The Role of TOMM40 in Cardiovascular Mortality and Conduction Disorders: An Observational Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Genetic Analysis
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| Cx | connexin |
| ICD | implantable cardiac defibrillator |
| LBBB | left bundle branch block |
| LVEF | left ventricle ejection fraction |
| MACE | major adverse clinical event |
| MAF | minor allele frequency |
| PM | pacemaker |
| RBBB | right bundle branch block |
| SNP | single nucleotide polymorphism |
| TOMM40 | translocase of outer mitochondrial membrane 40 |
References
- Johnson, S.C.; La Rue, A.; Hermann, B.P.; Xu, G.; Koscik, R.L.; Jonaitis, E.M.; Bendlin, B.B.; Hogan, K.J.; Roses, A.D.; Saunders, A.M.; et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE ε3/ε3 genotype. Alzheimers Dement. 2011, 7, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.W.; Crenshaw, D.G.; Saunders, A.M.; Roses, A.D. Genetic variation at a single locus and age of onset for Alzheimer's disease. Alzheimers Dement. 2010, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Aulchenko, Y.S.; Ripatti, S.; Lindqvist, I.; Boomsma, D.; Heid, I.M.; Pramstaller, P.P.; Penninx, B.W.J.H.; Janssens, A.C.J.W.; Wilson, J.F.; Spector, S.T.; et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 2009, 41, 47–55. [Google Scholar]
- Middelberg, R.P.; Ferreira, M.A.; Henders, A.K.; Heath, A.C.; Madden, P.A.; Montgomery, G.W.; Martin, N.G.; Whitfield, J.B. Genetic variants in LPL, OASL and TOMM40/APOE-C1-C2-C4 genes are associated with multiple cardiovascular-related traits. BMC Med. Genet. 2011, 12, 123. [Google Scholar] [CrossRef]
- Sandhu, M.S.; Waterworth, D.M.; Debenham, S.L.; Wheeler, E.; Papadakis, K.; Zhao, J.H.; Song, K.; Yuan, X.; Johnson, T.; Ashford, S.; et al. LDL-cholesterol concentrations: A genome-wide association study. Lancet 2008, 371, 483–491. [Google Scholar] [CrossRef]
- Zhang, Z.; Tao, L.; Chen, Z.; Zhou, D.; Kan, M.; Zhang, D.; Li, C.; He, L.; Liu, Y. Association of Genetic Loci with Blood Lipids in the Chinese Population. PLOS ONE 2011, 6, e27305. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lanktree, M.B.; Taylor, K.C.; Hakonarson, H.; Lange, L.A.; Keating, B.J. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Hum. Mol. Genet. 2013, 22, 184–201. [Google Scholar] [CrossRef]
- Salakhov, R.R.; Goncharovaa, I.A.; Makeeva, O.A.; Golubenko, M.V.; Kulish, E.V.; Kashtalap, V.V.; Barbarash, O.L.; Puzyrev, V.P. [TOMM40 gene polymorphism association with lipid profile]. Genetika 2014, 50, 222–229. [Google Scholar] [CrossRef]
