The Role of Galectin-3 Levels for Predicting Paroxysmal Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrocardiography
2.2. Echocardiography
2.3. Galectin-3
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-W.; Kim, C.K.; Kim, T.J.; An, S.J.; Oh, K.; Ko, S.-B.; Yoon, B.-W. Treatment of Cryptogenic Stroke with Active Cancer with a New Oral Anticoagulant. J. Stroke Cerebrovasc. Dis. 2017, 26, 2976–2980. [Google Scholar] [CrossRef]
- Elkind, M.S. Atrial Cardiopathy and Stroke Prevention. Curr. Cardiol. Rep. 2018, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, A.; Sawyer, L.M.; Lip, G.Y.; Witte, K.K.; Reynolds, M.R.; Fauchier, L.; Thijs, V.; Brown, B.; Quiroz Angulo, M.E.; Diener, H.-C. Cost-Effectiveness of an Insertable Cardiac Monitor to Detect Atrial Fibrillation in Patients with Cryptogenic Stroke. Int. J. Stroke 2016, 11, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kitsiou, A.; Rogalewski, A.; Kalyani, M.; Deelawar, S.; Tribunyan, S.; Greeve, I.; Minnerup, J.; Israel, C.; Schäbitz, W.-R. Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source during 3 Years of Prolonged Monitoring with an Implantable Loop Recorder. Thromb. Haemost. 2021, 121, 826–833. [Google Scholar] [PubMed]
- Lin, Y.-H.; Lin, L.-Y.; Wu, Y.-W.; Chien, K.-L.; Lee, C.-M.; Hsu, R.-B.; Chao, C.-L.; Wang, S.-S.; Hsein, Y.-C.; Liao, L.-C. The Relationship between Serum Galectin-3 and Serum Markers of Cardiac Extracellular Matrix Turnover in Heart Failure Patients. Clin. Chim. Acta 2009, 409, 96–99. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.; Olobatoke, A.; Vanhecke, T. Galectin-3: A Novel Blood Test for the Evaluation and Management of Patients With Heart Failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Canpinar, H.; Canpolat, U.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Hazirolan, T. The Association of Serum Galectin-3 Levels with Atrial Electrical and Structural Remodeling. J. Cardiovasc. Electrophysiol. 2015, 26, 635–640. [Google Scholar] [CrossRef]
