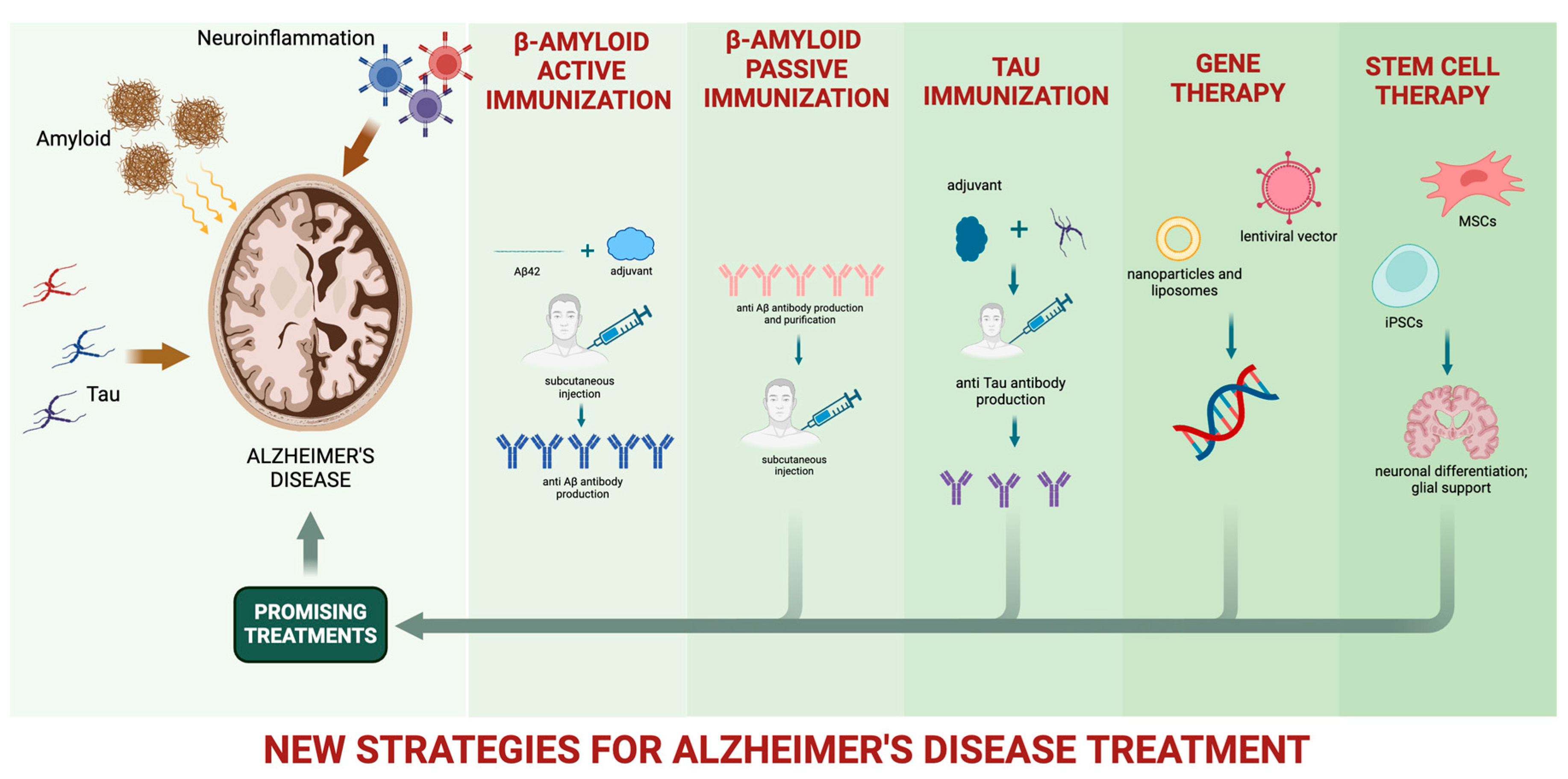
2. Overview of Novel Therapeutic Approaches for Slowing or Preventing AD
3. Amyloid-Beta Protein Immunotherapies
3.1. Anti-Amyloid Therapy
3.2. The Case of Aducanumab
| Intervening “Drug” | Trial Phase | Number of Patients Enrolled | Disease Stage | Duration | Primary Endpoint | Outcome | Authors, Year, and Country |
|---|---|---|---|---|---|---|---|
| Bapinezumab | III, placebo-controlled | 1121 ε4 allele APOE carriers and 1331 noncarriers | Mild-to-moderate AD | 78 weeks | ADAS-cog11; DAD | No group differences; low rate of ARIAs | Salloway et al., 2014, US [114] |
| Donanemab | II, placebo-controlled | 257 | Early symptomatic AD | 72 weeks | iADRS | Mild change from baseline in the iADRS score (p = 0.04); high rate of ARIAs | Mintun et al., 2021, US [115] |
| Lecanemab | IIa, ascending dose, placebo-controlled | 48 | Mild-to-moderate AD | 4 months | Safety and tolerability | Well-tolerated across all doses | Logovinsky et al., 2016, Sweden, US [121] |
| Lecanemab | IIb, placebo-controlled | 854 | Early AD, MCI due to AD, and mild AD dementia | 12 months | ADCOMS | No group differences; low rate of ARIAs | Swanson et al., 2021, Sweden, US [122] |
| Ponezumab | IIa and IIb, ascending dose, placebo-controlled | 77 + 26 (phase A) 63 + 32 (phase B) | Mild-to-moderate AD | 18 months | Safety and tolerability | Well-tolerated across all doses | Landen et al., 2021, US [123] |
| Crenezumab | III, placebo-controlled | 813 + 806 | Prodromal-to-mild AD | 100 weeks | CDR-SB | No clinical effects | Ostrowitzki et al., 2022, multicenter [126] |
| Gantenerumab | III, placebo-controlled | 985 + 980 | MCI due to AD, and mild AD dementia | 116 weeks | CDR-SB | No clinical effects | Bateman et al., 2023 [117] |
| Solanezumab | III, placebo-controlled | 2129 | Mild AD dementia | 76 weeks | ADAS-cog14 | No clinical effects | Honig et al., 2018, multicenter [130] |
| Aducanumab | III, placebo-controlled | 1638 + 1647 | MCI and early AD | 76 weeks | CDR-SB | Difference in CDR-SB in first 1638 patients | Haeberlein et al., 2022, multicenter [140] |
4. Beyond Amyloid: Tau and Neuroinflammation
4.1. Anti-Tau Therapy
4.2. Neuroinflammation
5. Gene Therapy
6. Stem Cell Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Chang, H.-Y.; Sang, T.-K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Zafar, S.; Zerr, I. Neurodegenerative Proteinopathies in the Proteoform Spectrum—Tools and Challenges. Int. J. Mol. Sci. 2021, 22, 1085. [Google Scholar] [CrossRef] [PubMed]
- Perani, D.; Caminiti, S.P.; Carli, G.; Tondo, G. PET Neuroimaging in Dementia Conditions. PET SPECT Neurol. 2021, 211–282. [Google Scholar] [CrossRef]
- Comi, C.; Tondo, G. Insights into the Protective Role of Immunity in Neurodegenerative Disease. Neural Regen. Res. 2017, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s Disease: Pathogenesis, Mechanisms, and Therapeutic Potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Kievit, R.A.; Passamonti, L.; Jones, P.S.; Tsvetanov, K.A.; Rittman, T.; Mak, E.; Nicastro, N.; Bevan-Jones, W.R.; Su, L. Microglial Activation and Tau Burden Predict Cognitive Decline in Alzheimer’s Disease. Brain 2020, 143, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F. The Relationships between Neuroinflammation, Beta-Amyloid and Tau Deposition in Alzheimer’s Disease: A Longitudinal PET Study. J. Neuroinflamm. 2020, 17, 151. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New Insight into Neurological Degeneration: Inflammatory Cytokines and Blood–Brain Barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Boccalini, C.; Caminiti, S.P.; Presotto, L.; Filippi, M.; Magnani, G.; Frisoni, G.B.; Iannaccone, S.; Perani, D. Brain Metabolism and Microglia Activation in Mild Cognitive Impairment: A Combined [18F] FDG and [11C]-(R)-PK11195 PET Study. J. Alzheimer’s Dis. 2021, 80, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Cope, T.E.; Street, D.; Jones, P.S.; Hezemans, F.H.; Mak, E.; Tsvetanov, K.A.; Rittman, T.; Bevan-Jones, W.R.; Patterson, K. Microglial Activation in the Frontal Cortex Predicts Cognitive Decline in Frontotemporal Dementia. Brain 2023, 146, 3221–3231. [Google Scholar] [CrossRef] [PubMed]
- Lavisse, S.; Goutal, S.; Wimberley, C.; Tonietto, M.; Bottlaender, M.; Gervais, P.; Kuhnast, B.; Peyronneau, M.-A.; Barret, O.; Lagarde, J. Increased Microglial Activation in Patients with Parkinson Disease Using [18F]-DPA714 TSPO PET Imaging. Park. Relat. Disord. 2021, 82, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Aprile, D.; De Marchi, F.; Sarasso, B.; Serra, P.; Borasio, G.; Rojo, E.; Arenillas, J.F.; Comi, C. Investigating the Prognostic Role of Peripheral Inflammatory Markers in Mild Cognitive Impairment. J. Clin. Med. 2023, 12, 4298. [Google Scholar] [CrossRef] [PubMed]
- Grassano, M.; Manera, U.; De Marchi, F.; Cugnasco, P.; Matteoni, E.; Daviddi, M.; Solero, L.; Bombaci, A.; Palumbo, F.; Vasta, R. The Role of Peripheral Immunity in ALS: A Population-based Study. Ann. Clin. Transl. Neurol. 2023, 10, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Hok-A-Hin, Y.S.; Del Campo, M.; Boiten, W.A.; Stoops, E.; Vanhooren, M.; Lemstra, A.W.; van der Flier, W.M.; Teunissen, C.E. Neuroinflammatory CSF Biomarkers MIF, STREM1, and STREM2 Show Dynamic Expression Profiles in Alzheimer’s Disease. J. Neuroinflamm. 2023, 20, 107. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Mendez, M.F. Early-Onset Alzheimer Disease and Its Variants. Continuum 2019, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.B.; Shadlen, M.-F.; Wang, L.; McCormick, W.C.; Bowen, J.D.; Teri, L.; Kukull, W.A. Survival after Initial Diagnosis of Alzheimer Disease. Ann. Intern. Med. 2004, 140, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Helzner, E.P.; Scarmeas, N.; Cosentino, S.; Tang, M.