- Chen, S.; Sarasua, S.M.; Davis, N.J.; DeLuca, J.M.; Boccuto, L.; Thielke, S.M.; Yu, C.E. TOMM40 genetic variants associated with healthy aging and longevity: A systematic review. BMC Geriatr. 2022, 22, 667. [Google Scholar] [CrossRef]
- Cruchaga, C.; Nowotny, P.; Kauwe, J.S.; Ridge, P.G.; Mayo, K.; Bertelsen, S.; Hinrichs, A.; Fagan, A.M.; Holtzman, D.M.; Morris, J.C.; et al. Association and expression analyses with single-nucleotide polymorphisms in TOMM40 in Alzheimer disease. Arch. Neurol. 2011, 68, 1013–1019. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Park, C.B.; Larsson, N.G. Mitochondrial DNA mutations in disease and aging. J. Cell Biol. 2011, 193, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Ruggiero, F.M.; Petrosillo, G.; Quagliariello, E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: Role of cardiolipin. FEBS Lett. 1997, 406, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Voos, W. Chaperone-protease networks in mitochondrial protein homeostasis. Biochim. Biophys. Acta 2013, 1833, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Langer, T.; López-Otín, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 2015, 16, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Anderson, A.J.; Stojanovski, D. Mitochondrial protein import dysfunction: Mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 2021, 595, 1107–1131. [Google Scholar] [CrossRef] [PubMed]
- Chacinska, A.; Koehler, C.M.; Milenkovic, D.; Lithgow, T.; Pfanner, N. Importing mitochondrial proteins: Machineries and mechanisms. Cell 2009, 138, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Baker, M.J.; Liem, M.; Louber, J.; McKenzie, M.; Atukorala, I.; Ang, C.S.; Keerthikumar, S.; Mathivanan, S.; Stojanovski, D. Tim29 is a novel subunit of the human TIM22 translocase and is involved in complex assembly and stability. Elife 2016, 5, e17463. [Google Scholar] [CrossRef]
- Pitt, A.S.; Buchanan, S.K. A Biochemical and Structural Understanding of TOM Complex Interactions and Implications for Human Health and Disease. Cells 2021, 10, 1164. [Google Scholar] [CrossRef]
- Guan, Z.; Yan, L.; Wang, Q.; Qi, L.; Hong, S.; Gong, Z.; Yan, C.; Yin, P. Structural insights into assembly of human mitochondrial translocase TOM complex. Cell Discov. 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.C.; Saenz, A.J.; Bornstein, P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J. Cell Biochem. 1999, 74, 11–22. [Google Scholar] [CrossRef]