- Jordan, K.; Yaghi, S.; Poppas, A.; Chang, A.D.; Mac Grory, B.; Cutting, S.; Burton, T.; Jayaraman, M.; Tsivgoulis, G.; Sabeh, M.K. Left Atrial Volume Index Is Associated with Cardioembolic Stroke and Atrial Fibrillation Detection after Embolic Stroke of Undetermined Source. Stroke 2019, 50, 1997–2001. [Google Scholar] [CrossRef]
- Sieweke, J.-T.; Biber, S.; Weissenborn, K.; Heuschmann, P.U.; Akin, M.; Zauner, F.; Gabriel, M.M.; Schuppner, R.; Berliner, D.; Bauersachs, J. Septal Total Atrial Conduction Time for Prediction of Atrial Fibrillation in Embolic Stroke of Unknown Source: A Pilot Study. Clin. Res. Cardiol. 2020, 109, 205–214. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Churchill, T.W.; Sharma, R.; Vandervoort, P.M.; Schwamm, L.H.; Sanborn, D.M.Y. Left Atrial Mechanics Assessed Early during Hospitalization for Cryptogenic Stroke Are Associated with Occult Atrial Fibrillation: A Speckle-Tracking Strain Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 156–165. [Google Scholar] [CrossRef]
- Öz, A.; Cinar, T.; Kızılto Güler, C.; Efe, S.Ç.; Emre, U.; Karabağ, T.; Ayça, B. Novel Electrocardiography Parameter for Paroxysmal Atrial Fibrillation in Acute Ischaemic Stroke Patients: P Wave Peak Time. Postgrad. Med. J. 2020, 96, 584–588. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Calapkorur, B.; Kelesoglu, S.; Sarli, B.; Turasan, A.; Arinc, H.; Kaya, M.G. Atrial Electromechanical Delay Is Impaired in Patients with Psoriasis. Med. Princ. Pract. 2015, 24, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Dorian, P.; Spring, M.; Panzov, V.; Mamdani, M.; Healey, J.S.; Thorpe, K.E.; EMBRACE Steering Committee and Investigators. Atrial Premature Beats Predict Atrial Fibrillation in Cryptogenic Stroke: Results from the EMBRACE Trial. Stroke 2015, 46, 936–941. [Google Scholar] [CrossRef]