-X.; Schupf, N.; Stern, Y. Survival in Alzheimer Disease: A Multiethnic, Population-Based Study of Incident Cases. Neurology 2008, 71, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Todd, S.; Barr, S.; Roberts, M.; Passmore, A.P. Survival in Dementia and Predictors of Mortality: A Review. Int. J. Geriatr. Psychiatry 2013, 28, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.P.; Benito-León, J.; Louis, E.D.; Bermejo-Pareja, F. Under Reporting of Dementia Deaths on Death Certificates: A Systematic Review of Population-Based Cohort Studies. J. Alzheimer’s Dis. 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.-Y. γ-Secretase in Alzheimer’s Disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Alldred, M.J.; Martini, A.C.; Patterson, D.; Hendrix, J.; Granholm, A.-C. Aging with Down Syndrome—Where Are We Now and Where Are We Going? J. Clin. Med. 2021, 10, 4687. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Rice, H.C.; De Malmazet, D.; Schreurs, A.; Frere, S.; Van Molle, I.; Volkov, A.N.; Creemers, E.; Vertkin, I.; Nys, J.; Ranaivoson, F.M. Secreted Amyloid-β Precursor Protein Functions as a GABABR1a Ligand to Modulate Synaptic Transmission. Science 2019, 363, eaao4827. [Google Scholar]
- Vecchi, G.; Sormanni, P.; Mannini, B.; Vandelli, A.; Tartaglia, G.G.; Dobson, C.M.; Hartl, F.U.; Vendruscolo, M. Proteome-Wide Observation of the Phenomenon of Life on the Edge of Solubility. Proc. Natl. Acad. Sci. USA 2020, 117, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Selkoe, D.J. A Mechanistic Hypothesis for the Impairment of Synaptic Plasticity by Soluble Aβ Oligomers from Alzheimer’s Brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef] [PubMed]
- O’Nuallain, B.; Freir, D.B.; Nicoll, A.J.; Risse, E.; Ferguson, N.; Herron, C.E.; Collinge, J.; Walsh, D.M. Amyloid β-Protein Dimers Rapidly Form Stable Synaptotoxic Protofibrils. J. Neurosci. 2010, 30, 14411–14419. [Google Scholar] [CrossRef] [PubMed]
- Gouwens, L.K.; Makoni, N.J.; Rogers, V.A.; Nichols, M.R. Amyloid-Β42 Protofibrils Are Internalized by Microglia More Extensively than Monomers. Brain Res. 2016, 1648, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fromholt, S.E.; Chakrabarty, P.; Zhu, F.; Liu, X.; Pace, M.C.; Koh, J.; Golde, T.E.; Levites, Y.; Lewis, J. Diversity in Aβ Deposit Morphology and Secondary Proteome Insolubility across Models of Alzheimer-Type Amyloidosis. Acta Neuropathol. Commun. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Meyer-Luehmann, M.; Spires-Jones, T.L.; Prada, C.; Garcia-Alloza, M.; De Calignon, A.; Rozkalne, A.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Bacskai, B.J.; Hyman, B.T. Rapid Appearance and Local Toxicity of Amyloid-β Plaques in a Mouse Model of Alzheimer’s Disease. Nature 2008, 451, 720–724. [Google Scholar] [CrossRef]
- Forloni, G.; Balducci, C. Alzheimer’s Disease, Oligomers, and Inflammation. J. Alzheimer’s Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef]
- Ezzat, K.; Sturchio, A.; Espay, A.J. The Shift to a Proteinopenia Paradigm in Neurodegeneration. Handb. Clin. Neurol. 2023, 193, 23–32. [Google Scholar] [PubMed]
- Granzotto, A.; Sensi, S.L. Once upon a Time, the Amyloid Cascade Hypothesis. Ageing Res. Rev. 2023, 93, 102161. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Resnick, S.M.; O’Brien, R.; Zonderman, A.B.; An, Y.; Pletnikova, O.; Rudow, G.; Crain, B.; Troncoso, J.C. Mild Cognitive Impairment and Asymptomatic Alzheimer Disease Subjects: Equivalent β-Amyloid and Tau Loads with Divergent Cognitive Outcomes. J. Neuropathol. Exp. Neurol. 2014, 73, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nievas, B.G.; Stein, T.D.; Tai, H.-C.; Dols-Icardo, O.; Scotton, T.C.; Barroeta-Espar, I.; Fernandez-Carballo, L.; De Munain, E.L.; Perez, J.; Marquie, M. Dissecting Phenotypic Traits Linked to Human Resilience to Alzheimer’s Pathology. Brain 2013, 136, 2510–2526. [Google Scholar] [CrossRef] [PubMed]
- Perani, D. FDG-PET and Amyloid-PET Imaging: The Diverging Paths. Curr. Opin. Neurol. 2014, 27, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Chételat, G. Neuroimaging Biomarkers in Alzheimer’s Disease and Other Dementias. Ageing Res. Rev. 2016, 30, 4–16. [Google Scholar] [CrossRef] [PubMed]
- van Harten, A.C.; Visser, P.J.; Pijnenburg, Y.A.L.; Teunissen, C.E.; Blankenstein, M.A.; Scheltens, P.; van der Flier, W.M. Cerebrospinal Fluid Aβ42 Is the Best Predictor of Clinical Progression in Patients with Subjective Complaints. Alzheimer’s Dement. 2013, 9, 481–487. [Google Scholar] [CrossRef] [PubMed]
- McDade, E.; Wang, G.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Holtzman, D.M.; Cairns, N.J.; Goate, A.M. Longitudinal Cognitive and Biomarker Changes in Dominantly Inherited Alzheimer Disease. Neurology 2018, 91, e1295–e1306. [Google Scholar] [CrossRef] [PubMed]
- Sturchio, A.; Dwivedi, A.K.; Young, C.B.; Malm, T.; Marsili, L.; Sharma, J.S.; Mahajan, A.; Hill, E.J.; Andaloussi, S.E.L.; Poston, K.L. High Cerebrospinal Amyloid-β 42 Is Associated with Normal Cognition in Individuals with Brain Amyloidosis. EClinicalMedicine 2021, 38, 100988. [Google Scholar] [CrossRef]
- Sturchio, A.; Dwivedi, A.K.; Malm, T.; Wood, M.J.A.; Cilia, R.; Sharma, J.S.; Hill, E.J.; Schneider, L.S.; Graff-Radford, N.R.; Mori, H. High Soluble Amyloid-β 42 Predicts Normal Cognition in Amyloid-Positive Individuals with Alzheimer’s Disease-Causing Mutations. J. Alzheimer’s Dis. 2022, 90, 333–348. [Google Scholar] [CrossRef]
- Rinauro, D.J.; Chiti, F.; Vendruscolo, M.; Limbocker, R. Misfolded Protein Oligomers: Mechanisms of Formation, Cytotoxic Effects, and Pharmacological Approaches against Protein Misfolding Diseases. Mol. Neurodegener. 2024, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Kreiser, R.P.; Wright, A.K.; Block, N.R.; Hollows, J.E.; Nguyen, L.T.; LeForte, K.; Mannini, B.; Vendruscolo, M.; Limbocker, R. Therapeutic Strategies to Reduce the Toxicity of Misfolded Protein Oligomers. Int. J. Mol. Sci. 2020, 21, 8651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.L.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C. Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M.-Y. Protein Transmission in Neurodegenerative Disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wan, H.; Chen, S.; Liu, G.-P. Targeting Tau in Alzheimer’s Disease: From Mechanisms to Clinical Therapy. Neural Regen. Res. 2024, 19, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713. [Google Scholar] [CrossRef]