- Taylor, R.D.; McHale, B.J.; Nargang, F.E. Characterization of Neurospora crassa Tom40-deficient mutants and effect of specific mutations on Tom40 assembly. J. Biol. Chem. 2003, 278, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, X.; Zhang, L.; Yi, J.; Ma, Q.; Yin, J.; Zhuo, W.; Gu, J.; Yang, M. Atomic structure of human TOM core complex. Cell Discov. 2020, 6, 67. [Google Scholar] [CrossRef]
- Bogorodskiy, A.; Okhrimenko, I.; Burkatovskii, D.; Jakobs, P.; Maslov, I.; Gordeliy, V.; Dencher, N.A.; Gensch, T.; Voos, W.; Altschmied, J.; et al. Role of Mitochondrial Protein Import in Age-Related Neurodegenerative and Cardiovascular Diseases. Cells 2021, 10, 3528. [Google Scholar] [CrossRef] [PubMed]
- Puschmann, A. Monogenic Parkinson’s disease and parkinsonism: Clinical phenotypes and frequencies of known mutations. Parkinsonism Relat. Disord. 2013, 19, 407–415. [Google Scholar] [CrossRef]
- Kasten, M.; Hartmann, C.; Hampf, J.; Schaake, S.; Westenberger, A.; Vollstedt, E.J.; Balck, A.; Domingo, A.; Vulinovic, F.; Dulovic, M.; et al. Genotype-Phenotype Relations for the Parkinson’s Disease Genes Parkin, PINK1, DJ1: MDSGene Systematic Review. Mov. Disord. 2018, 33, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Barrett, P.J.; Hoffman, E.K.; Barrett, C.W.; Zharikov, A.; Borah, A.; Hu, X.; McCoy, J.; Chu, C.T.; Burton, E.A.; et al. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016, 8, 342ra378. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Cenini, G.; Rüb, C.; Bruderek, M.; Voos, W. Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol. Biol. Cell 2016, 27, 3257–3272. [Google Scholar] [CrossRef]
- Hansson Petersen, C.A.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.F.; Alafuzoff, I.; Leinonen, V.; Ito, A.; Winblad, B.; Glaser, E.; et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2008, 105, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Heinemeyer, T.; Stemmet, M.; Bardien, S.; Neethling, A. Underappreciated Roles of the Translocase of the Outer and Inner Mitochondrial Membrane Protein Complexes in Human Disease. DNA Cell Biol. 2019, 38, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Gres, P.; Cabestrero, A.; Ruiz-Meana, M.; Garcia-Dorado, D.; Heusch, G.; Schulz, R. Prevention of the ischemia-induced decrease in mitochondrial Tom20 content by ischemic preconditioning. J. Mol. Cell Cardiol. 