- Yıldırım, E.; Günay, N.; Bayam, E.; Keskin, M.; Ozturkeri, B.; Selcuk, M. Relationship between Paroxysmal Atrial Fibrillation and a Novel Electrocardiographic Parameter P Wave Peak Time. J. Electrocardiol. 2019, 57, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G. P-Wave Morphology: Underlying Mechanisms and Clinical Implications. Ann. Noninvasive Electrocardiol. 2012, 17, 161–169. [Google Scholar] [CrossRef]
- Conen, D.; Glynn, R.J.; Sandhu, R.K.; Tedrow, U.B.; Albert, C.M. Risk Factors for Incident Atrial Fibrillation with and without Left Atrial Enlargement in Women. Int. J. Cardiol. 2013, 168, 1894–1899. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Keleşoğlu, Ş.; Yilmaz, Y.; Gökay, F.; Simsek, Y.; Calapkorur, B.; Elcik, D. Atrial Electromechanical Delay Is Impaired in Patients with Primary Hyperparathyroidism. Endokrynol. Pol. 2021, 72, 550–557. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; Yu, L.; van Veldhuisen, D.J. Galectin-3 in Cardiac Remodeling and Heart Failure. Curr. Heart Fail. Rep. 2010, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Tülüce, S.Y.; Tülüce, K.; Çil, Z.; Emren, S.V.; Akyıldız, Z.İ.; Ergene, O. Galectin-3 Levels in Patients with Hypertrophic Cardiomyopathy and Its Relationship with Left Ventricular Mass Index and Function. Anatol. J. Cardiol. 2016, 16, 344. [Google Scholar]
- Fashanu, O.E.; Norby, F.L.; Aguilar, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Chen, L.Y.; Soliman, E.Z.; Alonso, A.; Folsom, A.R. Galectin-3 and Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2017, 192, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Clementy, N.; Benhenda, N.; Piver, E.; Pierre, B.; Bernard, A.; Fauchier, L.; Pages, J.-C.; Babuty, D. Serum Galectin-3 Levels Predict Recurrences after Ablation of Atrial Fibrillation. Sci. Rep. 2016, 6, 34357. [Google Scholar] [CrossRef]