- Gauthier, S.; Boxer, A.; Knopman, D.; Sims, J.; Doody, R.; Aisen, P.; Iwatsubo, T.; Bateman, R.; Vellas, B. Therapeutic Targets for Alzheimer’s Disease: Amyloid vs. Non-Amyloid. Where Does Consensus Lie Today? An Ctad Task Force Report. J. Prev. Alzheimers Dis. 2022, 9, 231–235. [Google Scholar] [CrossRef]
- Tondo, G.; Iaccarino, L.; Caminiti, S.P.; Presotto, L.; Santangelo, R.; Iannaccone, S.; Magnani, G.; Perani, D. The Combined Effects of Microglia Activation and Brain Glucose Hypometabolism in Early-Onset Alzheimer’s Disease. Alzheimers Res. Ther. 2020, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C. Spatial Patterns of Neuroimaging Biomarker Change in Individuals from Families with Autosomal Dominant Alzheimer’s Disease: A Longitudinal Study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Li, Y.; Joseph-Mathurin, N.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Perrin, R.J.; Goate, A.M. A Soluble Phosphorylated Tau Signature Links Tau, Amyloid and the Evolution of Stages of Dominantly Inherited Alzheimer’s Disease. Nat. Med. 2020, 26, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Boccalini, C.; Vanoli, E.G.; Presotto, L.; Muscio, C.; Ciullo, V.; Banaj, N.; Piras, F.; Filippini, G.; Tiraboschi, P. Brain Metabolism and Amyloid Load in Individuals With Subjective Cognitive Decline or Pre–Mild Cognitive Impairment. Neurology 2022, 99, e258–e269. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for Neurodegenerative Diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Tondo, G.; De Marchi, F. From Biomarkers to Precision Medicine in Neurodegenerative Diseases: Where Are We? J. Clin. Med. 2022, 11, 4515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gahlawat, A.; Kumar, R.N.; Singh, Y.P.; Modi, G.; Garg, P. Drug Repurposing for Alzheimer’s Disease: In Silico and in Vitro Investigation of FDA-Approved Drugs as Acetylcholinesterase Inhibitors. J. Biomol. Struct. Dyn. 2022, 40, 2878–2892. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Orzeł-Sajdłowska, A. Aducanumab—Hope or Disappointment for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4367. [Google Scholar] [CrossRef] [PubMed]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; da Silva, B.S.; Passamani Ambrosio, R.; Castro, F.C.; Teixeira, S.F.; et al. Advances in Alzheimer’s Disease’s Pharmacological Treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current Anti-Amyloid-β Therapy for Alzheimer’s Disease Treatment: From Clinical Research to Nanomedicine. Int. J. Nanomed. 2023, 18, 7825–7845. [Google Scholar] [CrossRef]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s Disease: Targeting β-Amyloid and Beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, Present and Future of Therapeutic Strategies against Amyloid-β Peptides in Alzheimer’s Disease: A Systematic Review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Penke, B.; Szűcs, M.; Bogár, F. New Pathways Identify Novel Drug Targets for the Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 5383. [Google Scholar] [CrossRef]
- De Marchi, F.; Vignaroli, F.; Mazzini, L.; Comi, C.; Tondo, G. New Insights into the Relationship between Nutrition and Neuroinflammation in Alzheimer’s Disease: Preventive and Therapeutic Perspectives. CNS Neurol. Disord. -Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2024, 23, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.S.; Baker, S.L.; Dobyns, L.; Janabi, M.; Jagust, W.J.; Harrison, T.M. Tau Accumulation and Atrophy Predict Amyloid Independent Cognitive Decline in Aging. Alzheimer’s Dement. 2024, 20, 2526–2537. [Google Scholar] [CrossRef]
- Caminiti, S.P.; De Francesco, S.; Tondo, G.; Galli, A.; Redolfi, A.; Perani, D.; Initiative, A.D.N.; Project, I.; Cappa, S.F.; Cotelli, M. FDG-PET Markers of Heterogeneity and Different Risk of Progression in Amnestic MCI. Alzheimer’s Dement. 2024, 20, 159–172. [Google Scholar] [CrossRef]
- Atay, L.O.; Saka, E.; Akdemir, U.O.; Yetim, E.; Balci, E.; Arsava, E.M.; Topcuoglu, M.A. Hybrid PET/MRI with Flutemetamol and FDG in Alzheimer’s Disease Clinical Continuum. Curr. Alzheimer Res. 2023, 20, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K. Immunization with Amyloid-β Attenuates Alzheimer-Disease-like Pathology in the PDAPP Mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef]
- Monsonego, A.; Maron, R.; Zota, V.; Selkoe, D.J.; Weiner, H.L. Immune Hyporesponsiveness to Amyloid β-Peptide in Amyloid Precursor Protein Transgenic Mice: Implications for the Pathogenesis and Treatment of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2001, 98, 10273–10278. [Google Scholar] [CrossRef]
- Ferrer, I.; Rovira, M.B.; Guerra, M.L.S.; Rey, M.J.; Costa-Jussá, F. Neuropathology and Pathogenesis of Encephalitis Following Amyloid β Immunization in Alzheimer’s Disease. Brain Pathol. 2004, 14, 11–20. [Google Scholar] [CrossRef]
- Vellas, B.; Black, R.; Thal, L.J.; Fox, N.C.; Daniels, M.; McLennan, G.; Tompkins, C.; Leibman, C.; Pomfret, M.; Grundman, M. Long-Term Follow-up of Patients Immunized with AN1792: Reduced Functional Decline in Antibody Responders. Curr. Alzheimer Res. 2009, 6, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.J.; Bullock, R.; Jones, R.W.; Wilkinson, D.; Paterson, K.R.; Jenkins, L.; Millais, S.B.; Donoghue, S. Evaluation of the Safety and Immunogenicity of Synthetic Aβ42 (AN1792) in Patients with AD. Neurology 2005, 64, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Lemere, C.A. Developing Novel Immunogens for a Safe and Effective Alzheimer’s Disease Vaccine. Prog. Brain Res. 2009, 175, 83–93. [Google Scholar] [PubMed]