2006, 41, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, H.; Shiota, T.; Ishizaka, N.; Kawano, S.; Tamura, Y.; Tan, K.S.; Imai, K.; Motono, C.; Hirokawa, T.; Taki, K.; et al. Porin Associates with Tom22 to Regulate the Mitochondrial Protein Gate Assembly. Mol. Cell 2019, 73, 1044–1055.e1048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Zhang, Z.; Zhu, R.; Olcese, R.; Stefani, E.; Toro, L. The mitochondrial BK(Ca) channel cardiac interactome reveals BK(Ca) association with the mitochondrial import receptor subunit Tom22, and the adenine nucleotide translocator. Mitochondrion 2017, 33, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Deelen, J.; Beekman, M.; Uh, H.W.; Broer, L.; Ayers, K.L.; Tan, Q.; Kamatani, Y.; Bennet, A.M.; Tamm, R.; Trompet, S.; et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum. Mol. Genet. 2014, 23, 4420–4432. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.I.; Arbeev, K.G.; Wu, D.; Arbeeva, L.S.; Bagley, O.; Stallard, E.; Kulminski, A.M.; Akushevich, I.; Fang, F.; Wojczynski, M.K.; et al. Genetics of Human Longevity From Incomplete Data: New Findings From the Long Life Family Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Shadyab, A.H.; Kooperberg, C.; Reiner, A.P.; Jain, S.; Manson, J.E.; Hohensee, C.; Macera, C.A.; Shaffer, R.A.; Gallo, L.C.; LaCroix, A.Z. Replication of Genome-Wide Association Study Findings of Longevity in White, African American, and Hispanic Women: The Women’s Health Initiative. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, Y.; Yan, D.; Liao, X.; Gong, G.; Hu, J.; Fu, Y.; Cai, W. Association of common variants in TOMM40/APOE/APOC1 region with human longevity in a Chinese population. J. Hum. Genet. 2016, 61, 323–328. [Google Scholar] [CrossRef]
- Lu, F.; Guan, H.; Gong, B.; Liu, X.; Zhu, R.; Wang, Y.; Qian, J.; Zhou, T.; Lan, X.; Wang, P.; et al. Genetic variants in PVRL2-TOMM40-APOE region are associated with human longevity in a Han Chinese population. PLoS ONE 2014, 9, e99580. [Google Scholar] [CrossRef]
- Arpawong, T.E.; Pendleton, N.; Mekli, K.; McArdle, J.J.; Gatz, M.; Armoskus, C.; Knowles, J.A.; Prescott, C.A. Genetic variants specific to aging-related verbal memory: Insights from GWASs in a population-based cohort. PLoS ONE 2017, 12, e0182448. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pappas, C.; Le, S.T.; Wang, Q.; Klinedinst, B.S.; Larsen, B.A.; Pollpeter, A.; Lee, L.Y.; Lutz, M.W.; Gottschalk, W.K.; et al. APOE, TOMM40, and sex interactions on neural network connectivity. Neurobiol. Aging 2022, 109, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Loika, Y.; Culminskaya, I.; Huang, J.; Arbeev, K.G.; Bagley, O.; Feitosa, M.F.; Zmuda, J.M.; Christensen, K.; Yashin, A.I. Independent associations of TOMM40 and APOE variants with body mass index. Aging Cell 2019, 18, e12869. [Google Scholar] [CrossRef]