- Doverhag, C.; Hedtjärn, M.; Poirier, F.; Mallard, C.; Hagberg, H.; Karlsson, A.; Sävman, K. Galectin-3 Contributes to Neonatal Hypoxic–Ischemic Brain Injury. Neurobiol. Dis. 2010, 38, 36–46. [Google Scholar] [CrossRef]
| ESUS-PAF Group | ESUS + PAF Group | Control Group | p Value * | p Value ** | |
|---|---|---|---|---|---|
| Age | 64.16 ± 11.88 | 67.53 ± 9.60 | 62.37 ± 9.82 | 0.152 | 0.187 |
| Female sex, (n%) | 56 (46%) | 13 (43%) | 12 (40%) | 0.839 | 0.790 |
| Diabetes, (n%) | 45 (37.5%) | 17 (56%) | 8 (26%) | 0.065 | 0.050 |
| Heart Failure, (n%) | 9 (7.5%) | 3 (10%) | 4 (13.3%) | 0.707 | 0.588 |
| Coronary artery disease, (n%) | 29 (24%) | 11 (36%) | 7 (23%) | 0.173 | 0.352 |
| Hypertension (n%) | 84 (70%) | 26 (86%) | 21 (70%) | 0.070 | 0.173 |
| BMI (kg/m2) | 25.35 ± 3.25 | 26.31 ± 4.24 | 25.79 ± 3.42 | 0.180 | 0.380 |
| CHADS-VASc Score | 3.06 ± 1.71 | 3.40 ± 1.45 | 2.27 ± 1.63 | 0.316 | |
| Heart Rate (beats/min) | 73.41 ± 12.35 | 75.90 ± 12.21 | 74.43 ± 12.37 | 0.324 | 0.600 |
| Holter duration (h) | 47.63 ± 8.85 | 51.27 ± 7.58 | NA | 0.041 | NA |
| NIHSS | 5.98 ± 3.45 | 6.90 ± 3.05 | NA | 0.186 | NA |
| mRS | 1.88 ± 1.07 | 2.20 ± 0.925 | NA | 0.139 | NA |
| ESUS-PAF Group | ESUS + PAF Group | Control Group | p Value * | p Value ** | |
|---|---|---|---|---|---|
| PR interval (ms) | 163.2 ± 22.7 | 170.8 ± 21.3 | 155.2 ± 16.8 | 0.099 | 0.021 |
| P wave time (ms) | 98.4 ± 14.3 | 105.3 ± 17.2 | 89.9 ±16.7 | 0.027 | 0.001 |
| P wave peak time (ms) | 53.3 ± 11.0 | 59.2 ± 12.7 | 49.3 ± 8.7 | 0.013 | 0.002 |
| ESUS-PAF Group | ESUS + PAF Group | Control Group | p Value * | p Value ** | |
|---|---|---|---|---|---|
| LVSd (cm) | 3.36 ± 0.49 | 3.58 ± 0.84 | 3.33 ± 0.31 | 0.061 | 0.106 |
| LVDd (cm) | 4.89 ± 0.59 | 4.67 ± 0.83 | 5.05 ± 0.44 | 0.090 | 0.061 |
| IVSd (cm) | 1.06 ± 0.16 | 1.18 ± 0.14 | 1.05 ± 0.13 | <0.001 | <0.001 |