- Hock, C.; Konietzko, U.; Papassotiropoulos, A.; Wollmer, A.; Streffer, J.; von Rotz, R.C.; Davey, G.; Moritz, E.; Nitsch, R.M. Generation of Antibodies Specific for β-Amyloid by Vaccination of Patients with Alzheimer Disease. Nat. Med. 2002, 8, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Zieneldien, T.; Kim, J.; Sawmiller, D.; Cao, C. The Immune System as a Therapeutic Target for Alzheimer’s Disease. Life 2022, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Bates, K.A.; Verdile, G.; Li, Q.X.; Ames, D.; Hudson, P.; Masters, C.L.; Martins, R. Clearance Mechanisms of Alzheimer’s Amyloid-β Peptide: Implications for Therapeutic Design and Diagnostic Tests. Mol. Psychiatry 2009, 14, 469–486. [Google Scholar] [CrossRef]
- Lu, D.; Dou, F.; Gao, J. Development of Amyloid Beta-Directed Antibodies against Alzheimer’s Disease: Twists and Turns. Drug Discov. Ther. 2023, 17, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and Hopes for Alzheimer’s Disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J. Potential of Low Dose Leuco-Methylthioninium Bis (Hydromethanesulphonate)(LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimer’s Dis. 2018, 61, 435–457. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Schindler, S.E.; Christensen, J.; McKay, N.S.; Liu, J.; Wang, S.; Sun, Z.; Hassenstab, J.; Su, Y. Baseline Microglial Activation Correlates with Brain Amyloidosis and Longitudinal Cognitive Decline in Alzheimer Disease. Neurol. Neuroimmunol. Neuroinflamm 2022, 9, e1152. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Neuroinflammation for Alzheimer’s Disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, B.P.; Solfrizzi, V.; Panza, F. Are NSAIDs Useful to Treat Alzheimer’s Disease or Mild Cognitive Impairment? Front. Aging Neurosci. 2010, 2, 1517. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Strandberg, O.; Whelan, C.D.; Zetterberg, H.; Blennow, K.; Palmqvist, S.; Stomrud, E.; Mattsson-Carlgren, N.; Hansson, O. Microglial Activation Protects against Accumulation of Tau Aggregates in Nondemented Individuals with Underlying Alzheimer’s Disease Pathology. Nat. Aging 2022, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Du, H. TREM2 Mediates Microglial Anti-Inflammatory Activations in Alzheimer’s Disease: Lessons Learned from Transcriptomics. Cells 2021, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Tondo, G.; Perani, D.; Comi, C. TAM Receptor Pathways at the Crossroads of Neuroinflammation and Neurodegeneration. Dis. Markers 2019, 2019, 2387614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, Y.; Zhang, Z.; Yuan, Y. An Insight into the TAM System in Alzheimer’s Disease. Int. Immunopharmacol. 2023, 116, 109791. [Google Scholar] [CrossRef] [PubMed]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K. SYK Coordinates Neuroprotective Microglial Responses in Neurodegenerative Disease. Cell 2022, 185, 4135–4152. [Google Scholar] [CrossRef]
- Puntambekar, S.S.; Moutinho, M.; Lin, P.B.-C.; Jadhav, V.; Tumbleson-Brink, D.; Balaji, A.; Benito, M.A.; Xu, G.; Oblak, A.; Lasagna-Reeves, C.A. CX3CR1 Deficiency Aggravates Amyloid Driven Neuronal Pathology and Cognitive Decline in Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 47. [Google Scholar] [CrossRef]
- Claes, C.; England, W.E.; Danhash, E.P.; Kiani Shabestari, S.; Jairaman, A.; Chadarevian, J.P.; Hasselmann, J.; Tsai, A.P.; Coburn, M.A.; Sanchez, J. The P522R Protective Variant of PLCG2 Promotes the Expression of Antigen Presentation Genes by Human Microglia in an Alzheimer’s Disease Mouse Model. Alzheimer’s Dement. 2022, 18, 1765–1778. [Google Scholar] [CrossRef]
- Birch, A.M. The Contribution of Astrocytes to Alzheimer’s Disease. Biochem. Soc. Trans. 2014, 42, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Giraldo, M.; González-Reyes, R.E.; Ramírez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a Therapeutic Target in Alzheimer’s Disease–Comprehensive Review and Recent Developments. Int. J. Mol. Sci. 2022, 23, 13630. [Google Scholar] [CrossRef]
- Orgogozo, J.-M.; Gilman, S.; Dartigues, J.-F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S. Subacute Meningoencephalitis in a Subset of Patients with AD after Aβ42 Immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Koller, M.; Black, R.S.; Jenkins, L.; Griffith, S.G.; Fox, N.C.; Eisner, L.; Kirby, L.; Rovira, M.B.; Forette, F. Clinical Effects of Aβ Immunization (AN1792) in Patients with AD in an Interrupted Trial. Neurology 2005, 64, 1553–1562. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of Human Alzheimer Disease after Immunization with Amyloid-β Peptide: A Case Report. Nat. Med. 2003, 9, 448–452. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Buckland, G.R.; Harrison, C.H.; Page, A.; Harris, S.; Love, S.; Neal, J.W.; Holmes, C.; Boche, D. Persistent Neuropathological Effects 14 Years Following Amyloid-β Immunization in Alzheimer’s Disease. Brain 2019, 142, 2113–2126. [Google Scholar] [CrossRef]
- Kwan, P.; Konno, H.; Chan, K.Y.; Baum, L. Rationale for the Development of an Alzheimer’s Disease Vaccine. Hum. Vaccin. Immunother. 2020, 16, 645–653. [Google Scholar] [CrossRef]
- Winblad, B.; Andreasen, N.; Minthon, L.; Floesser, A.; Imbert, G.; Dumortier, T.; Maguire, R.P.; Blennow, K.; Lundmark, J.; Staufenbiel, M. Safety, Tolerability, and Antibody Response of Active Aβ Immunotherapy with CAD106 in Patients with Alzheimer’s Disease: Randomised, Double-Blind, Placebo-Controlled, First-in-Human Study. Lancet Neurol. 2012, 11, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Wang, P.-N.; Chiu, M.-J.; Finstad, C.L.; Lin, F.; Lynn, S.; Tai, Y.-H.; De Fang, X.; Zhao, K.; Hung, C.-H. UB-311, a Novel UBITh® Amyloid β Peptide Vaccine for Mild Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 262–272. [Google Scholar] [CrossRef]
- Yu, H.J.; Dickson, S.P.; Wang, P.-N.; Chiu, M.-J.; Huang, C.-C.; Chang, C.-C.; Liu, H.; Hendrix, S.B.; Dodart, J.-C.; Verma, A. Safety, Tolerability, Immunogenicity, and Efficacy of UB-311 in Participants with Mild Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase 2a Study. EBioMedicine 2023, 94, 104665. [Google Scholar] [CrossRef]