- Lamparello, A.J.; Namas, R.A.; Schimunek, L.; Cohen, M.; El-Dehaibi, F.; Yin, J.; Barclay, D.; Zamora, R.; Billiar, T.R.; Vodovotz, Y. An Aging-Related Single-Nucleotide Polymorphism is Associated With Altered Clinical Outcomes and Distinct Inflammatory Profiles in Aged Blunt Trauma Patients. Shock 2020, 53, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.; Qiu, C.; Shao, Q.; Li, J. Associations of Vascular Risk Factors, APOE and TOMM40 Polymorphisms With Cognitive Function in Dementia-Free Chinese Older Adults: A Community-Based Study. Front. Psychiatry 2021, 12, 617773. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Drenos, F.; Shah, S.; Shah, T.; Palmen, J.; Verzilli, C.; Gaunt, T.R.; Pallas, J.; Lovering, R.; Li, K.; et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J. Hum. Genet. 2009, 85, 628–642. [Google Scholar] [CrossRef]
- Clark, D.; Skrobot, O.A.; Adebiyi, I.; Susce, M.T.; de Leon, J.; Blakemore, A.F.; Arranz, M.J. Apolipoprotein-E gene variants associated with cardiovascular risk factors in antipsychotic recipients. Eur. Psychiatry 2009, 24, 456–463. [Google Scholar] [CrossRef]
- Weidmann, S. The electrical constants of Purkinje fibres. J. Physiol. 1952, 118, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Baruteau, A.E.; Probst, V.; Abriel, H. Inherited progressive cardiac conduction disorders. Curr. Opin. Cardiol. 2015, 30, 33–39. [Google Scholar] [CrossRef]
- Kléber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef]
- Michela, P.; Velia, V.; Aldo, P.; Ada, P. Role of connexin 43 in cardiovascular diseases. Eur. J. Pharmacol. 2015, 768, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Del Ry, S.; Moscato, S.; Bianchi, F.; Morales, M.A.; Dolfi, A.; Burchielli, S.; Cabiati, M.; Mattii, L. Altered expression of connexin 43 and related molecular partners in a pig model of left ventricular dysfunction with and without dipyrydamole therapy. Pharmacol. Res. 2015, 95–96, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.K.; Nishida, K.; Kato, T.; Nattel, S. Atrial fibrillation pathophysiology: Implications for management. Circulation 2011, 124, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Makita, N.; Seki, A.; Sumitomo, N.; Chkourko, H.; Fukuhara, S.; Watanabe, H.; Shimizu, W.; Bezzina, C.R.; Hasdemir, C.; Mugishima, H.; et al. A connexin40 mutation associated with a malignant variant of progressive familial heart block type I. Circ. Arrhythm. Electrophysiol. 2012, 5, 163–172. [Google Scholar] [CrossRef]