| PWd (cm) | 1.01 ± 0.14 | 1.12 ± 0.13 | 1.01 ± 0.14 | <0.001 | 0.001 |
| LVEF (%) | 62.9 ± 3.65 | 64.0 ± 4.14 | 62.73 ± 3.64 | 0.153 | 0.296 |
| LA diameter (cm) | 3.41 ± 0.36 | 3.80 ± 0.36 | 3.30 ± 0.31 | <0.001 | <0.001 |
| LA area (cm2) | 13.56 ± 3.26 | 18.38 ± 4.09 | 12.36 ± 2.54 | <0.001 | <0.001 |
| LA Volume Index (mL/m2) | 32.13 ± 6.84 | 39.06 ± 5.93 | 26.00 ± 4.57 | <0.001 | <0.001 |
| PA lateral (ms) | 73.94 ± 10.74 | 97.83 ± 10.95 | 73.7 ± 7.56 | <0.001 | <0.001 |
| PA septum (ms) | 64.01 ± 10.75 | 84.80 ± 11.23 | 61.83 ± 5.94 | <0.001 | <0.001 |
| PA tricuspid (ms) | 54.76 ± 10.19 | 75.27 ± 11.02 | 51.33 ± 5.96 | <0.001 | <0.001 |
| LA global peak strain (%) | 23.68 ± 6.30 | 16.87 ± 4.51 | 29.40 ± 3.61 | <0.001 | <0.001 |
| Serum galectin-3 (pg/mL) | 297.8 ± 280.3 | 479.0 ± 435.8 | 125.4 ± 87.0 | 0.006 | <0.001 |
| LAVI (mL/m2) | PA Lateral (ms) | Global LA Strain% | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
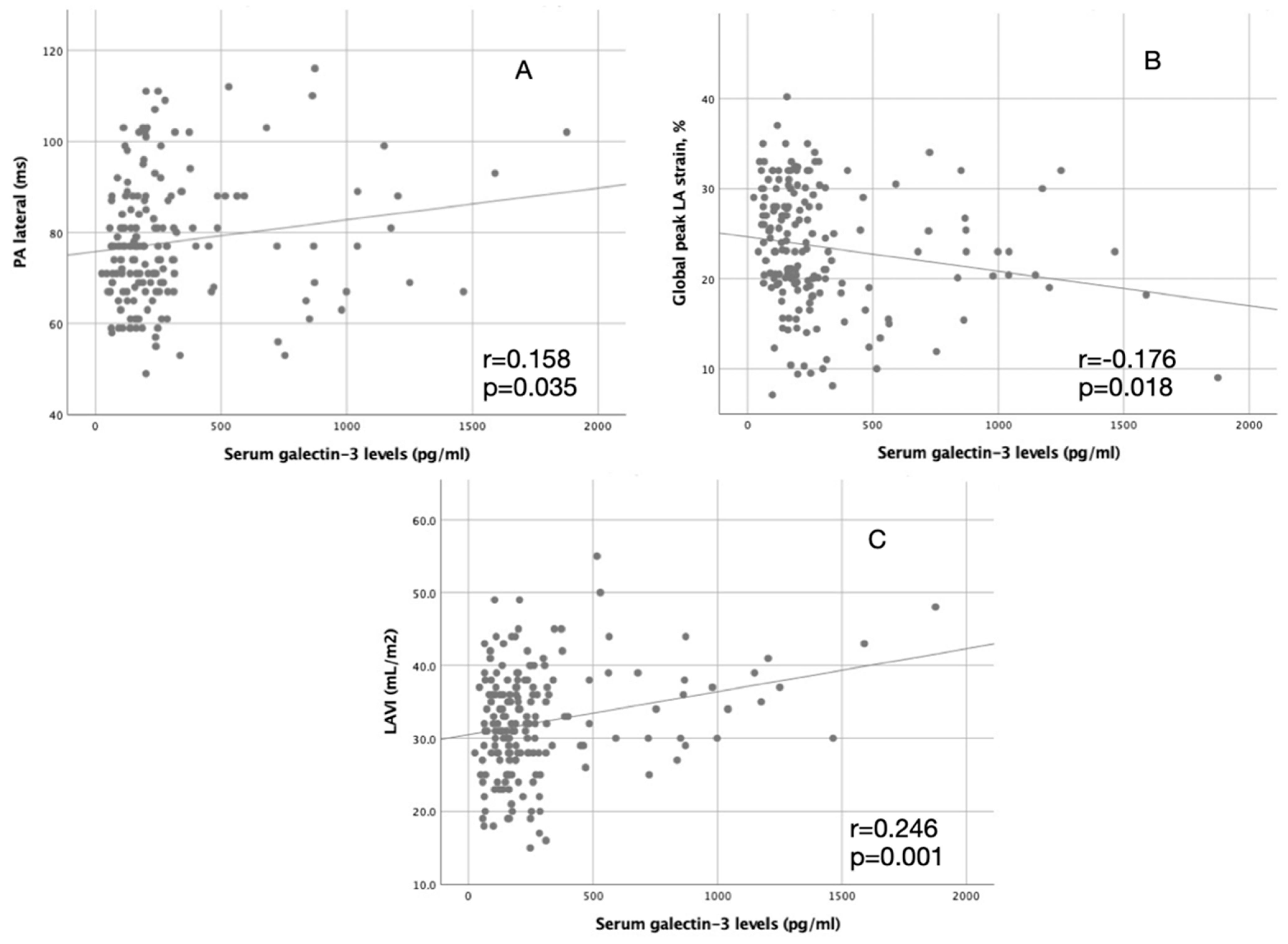
| Serum galectin-3 levels (pg/mL) | 0.246 | 0.001 | 0.158 | 0.035 | −0.176 | 0.018 |
| Univariate Regression Model | Multivariate Regression Model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (year) | 1.028 | 0.990–1.068 | 0.153 | |||
| Female Sex | 0.874 | 0.390–1.957 | 0.743 | |||
| Heart failure | 1.370 | 0.347–5.407 | 0.653 | |||
| Hypertension | 2.786 | 0.906–8.561 | 0.074 | |||
| Diabetes | 2.179 | 0.968–4.905 | 0.060 | |||
| Coronary artery disease | 1.817 | 0.775–4.259 | 0.170 | |||
| NIHSS | 1.080 | 0.963–1.210 | 0.187 | |||
| mRS | 1.316 | 0.913–1.898 | 0.141 | |||
| CHADS-VASc Score | 1.130 | 0.891–1.433 | 0.315 | |||
| PR interval (ms) | 1.015 | 0.997–1.033 | 0.102 | |||
| Holter Monitoring Duration (h) | 1.050 | 1.002–1.101 | 0.043 | 1.080 | 0.978–1.192 | 0.127 |
| P wave duration (ms) | 1.032 | 1.003–1.061 | 0.029 | 0.962 | 0.891–1.039 | 0.323 |