- Lacosta, A.-M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I. Safety, Tolerability and Immunogenicity of an Active Anti-Aβ 40 Vaccine (ABvac40) in Patients with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase I Trial. Alzheimers Res. Ther. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.-L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K. Peripherally Administered Antibodies against Amyloid β-Peptide Enter the Central Nervous System and Reduce Pathology in a Mouse Model of Alzheimer Disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Esquer, A.; Blanc, F.; Collongues, N. Immunotherapies Targeting Amyloid and Tau Protein in Alzheimer’s Disease: Should We Move Away from Diseases and Focus on Biological Targets? A Systematic Review and Expert Opinion. Neurol. Ther. 2023, 12, 1883–1907. [Google Scholar] [CrossRef]
- Piazza, F.; Winblad, B. Amyloid-Related Imaging Abnormalities (ARIA) in Immunotherapy Trials for Alzheimer’s Disease: Need for Prognostic Biomarkers? J. Alzheimer’s Dis. 2016, 52, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef]
- Bateman, R.J.; Smith, J.; Donohue, M.C.; Delmar, P.; Abbas, R.; Salloway, S.; Wojtowicz, J.; Blennow, K.; Bittner, T.; Black, S.E. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1862–1876. [Google Scholar] [CrossRef] [PubMed]
- Bouter, Y.; Noguerola, J.S.L.; Tucholla, P.; Crespi, G.A.N.; Parker, M.W.; Wiltfang, J.; Miles, L.A.; Bayer, T.A. Abeta Targets of the Biosimilar Antibodies of Bapineuzumab, Crenezumab, Solanezumab in Comparison to an Antibody against N-Truncated Abeta in Sporadic Alzheimer Disease Cases and Mouse Models. Acta Neuropathol. 2015, 130, 713–729. [Google Scholar] [CrossRef]
- Gueorguieva, I.; Willis, B.A.; Chua, L.; Chow, K.; Ernest, C.S.; Shcherbinin, S.; Ardayfio, P.; Mullins, G.R.; Sims, J.R. Donanemab Population Pharmacokinetics, Amyloid Plaque Reduction, and Safety in Participants with Alzheimer’s Disease. Clin. Pharmacol. Ther. 2023, 113, 1258–1267. [Google Scholar] [CrossRef]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and Tolerability of BAN2401-a Clinical Study in Alzheimer’s Disease with a Protofibril Selective Aβ Antibody. Alzheimers Res. Ther. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A. A Randomized, Double-Blind, Phase 2b Proof-of-Concept Clinical Trial in Early Alzheimer’s Disease with Lecanemab, an Anti-Aβ Protofibril Antibody. Alzheimers Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Landen, J.W.; Cohen, S.; Billing Jr, C.B.; Cronenberger, C.; Styren, S.; Burstein, A.H.; Sattler, C.; Lee, J.; Jack Jr, C.R.; Kantarci, K. Multiple-dose Ponezumab for Mild-to-moderate Alzheimer’s Disease: Safety and Efficacy. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Dang, Y.; Ostaszewski, B.; Mengel, D.; Steffen, V.; Rabe, C.; Bittner, T.; Walsh, D.M.; Selkoe, D.J. Target Engagement in an Alzheimer Trial: Crenezumab Lowers Amyloid β Oligomers in Cerebrospinal Fluid. Ann. Neurol. 2019, 86, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Cohen, S.; van Dyck, C.H.; Brody, M.; Curtis, C.; Cho, W.; Ward, M.; Friesenhahn, M.; Rabe, C.; Brunstein, F. ABBY: A Phase 2 Randomized Trial of Crenezumab in Mild to Moderate Alzheimer Disease. Neurology 2018, 90, e1889–e1897. [Google Scholar] [CrossRef] [PubMed]
- Ostrowitzki, S.; Bittner, T.; Sink, K.M.; Mackey, H.; Rabe, C.; Honig, L.S.; Cassetta, E.; Woodward, M.; Boada, M.; van Dyck, C.H. Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults with Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials. JAMA Neurol. 2022, 79, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Farlow, M.; McDade, E.; Clifford, D.B.; Wang, G.; Llibre-Guerra, J.J.; Hitchcock, J.M.; Mills, S.L.; Santacruz, A.M.; Aschenbrenner, A.J. A Trial of Gantenerumab or Solanezumab in Dominantly Inherited Alzheimer’s Disease. Nat. Med. 2021, 27, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P. A Phase III Randomized Trial of Gantenerumab in Prodromal Alzheimer’s Disease. Alzheimers Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef]
- Sperling, R.A.; Donohue, M.C.; Raman, R.; Rafii, M.S.; Johnson, K.; Masters, C.L.; van Dyck, C.H.; Iwatsubo, T.; Marshall, G.A.; Yaari, R. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N. Engl. J. Med. 2023, 389, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, J.; Williams, L.; Stella, H.; Leitermann, K.; Mikulskis, A.; O’Gorman, J.; Sevigny, J. First-in-Human, Double-Blind, Placebo-Controlled, Single-Dose Escalation Study of Aducanumab (BIIB037) in Mild-to-Moderate Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S. Structural and Kinetic Basis for the Selectivity of Aducanumab for Aggregated Forms of Amyloid-β. Sci. Rep. 2018, 8, 6412. [Google Scholar] [CrossRef]
- Heidebrink, J.L.; Paulson, H.L. Lessons Learned from Approval of Aducanumab for Alzheimer’s Disease. Annu. Rev. Med. 2024, 75, 99–111. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; Von Hehn, C. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer Disease and Aducanumab: Adjusting Our Approach. Nat. Rev. Neurol. 2019, 15, 365–366. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Mullard, A. Alzheimers Drug Approval Could Affect Other Diseases. Nature 2021, 595, 162–163. [Google Scholar] [CrossRef]
- Haeberlein, S.B.; von Hehn, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R. Emerge and Engage Topline Results: Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease: Developments in Clinical Trials and Cognitive Assessment. Alzheimer’s Dement. 2020, 16, e047259. [Google Scholar] [CrossRef]