- Lampe, P.D.; Lau, A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004, 36, 1171–1186. [Google Scholar] [CrossRef]
- Rohr, S.; Kucera, J.P.; Kléber, A.G. Slow conduction in cardiac tissue, I: Effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ. Res. 1998, 83, 781–794. [Google Scholar] [CrossRef]
- Cole, W.C.; Picone, J.B.; Sperelakis, N. Gap junction uncoupling and discontinuous propagation in the heart. A comparison of experimental data with computer simulations. Biophys. J. 1988, 53, 809–818. [Google Scholar] [CrossRef]
- Boengler, K.; Schulz, R.; Heusch, G. Connexin 43 signalling and cardioprotection. Heart 2006, 92, 1724–1727. [Google Scholar] [CrossRef]
- van Rijen, H.V.; Eckardt, D.; Degen, J.; Theis, M.; Ott, T.; Willecke, K.; Jongsma, H.J.; Opthof, T.; de Bakker, J.M. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004, 109, 1048–1055. [Google Scholar] [CrossRef]
- Agullo-Pascual, E.; Cerrone, M.; Delmar, M. Arrhythmogenic cardiomyopathy and Brugada syndrome: Diseases of the connexome. FEBS Lett. 2014, 588, 1322–1330. [Google Scholar] [CrossRef]
| All Patients | TOMM40 Genotypes | |||
|---|---|---|---|---|
| AA | Xg | p Value | ||
| Number of subjects | 276 | 227 | 49 | |
| Age (y) | 70.16 ± 7.96 | 69.98 ± 7.80 | 71.0 ± 8.72 | 0.42 |
| Gender (male/female) | 209/67 | 176/33 | 51/16 | 0.13 |
| BMI (kg/m2) | 28.42 ± 3.95 | 28.55 ± 3.97 | 27.79 ± 3.87 | 0.22 |
| Waist circumference (cm) | 100.35 ± 10.25 | 100.89 ± 10.0 | 97.75 ± 11.12 | 0.064 |
| Waist-hip ratio | 0.96 ± 0.07 | 0.97 ± 0.07 | 0.95 ± 0.08 | 0.13 |
| Systolic blood pressure (mmHg) | 132.95 ± 17.22 | 133.04 ± 17.62 | 132.50 ± 15.36 | 0.87 |
| Diastolic blood pressure (mmHg) | 79.25 ± 6.64 | 79.39 ± 6.44 | 78.53 ± 7.62 | 0.49 |
| Pulse pressure (mmHg) | 53.71 ± 14.96 | 53.65 ± 15.56 | 53.97 ± 11.78 | 0.95 |
| Fasting glucose (mg/dL) | 117.22 ± 37.88 | 117.49 ± 36.79 | 116.00 ± 42.87 | 0.80 |
| HOMA-IR | 4.74 ± 7.17 | 4.81 ± 7.45 | 4.39 ± 5.75 | 0.66 |
| Triglycerides (mg/dL) | 121.36 ± 57.15 | 122.27 ± 58.86 | 117.21 ± 58.89 | 0.79 |
| Total Ch (mg/dL) | 166.51 ± 41.42 | 167.29 ± 42.26 | 162.92 ± 37.54 | 0.51 |
| HDL-Ch (mg/dL) | 49.35 ± 12.38 | 49.31 ± 12.56 | 49.52 ± 12.62 | 0.91 |