| P wave peak duration (ms) | 1.045 | 1.009–1.083 | 0.015 | 0.962 | 0.891–1.039 | 0.203 |
| Global Peak LA Strain% | 0.821 | 0.754–0.894 | <0.001 | 0.821 | 0.700–0.963 | 0.015 |
| PA Lateral (ms) | 1.206 | 1.130–1.287 | <0.001 | 1.500 | 1.194–1.885 | <0001 |
| PA Septal (ms) | 1.190 | 1.118–1.267 | <0.001 | 0.739 | 0.545–1.002 | 0.052 |
| PA Tricuspit (ms) | 1.196 | 1.123–1.273 | <0.001 | 1.130 | 0.906–1.409 | 0.279 |
| LAVI (mL/m2) | 1.180 | 1.094–1.273 | <0.001 | 0.920 | 0.798–1.060 | 0.248 |
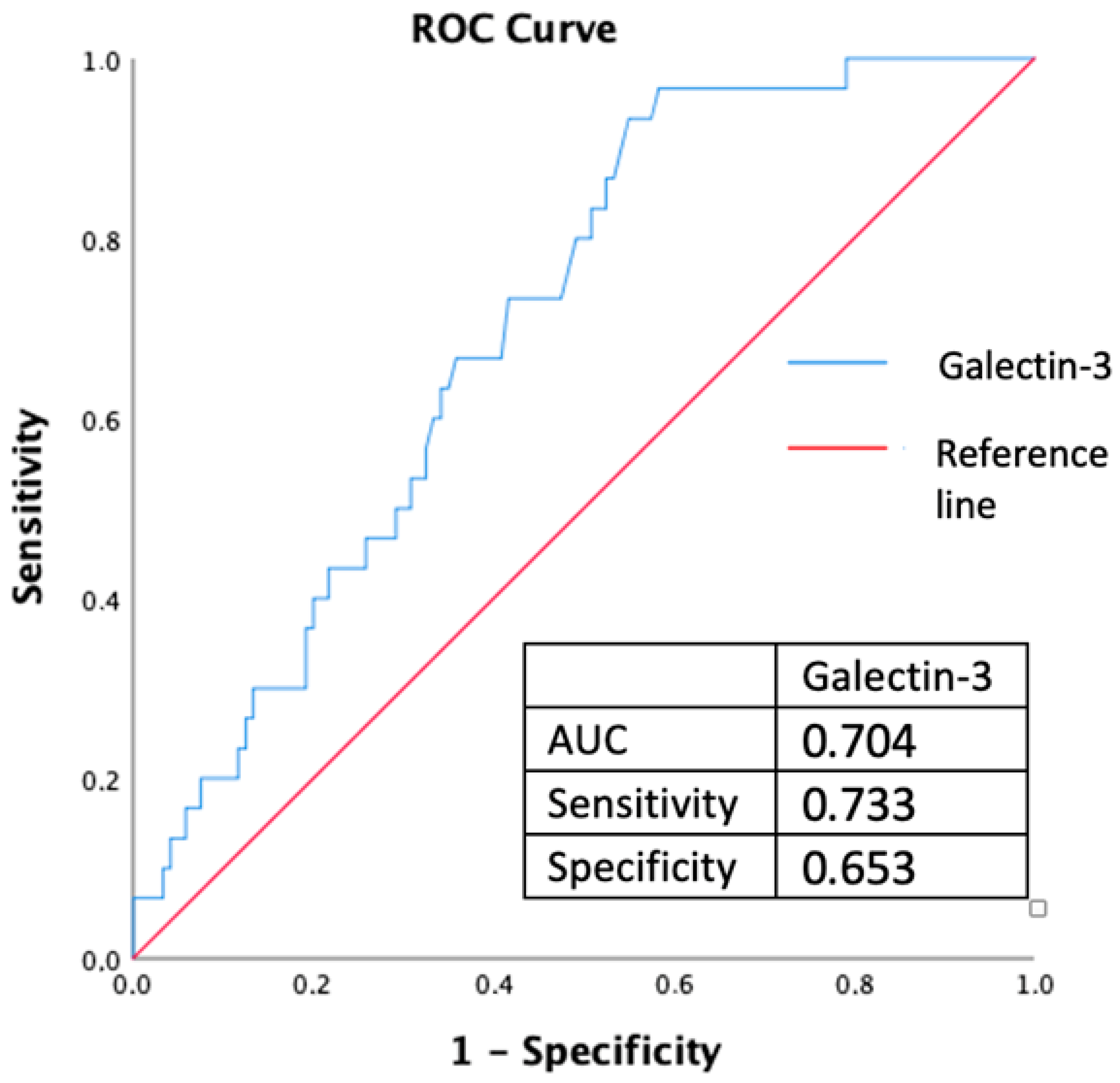
| Serum galectin-3 levels (pg/mL) | 1.001 | 1.000–1.003 | 0.010 | 1.003 | 1.000–1.006 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalapkorur, B.; Demirci, E.; Baran, O.; Ulusoy, E.K.; Koçer, D.; Demirelli, S.; Gök, M.; Şimşek, Z. The Role of Galectin-3 Levels for Predicting Paroxysmal Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source. J. Clin. Med. 2024, 13, 3175. https://doi.org/10.3390/jcm13113175
Çalapkorur B, Demirci E, Baran O, Ulusoy EK, Koçer D, Demirelli S, Gök M, Şimşek Z. The Role of Galectin-3 Levels for Predicting Paroxysmal Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source. Journal of Clinical Medicine. 2024; 13(11):3175. https://doi.org/10.3390/jcm13113175
Chicago/Turabian StyleÇalapkorur, Bekir, Erkan Demirci, Oğuzhan Baran, Ersin Kasım Ulusoy, Derya Koçer, Selami Demirelli, Mustafa Gök, and Ziya Şimşek. 2024. "The Role of Galectin-3 Levels for Predicting Paroxysmal Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source" Journal of Clinical Medicine 13, no. 11: 3175. https://doi.org/10.3390/jcm13113175
APA StyleÇalapkorur, B., Demirci, E., Baran, O., Ulusoy, E. K., Koçer, D., Demirelli, S., Gök, M., & Şimşek, Z. (2024). The Role of Galectin-3 Levels for Predicting Paroxysmal Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source. Journal of Clinical Medicine, 13(11), 3175. https://doi.org/10.3390/jcm13113175