- Alexander, G.C.; Knopman, D.S.; Emerson, S.S.; Ovbiagele, B.; Kryscio, R.J.; Perlmutter, J.S.; Kesselheim, A.S. Revisiting FDA Approval of Aducanumab. N. Engl. J. Med. 2021, 385, 769–771. [Google Scholar] [CrossRef]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. Disease-Modifying Therapies for Alzheimer’s Disease: More Questions than Answers. Neurotherapeutics 2023, 19, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Ait-Bouziad, N.; Chiki, A.; Limorenko, G.; Xiao, S.; Eliezer, D.; Lashuel, H.A. Phosphorylation of the Overlooked Tyrosine 310 Regulates the Structure, Aggregation, and Microtubule-and Lipid-Binding Properties of Tau. J. Biol. Chem. 2020, 295, 7905–7922. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.A.; Carare, R.O.; Weller, R.O. Amyloid and Tau in the Brain in Sporadic Alzheimer’s Disease: Defining the Chicken and the Egg. Acta Neuropathol. 2014, 127, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Bittar, A.; Bhatt, N.; Kayed, R. Advances and Considerations in AD Tau-Targeted Immunotherapy. Neurobiol. Dis. 2020, 134, 104707. [Google Scholar] [CrossRef] [PubMed]
- Asuni, A.A.; Boutajangout, A.; Quartermain, D.; Sigurdsson, E.M. Immunotherapy Targeting Pathological Tau Conformers in a Tangle Mouse Model Reduces Brain Pathology with Associated Functional Improvements. J. Neurosci. 2007, 27, 9115–9129. [Google Scholar] [CrossRef] [PubMed]
- Kontsekova, E.; Zilka, N.; Kovacech, B.; Novak, P.; Novak, M. First-in-Man Tau Vaccine Targeting Structural Determinants Essential for Pathological Tau–Tau Interaction Reduces Tau Oligomerisation and Neurofibrillary Degeneration in an Alzheimer’s Disease Model. Alzheimers Res. Ther. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Schmidt, R.; Kontsekova, E.; Kovacech, B.; Smolek, T.; Katina, S.; Fialova, L.; Prcina, M.; Parrak, V.; Dal-Bianco, P. FUNDAMANT: An Interventional 72-Week Phase 1 Follow-up Study of AADvac1, an Active Immunotherapy against Tau Protein Pathology in Alzheimer’s Disease. Alzheimers Res. Ther. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Novak, P.; Kontsekova, E.; Zilka, N.; Novak, M. Ten Years of Tau-Targeted Immunotherapy: The Path Walked and the Roads Ahead. Front. Neurosci. 2018, 12, 798. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Lozupone, M.; Santamato, A.; Tortelli, R.; Galizia, I.; Prete, C.; Daniele, A. Tau-Based Therapeutics for Alzheimer’s Disease: Active and Passive Immunotherapy. Immunotherapy 2016, 8, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N. ADAMANT: A Placebo-Controlled Randomized Phase 2 Study of AADvac1, an Active Immunotherapy against Pathological Tau in Alzheimer’s Disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Cullen, N.C.; Novak, P.; Tosun, D.; Kovacech, B.; Hanes, J.; Kontsekova, E.; Fresser, M.; Ropele, S.; Feldman, H.H.; Schmidt, R. Efficacy Assessment of an Active Tau Immunotherapy in Alzheimer’s Disease Patients with Amyloid and Tau Pathology: A Post Hoc Analysis of the “ADAMANT” Randomised, Placebo-Controlled, Double-Blind, Multi-Centre, Phase 2 Clinical Trial. EBioMedicine 2024, 99, 104923. [Google Scholar] [CrossRef] [PubMed]
- Theunis, C.; Crespo-Biel, N.; Gafner, V.; Pihlgren, M.; López-Deber, M.P.; Reis, P.; Hickman, D.T.; Adolfsson, O.; Chuard, N.; Ndao, D.M. Efficacy and Safety of a Liposome-Based Vaccine against Protein Tau, Assessed in Tau. P301L Mice That Model Tauopathy. PLoS ONE 2013, 8, e72301. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s Disease: Recent Treatment Strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Ayalon, G.; Lee, S.-H.; Adolfsson, O.; Foo-Atkins, C.; Atwal, J.K.; Blendstrup, M.; Booler, H.; Bravo, J.; Brendza, R.; Brunstein, F. Antibody Semorinemab Reduces Tau Pathology in a Transgenic Mouse Model and Engages Tau in Patients with Alzheimer’s Disease. Sci. Transl. Med. 2021, 13, eabb2639. [Google Scholar] [CrossRef]
- Mullard, A. Failure of First Anti-Tau Antibody in Alzheimer Disease Highlights Risks of History Repeating. Nat. Rev. Drug Discov. 2021, 20, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Bohorquez, S.S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V. Safety and Efficacy of Semorinemab in Individuals with Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef]
- Monteiro, C.; Toth, B.; Brunstein, F.; Bobbala, A.; Datta, S.; Ceniceros, R.; Sanabria Bohorquez, S.M.; Anania, V.G.; Wildsmith, K.R.; Schauer, S.P. Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants with Mild-to-Moderate Alzheimer Disease: Lauriet. Neurology 2023, 101, e1391–e1401. [Google Scholar] [CrossRef]
- Sopko, R.; Golonzhka, O.; Arndt, J.; Quan, C.; Czerkowicz, J.; Cameron, A.; Smith, B.; Murugesan, Y.; Gibbons, G.; Kim, S.-J. Characterization of Tau Binding by Gosuranemab. Neurobiol. Dis. 2020, 146, 105120. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.-C.; Aiba, I.; Grundman, M. Safety and Efficacy of Anti-Tau Monoclonal Antibody Gosuranemab in Progressive Supranuclear Palsy: A Phase 2, Randomized, Placebo-Controlled Trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J. TANGO: A Placebo-Controlled Randomized Phase 2 Study of Efficacy and Safety of the Anti-Tau Monoclonal Antibody Gosuranemab in Early Alzheimer’s Disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Kfoury, N.; Jiang, H.; Mahan, T.E.; Ma, S.; Maloney, S.E.; Wozniak, D.F.; Diamond, M.I.; Holtzman, D.M. Anti-Tau Antibodies That Block Tau Aggregate Seeding in Vitro Markedly Decrease Pathology and Improve Cognition in Vivo. Neuron 2013, 80, 402–414. [Google Scholar] [CrossRef]
- Florian, H.; Wang, D.; Arnold, S.E.; Boada, M.; Guo, Q.; Jin, Z.; Zheng, H.; Fisseha, N.; Kalluri, H.V.; Rendenbach-Mueller, B. Tilavonemab in Early Alzheimer’s Disease: Results from a Phase 2, Randomized, Double-Blind Study. Brain 2023, 146, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.A.; Lo, A.C.; Dage, J.L.; Shcherbinin, S.; Chinchen, L.; Andersen, S.W.; LaBell, E.S.; Perahia, D.G.S.; Hauck, P.M.; Lowe, S.L. Safety, Tolerability, and Pharmacokinetics of Zagotenemab in Participants with Symptomatic Alzheimer’s Disease: A Phase I Clinical Trial. J. Alzheimers Dis. Rep. 2023, 7, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Stopschinski, B.E.; Weideman, R.A.; McMahan, D.; Jacob, D.A.; Little, B.B.; Chiang, H.-S.; Saez Calveras, N.; Stuve, O. Microglia as a Cellular Target of Diclofenac Therapy in Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2023, 16, 17562864231156674. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Kim, D.; Kim, H.-Y.; Jeong, S.W.; Lee, S.-G.; Kim, H.