| LDL-Ch (mg/dL) | 93.91 ± 35.69 | 94.73 ± 36.35 | 90.16 ± 32.58 | 0.42 |
| Serum creatine (mg/dL) | 1.03 ± 0.58 | 1.03 ± 0.62 | 1.01 ± 0.33 | 0.87 |
| Microalbuminuria (µg/min) | 55.30 ± 127.30 | 58.72 ± 127.24 | 39.18 ± 127.83 | 0.10 |
| Sinus rhythm (n, %) | 262 (94.9%) | 215 (94.7%) | 47 (95.9%) | 0.73 |
| Right bundle branch block (n, %) | 15 (5.4%) | 10 (4.4%) | 5 (10.2%) | 0.10 |
| Left bundle branch block (n, %) | 12 (4.3%) | 7 (3.1%) | 5 (10.2%) | 0.027 |
| Implantation age of cardiac device (y) | 70.96 ± 11.30 | 72.14 ± 11.11 | 58.50 ± 0.71 | 0.10 |
| PR interval (ms) | 162.74 ± 27.42 | 163.26 ± 28.44 | 160.32 ± 22.21 | 0.55 |
| QRS interval (ms) | 100.16 ± 21.20 | 100.05 ± 28.44 | 100.68 ± 21.78 | 0.87 |
| QTc interval (ms) | 415.58 ± 23.54 | 414.58 ± 23.75 | 414.58 ± 22.78 | 1.00 |
| Aortic bulb diameter (mm) | 33.07 ± 4.24 | 33.0 ± 4.13 | 33.34 ± 4.75 | 0.62 |
| Left atrium diameter (mm) | 35.52 ± 5.72 | 35.6 ± 5.74 | 35.19 ± 5.65 | 0.66 |
| End-diastolic left ventricle (mm) | 46.01 ± 6.25 | 45.96 ± 6.34 | 46.27 ± 5.86 | 0.75 |
| Interventricular septum (mm) | 11.76 ± 1.24 | 11.76 ± 1.26 | 11.75 ± 1.14 | 0.97 |
| Posterior wall (mm) | 11.13 ± 1.04 | 11.71 ± 1.03 | 11.11 ± 1.08 | 0.87 |
| Heart rate (bpm) | 70.0 ± 10.67 | 70.41 ± 10.52 | 68.27 ± 11.20 | 0.21 |
| LVEF (%) | 58.75 ± 5.61 | 58.64 ± 5.47 | 59.23 ± 6.23 | 0.51 |
| LVMI (gr/m2) | 76.29 ± 20.22 | 76.39 ± 20.71 | 75.84 ± 17.96 | 0.88 |
| All Patients | TOMM40 Genotypes | ||||||
|---|---|---|---|---|---|---|---|
| AA | Xg | p Value | |||||
| Comorbidities | |||||||
| Hypertension | 239 | (86.6%) | 197 | (86.8%) | 42 | (85.7%) | 0.84 |
| Dyslipidemia | 220 | (80.9%) | 180 | (80.7%) | 40 | (81.6%) | 0.88 |
| Type 2 diabetes | 122 | (44.2%) | 103 | (45.4%) | 19 | (38.8%) | 0.40 |
| Smoking | 59 | (21.4%) | 49 | (21.6%) | 10 | (20.4%) | 0.88 |
| Cardiac device implantation | 23 | (8.7%) | 21 | (9.6%) | 2 | (4.4%) | 0.27 |
| Ischemic heart disease | 102 | (37.9%) | 86 | (37.9%) | 16 | (32.7%) | 0.49 |
| Stroke | 40 | (14.5%) | 31 | (13.7%) | 9 | (18.4%) | 0.39 |
| Cancer | 40 | (14.5%) | 35 | (15.4%) | 5 | (10.2%) | 0.35 |
| Medical treatments | |||||||
| ARBs | 105 | (38.0%) | 86 | (37.9%) | 19 | (38.8%) | 0.91 |
| ACE inhibitors | 103 | (37.3%) | 86 | (37.9%) | 17 | (34.7%) | 0.67 |
| Calcium channel blockers | 72 | (26.1%) | 58 | (25.6%) | 14 | (28.6%) | 0.66 |
| β-blockers | 77 | (26.4%) | 60 | (26.4%) | 17 | (27.9%) | 0.26 |