-C.; Lee, Y.-J.; Kwon, M.K.; Hwang, J.-S.; Han, J.E. Microglial Phagocytosis of Polystyrene Microplastics Results in Immune Alteration and Apoptosis in Vitro and in Vivo. Sci. Total Environ. 2022, 807, 150817. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schmeidler, J.; Pasinetti, G.M. Randomized Pilot Study of Nimesulide Treatment in Alzheimer’s Disease. Neurology 2002, 58, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.; Jansen, R.; Hoefnagels, W.; Jellesma-Eggenkamp, M.; Verbeek, M.; Borm, G.; Kremer, B. No Effect of One-Year Treatment with Indomethacin on Alzheimer’s Disease Progression: A Randomized Controlled Trial. PLoS ONE 2008, 3, e1475. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.; Mander, A.; Ugoni, A.; Vajda, F.; Christophidis, N. A Double-Blind, Placebo-Controlled Trial of Diclofenac/Misoprostol in Alzheimer’s Disease. Neurology 1999, 53, 197. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-Inflammatories in Alzheimer’s Disease—Potential Therapy or Spurious Correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Dikmen, H.O.; Hemmerich, M.; Lewen, A.; Hollnagel, J.-O.; Chausse, B.; Kann, O. GM-CSF Induces Noninflammatory Proliferation of Microglia and Disturbs Electrical Neuronal Network Rhythms in Situ. J. Neuroinflamm. 2020, 17, 235. [Google Scholar] [CrossRef]
- Potter, H.; Woodcock, J.H.; Boyd, T.D.; Coughlan, C.M.; O’Shaughnessy, J.R.; Borges, M.T.; Thaker, A.A.; Raj, B.A.; Adamszuk, K.; Scott, D. Safety and Efficacy of Sargramostim (GM-CSF) in the Treatment of Alzheimer’s Disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12158. [Google Scholar] [CrossRef] [PubMed]
- Tobeh, N.S.; Bruce, K.D. Emerging Alzheimer’s Disease Therapeutics: Promising Insights from Lipid Metabolism and Microglia-Focused Interventions. Front. Aging Neurosci. 2023, 15, 1259012. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Rawji, K.S.; Young, A.M.H.; Ghosh, T.; Michaels, N.J.; Mirzaei, R.; Kappen, J.; Kolehmainen, K.L.; Alaeiilkhchi, N.; Lozinski, B.; Mishra, M.K. Niacin-Mediated Rejuvenation of Macrophage/Microglia Enhances Remyelination of the Aging Central Nervous System. Acta Neuropathol. 2020, 139, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Remolina Serrano, J.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 Deficiency Attenuates Neuroinflammation and Protects against Neurodegeneration in a Mouse Model of Tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S. Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef] [PubMed]
- Faridar, A.; Thome, A.D.; Zhao, W.; Thonhoff, J.R.; Beers, D.R.; Pascual, B.; Masdeu, J.C.; Appel, S.H. Restoring Regulatory T-Cell Dysfunction in Alzheimer’s Disease through Ex Vivo Expansion. Brain Commun. 2020, 2, fcaa112. [Google Scholar] [CrossRef]
- Faridar, A.; Vasquez, M.; Thome, A.D.; Yin, Z.; Xuan, H.; Wang, J.H.; Wen, S.; Li, X.; Thonhoff, J.R.; Zhao, W. Ex Vivo Expanded Human Regulatory T Cells Modify Neuroinflammation in a Preclinical Model of Alzheimer’s Disease. Acta Neuropathol. Commun. 2022, 10, 144. [Google Scholar] [CrossRef]
- Faridar, A.; Eid, A.M.; Thome, A.D.; Zhao, W.; Beers, D.R.; Pascual, M.B.; Nakawah, M.O.; Roman, G.C.; Davis, C.S.; Grundman, M. A Phase 1 Open-Label Pilot Study of Low-Dose Interleukine-2 Immunotherapy in Patients with Alzheimer’s Disease. Transl. Neurodegener. 2023, 12, 54. [Google Scholar] [CrossRef]
- Tarkowski, E.; Andreasen, N.; Tarkowski, A.; Blennow, K. Intrathecal Inflammation Precedes Development of Alzheimer’s Disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, R.; Kaelber, D.C.; Gurney, M.E. Tumor Necrosis Factor (TNF) Blocking Agents Are Associated with Lower Risk for Alzheimer’s Disease in Patients with Rheumatoid Arthritis and Psoriasis. PLoS ONE 2020, 15, e0229819. [Google Scholar] [CrossRef]
- Paganoni, S.; Berry, J.D.; Quintana, M.; Macklin, E.; Saville, B.R.; Detry, M.A.; Chase, M.; Sherman, A.V.; Yu, H.; Drake, K. Adaptive Platform Trials to Transform Amyotrophic Lateral Sclerosis Therapy Development. Ann. Neurol. 2022, 91, 165–175. [Google Scholar] [CrossRef]
- Dhimolea, E. Canakinumab. In Proceedings of the MAbs; Taylor & Francis: Boca Raton, FL, USA, 2010; Volume 2, pp. 3–13. [Google Scholar]
- Robinson, M.; Lee, B.Y.; Hane, F.T. Recent Progress in Alzheimer’s Disease Research, Part 2: Genetics and Epidemiology. J. Alzheimer’s Dis. 2017, 57, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, L.; Tanzi, R.E.; Kovacs, D.M. Alzheimer’s Disease: The Cholesterol Connection. Nat. Neurosci. 2003, 6, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Doshi, V.; Joshi, G.; Sharma, S.; Choudhary, D. Gene therapy: An alternative to treat Alzheimer’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3675–3693. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Piras, A.; Ismail, M.A.M.; Manchanda, S.; Eyjolfsdottir, H.; Saido, T.C.; Johansson, J.; Eriksdotter, M.; Winblad, B.; Nilsson, P. Targeting Alzheimer’s Disease with Gene and Cell Therapies. J. Intern. Med. 2018, 284, 2–36. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; U, H.S.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G. A Phase 1 Clinical Trial of Nerve Growth Factor Gene Therapy for Alzheimer Disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.S.; Tuszynski, M.H.; Thomas, R.G.; Barba, D.; Brewer, J.B.; Rissman, R.A.; Siffert, J.; Aisen, P.S.; AAV2-NGF Study Team. Adeno-Associated Viral Vector (Serotype 2)–Nerve Growth Factor for Patients with Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol 2018, 75, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.; Iwata, N.; Muramatsu, S.; Tjernberg, L.O.; Winblad, B.; Saido, T.C. Gene Therapy in Alzheimer’s Disease–Potential for Disease Modification. J. Cell Mol. Med. 2010, 14, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Takaki, Y.; Fukami, S.; Tsubuki, S.; Saido, T.C. Region-specific Reduction of Aβ-degrading Endopeptidase, Neprilysin, in Mouse Hippocampus upon Aging. J. Neurosci. Res. 2002, 70, 493–500. [Google Scholar] [CrossRef]
- Kaminari, A.; Tsilibary, E.C.; Tzinia, A. A New Perspective in Utilizing MMP-9 as a Therapeutic Target for Alzheimer’s Disease and Type 2 Diabetes Mellitus. J. Alzheimer’s Dis. 2018, 64, 1–16. [Google Scholar] [CrossRef]
- Hernandes-Alejandro, M.; Montaño, S.; Harrington, C.R.; Wischik, C.M.; Salas-Casas, A.; Cortes-Reynosa, P.; Pérez Salazar, E.; Cazares-Apatiga, J.; Apatiga-Perez, R.; Ontiveros Torres, M.Á. Analysis of the Relationship between Metalloprotease-9 and Tau Protein in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Chiba, Y.; Hattori, S.; Yoshimi, A.; Asami, T.; Katsuse, O.; Suda, A.; Hishimoto, A.; Initiative, A.D.N. Influence of Plasma Matrix Metalloproteinase Levels on Longitudinal Changes in Alzheimer’s Disease (AD) Biomarkers and Cognitive Function in Patients with Mild Cognitive Impairment Due to AD Registered in the Alzheimer’s Disease Neuroimaging Initiative Database. J. Neurol. Sci. 2020, 416, 116989. [Google Scholar]
- Yan, P.; Hu, X.; Song, H.; Yin, K.; Bateman, R.J.; Cirrito, J.R.; Xiao, Q.; Hsu, F.F.; Turk, J.W.; Xu, J. Matrix Metalloproteinase-9 Degrades Amyloid-β Fibrils in Vitro and Compact Plaques in Situ. J. Biol. Chem. 2006, 281, 24566–24574. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, J.R.; Lim, G.P.; Cullen, M.J.; Tökés, Z.A. Matrix Metalloproteinase-9 (MMP-9) Is Synthesized in Neurons of the Human Hippocampus and Is Capable of Degrading the Amyloid-β Peptide (1–40). J. Neurosci. 1996, 16, 7910–7919. [Google Scholar] [CrossRef] [PubMed]
- Dhanavade, M.J.; Sonawane, K.D. Amyloid Beta Peptide-Degrading Microbial Enzymes and Its Implication in Drug Design. 3 Biotech 2020, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Ringland, C.; Schweig, J.E.; Paris, D.; Shackleton, B.; Lynch, C.E.; Eisenbaum, M.; Mullan, M.; Crawford, F.; Abdullah, L.; Bachmeier, C. Apolipoprotein E Isoforms Differentially Regulate Matrix Metallopeptidase 9 Function in Alzheimer’s Disease. Neurobiol. Aging 2020, 95, 56–68. [Google Scholar] [CrossRef]
- Ringland, C.; Schweig, J.E.; Eisenbaum, M.; Paris, D.; Ait-Ghezala, G.; Mullan, M.; Crawford, F.; Abdullah, L.; Bachmeier, C. MMP9 Modulation Improves Specific Neurobehavioral Deficits in a Mouse Model of Alzheimer’s Disease. BMC Neurosci. 2021, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C. Tau-Targeting Antisense Oligonucleotide MAPTRx in Mild Alzheimer’s Disease: A Phase 1b, Randomized, Placebo-Controlled Trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.L.; Collins, J.A.; Junge, C.; Kordasiewicz, H.; Mignon, L.; Wu, S.; Li, Y.; Lin, L.; DuBois, J.; Hutchison, R.M. Exploratory Tau Biomarker Results From a Multiple Ascending-Dose Study of BIIB080 in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2023, 80, 1344–1352. [Google Scholar] [CrossRef]
- Sironi, F.; De Marchi, F.; Mazzini, L.; Bendotti, C. Cell Therapy in ALS: An Update on Preclinical and Clinical Studies. Brain Res. Bull. 2023, 194, 64–81. [Google Scholar] [CrossRef]
- De Marchi, F.; Munitic, I.; Vidatic, L.; Papić, E.; Rački, V.; Nimac, J.; Jurak, I.; Novotni, G.; Rogelj, B.; Vuletic, V. Overlapping Neuroimmune Mechanisms and Therapeutic Targets in Neurodegenerative Disorders. Biomedicines 2023, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yoon, S.; Choi, J.; Kim, Y.J.; Lee, G. Stem Cell-Based Approaches in Parkinson’s Disease Research. Int. J. Stem. Cells 2024. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Lyu, L.; Zhan, S. Stem Cell Therapy for Alzheimer’s Disease: A Scoping Review for 2017–2022. Biomedicines 2023, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Brody, M.; Agronin, M.; Herskowitz, B.J.; Bookheimer, S.Y.; Small, G.W.; Hitchinson, B.; Ramdas, K.; Wishard, T.; McInerney, K.F.; Vellas, B. Results and Insights from a Phase I Clinical Trial of Lomecel-B for Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 261–273. [Google Scholar] [CrossRef]
| Intervening “Drug” | Trial Phase | Number of Patients Enrolled | Disease Stage | Duration | Primary Endpoint | Outcome | Authors, Year, and Country |
|---|---|---|---|---|---|---|---|
| Semorinemab | Phase II | 272 | Prodromal-to-mild AD | 48/60 weeks | ADAS-Cog11; ADCS-ADL | No clinical effects | Monteiro et al., 2023, US [160] |
| Gosuranemab | Phase II, dose finding | 654 | Early AD | 78 weeks | Safety and efficacy | Safe profile and well-tolerated | Shulman et al., 2023, US [163] |
| Tilavonemab | Phase II | 453 | Early AD | 96 weeks | Safety and efficacy; CDR-SB | Well-tolerated but no clinical effects | Florian et al., 2023, multicenter [165] |
| Zagotenemab | Phase Ib, placebo-controlled | 22 | MCI or early AD | 49 weeks | Safety and pharmacokinetics | No adverse events | Willis et al., 2023, US [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tondo, G.; De Marchi, F.; Bonardi, F.; Menegon, F.; Verrini, G.; Aprile, D.; Anselmi, M.; Mazzini, L.; Comi, C. Novel Therapeutic Strategies in Alzheimer’s Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond. J. Clin. Med. 2024, 13, 3098. https://doi.org/10.3390/jcm13113098
Tondo G, De Marchi F, Bonardi F, Menegon F, Verrini G, Aprile D, Anselmi M, Mazzini L, Comi C. Novel Therapeutic Strategies in Alzheimer’s Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond. Journal of Clinical Medicine. 2024; 13(11):3098. https://doi.org/10.3390/jcm13113098
Chicago/Turabian StyleTondo, Giacomo, Fabiola De Marchi, Francesca Bonardi, Federico Menegon, Gaia Verrini, Davide Aprile, Matteo Anselmi, Letizia Mazzini, and Cristoforo Comi. 2024. "Novel Therapeutic Strategies in Alzheimer’s Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond" Journal of Clinical Medicine 13, no. 11: 3098. https://doi.org/10.3390/jcm13113098
APA StyleTondo, G., De Marchi, F., Bonardi, F., Menegon, F., Verrini, G., Aprile, D., Anselmi, M., Mazzini, L., & Comi, C. (2024). Novel Therapeutic Strategies in Alzheimer’s Disease: Pitfalls and Challenges of Anti-Amyloid Therapies and Beyond. Journal of Clinical Medicine, 13(11), 3098. https://doi.org/10.3390/jcm13113098