| Diuretics | 120 | (43.5%) | 100 | (44.1%) | 20 | (40.8%) | 0.68 |
| Antiplatelet | 246 | (89.1%) | 205 | (90.3%) | 41 | (83.7%) | 0.18 |
| Lipid-lowering drugs | 2242 | (87.7%) | 197 | (86.8%) | 45 | (91.8%) | 0.33 |
| Antidiabetic therapy | 90 | (32.6%) | 75 | (33.0%) | 15 | (30.6%) | 0.74 |
| All Patients | TOMM40 Genotypes | ||||
|---|---|---|---|---|---|
| AA | Xg | HR (95% CI) | p Value | ||
| Cardiovascular events | |||||
| MACE | 65 (23.6%) | 54 (23.8%) | 11 (22.4%) | 0.94 (0.493–1.802) | 0.86 |
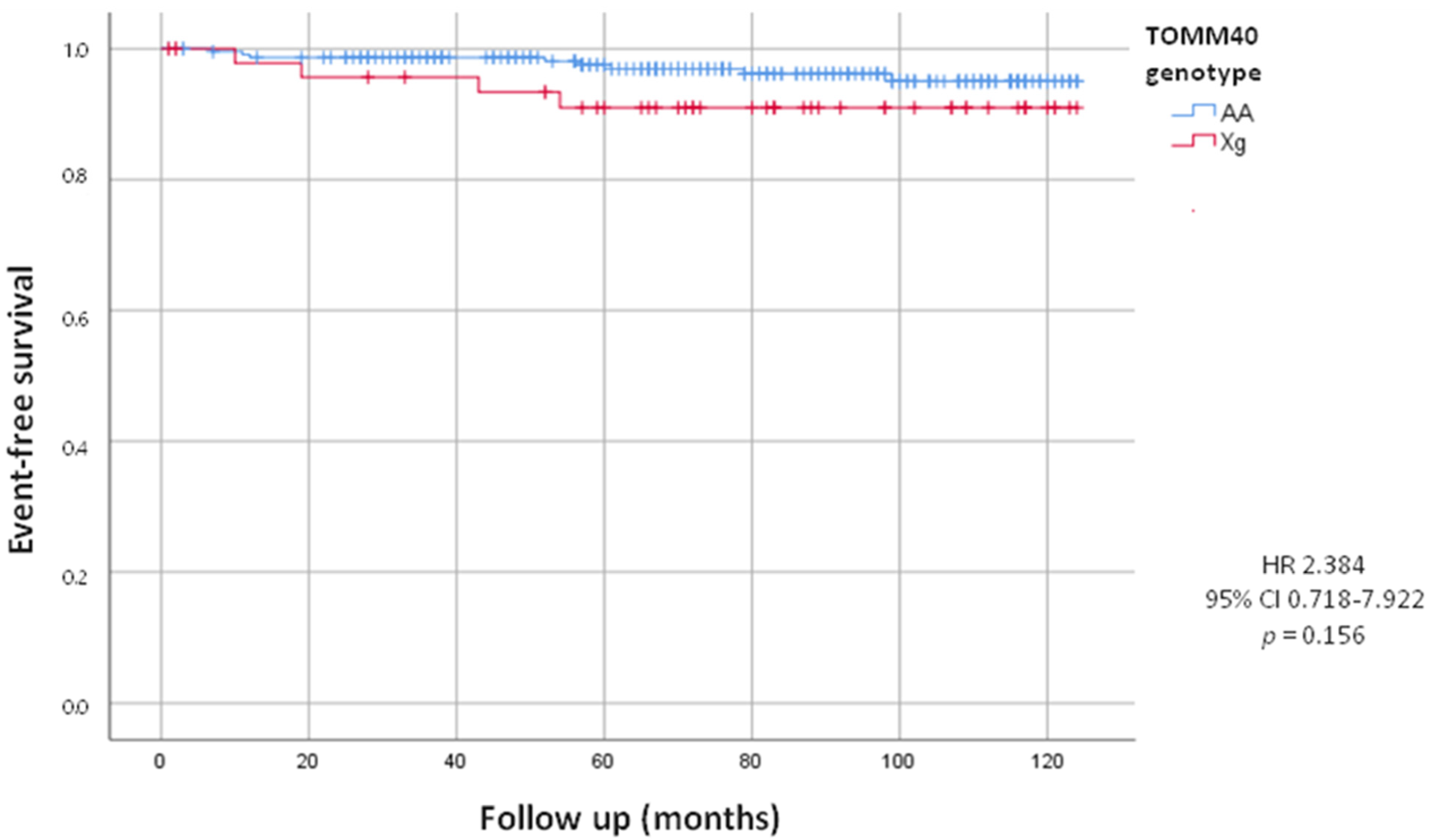
| Cardiac device implantation | 12 (4.3%) | 8 (3.5%) | 4 (8.2%) | 2.384 (0.718–7.922) | 0.156 |
| Fatal events | |||||
| Total death | 52 (18.8%) | 44 (19.4%) | 8 (16.3%) | 0.84 (0.395–1.784) | 0.62 |
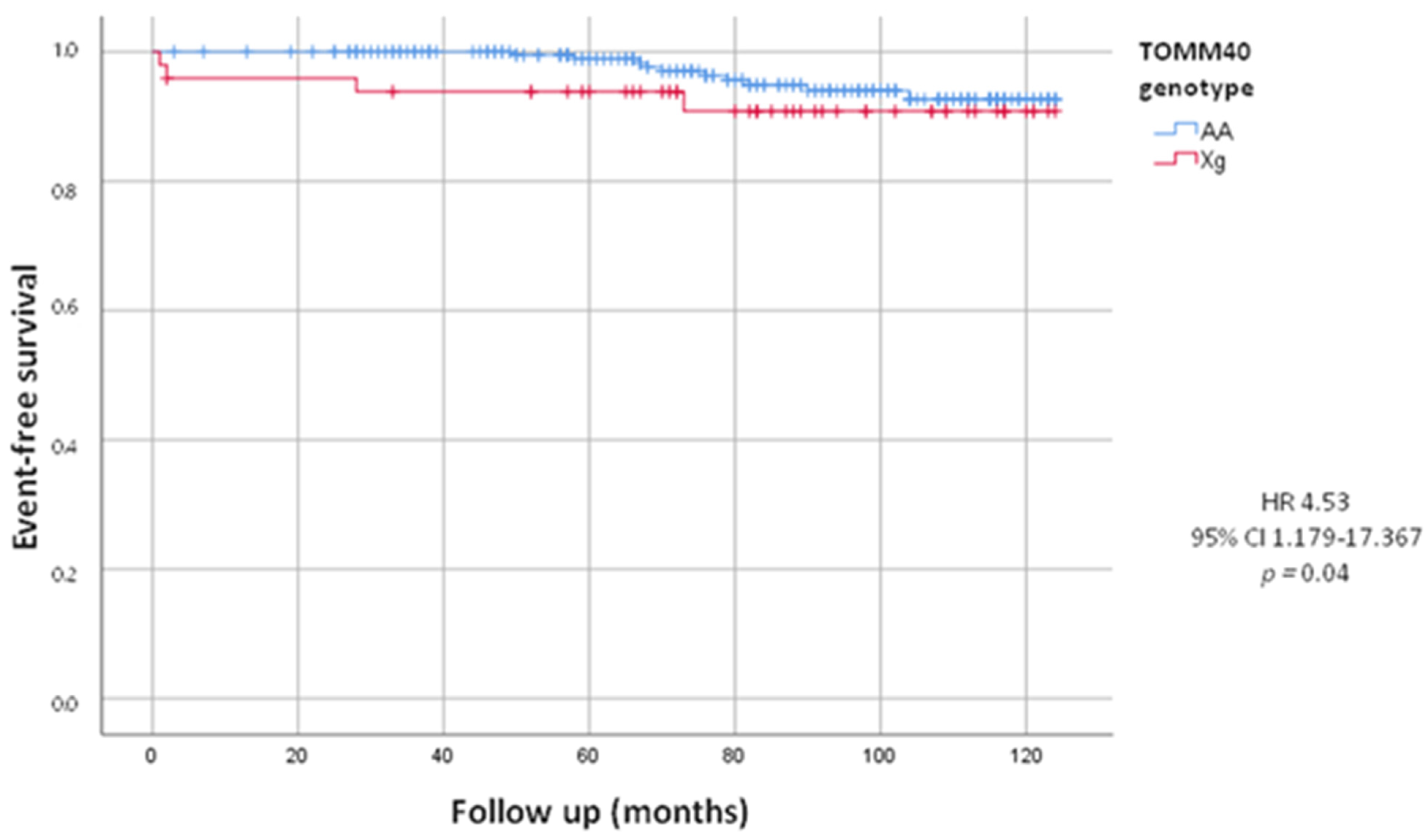
| Cardiovascular death | 14 (5.1%) | 10 (4.4%) | 4 (8.2%) | 4.53 (1.179–17.367) | 0.04 |
| Cancer death | 17 (6.2%) | 15 (6.6%) | 2 (4.1%) | 1.79 (0.387–8.265) | 0.46 |
| All Patients | TOMM40 Genotypes | |||
|---|---|---|---|---|
| AA | Xg | p Value | ||
| T0 (time of enrollment) | Number of implants | 21 (9.6) | 2 (4.4%) | 0.27 |
| Age of implantation | 72.14 ± 11.11 | 58.50 ± 0.71 | 0.10 | |
| During follow-up | Number of implants | 8 (3.5%) | 4 (8.2%) | 0.156 |
| Age of implantation | 75.63 ± 8.35 | 69.50 ± 2.89 | 0.074 | |
| Total | Number of implants | 29 (12.8) | 6 (12.2) | 0.919 |
| Age of implantation | 73.10 ± 10.39 | 65.83 ± 6.11 | 0.049 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stolfo, G.; Mastroianno, S.; Soldato, N.; Massaro, R.S.; De Luca, G.; Seripa, D.; Urbano, M.; Gravina, C.; Greco, A.; Siena, P.; et al. The Role of TOMM40 in Cardiovascular Mortality and Conduction Disorders: An Observational Study. J. Clin. Med. 2024, 13, 3177. https://doi.org/10.3390/jcm13113177
Di Stolfo G, Mastroianno S, Soldato N, Massaro RS, De Luca G, Seripa D, Urbano M, Gravina C, Greco A, Siena P, et al. The Role of TOMM40 in Cardiovascular Mortality and Conduction Disorders: An Observational Study. Journal of Clinical Medicine. 2024; 13(11):3177. https://doi.org/10.3390/jcm13113177
Chicago/Turabian StyleDi Stolfo, Giuseppe, Sandra Mastroianno, Nicolò Soldato, Raimondo Salvatore Massaro, Giovanni De Luca, Davide Seripa, Maria Urbano, Carolina Gravina, Antonio Greco, Paola Siena, and et al. 2024. "The Role of TOMM40 in Cardiovascular Mortality and Conduction Disorders: An Observational Study" Journal of Clinical Medicine 13, no. 11: 3177. https://doi.org/10.3390/jcm13113177






