Efficacy of Continuous Lumbar Plexus Blockade in Managing Post-Operative Pain after Hip or Femur Orthopedic Surgeries: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search and Identification of Studies
2.3. Study Selection
2.4. Data Extraction
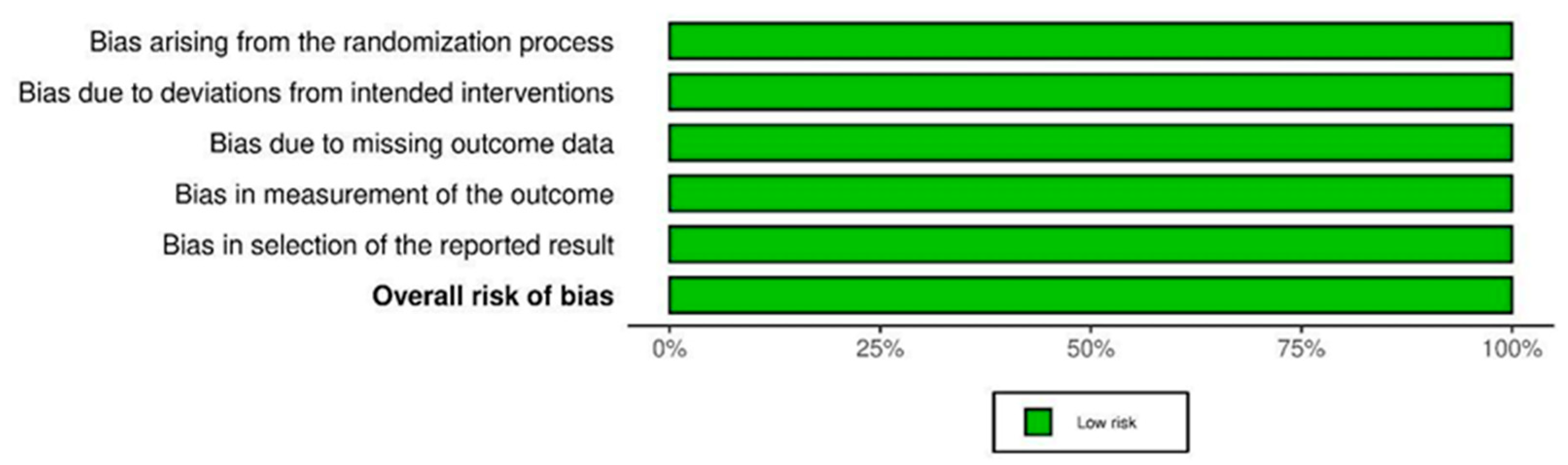
2.5. Methodological Quality Assessment
2.6. Data Analysis
3. Results
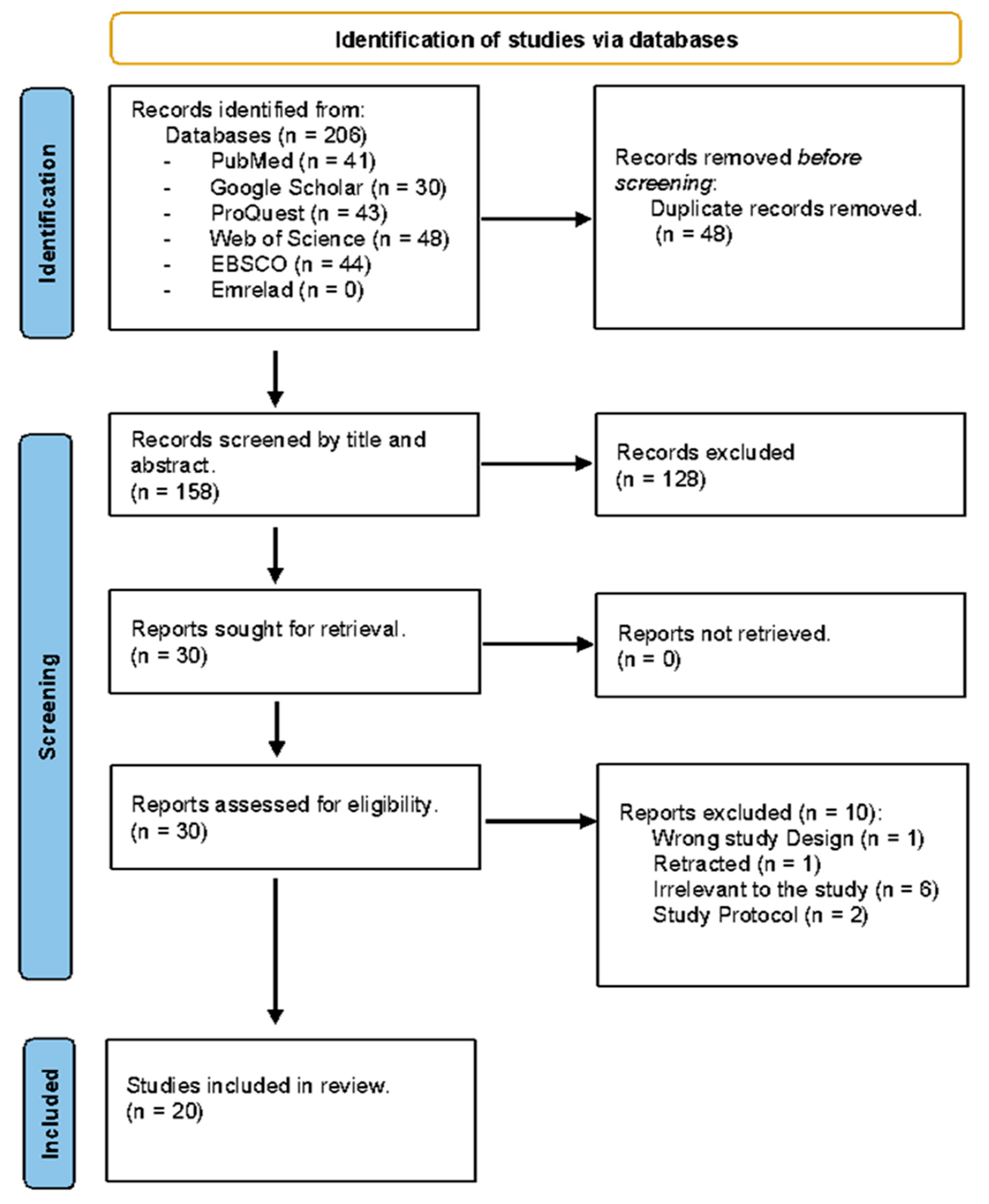
3.1. Study Selection
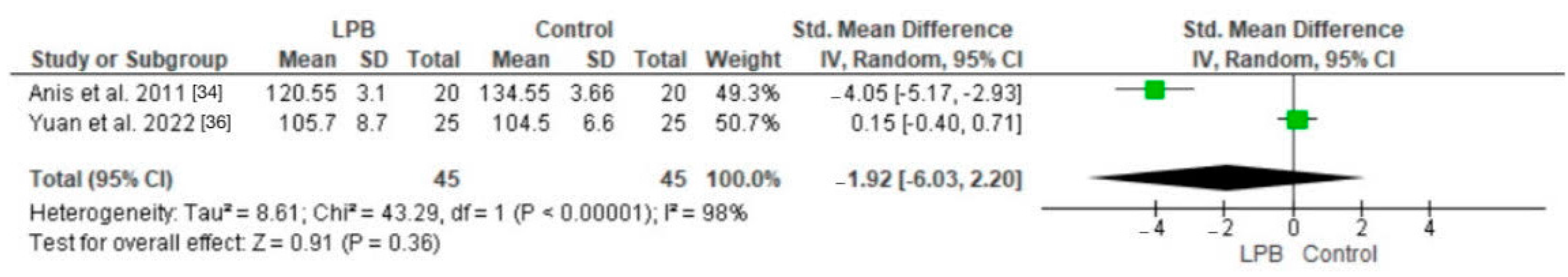
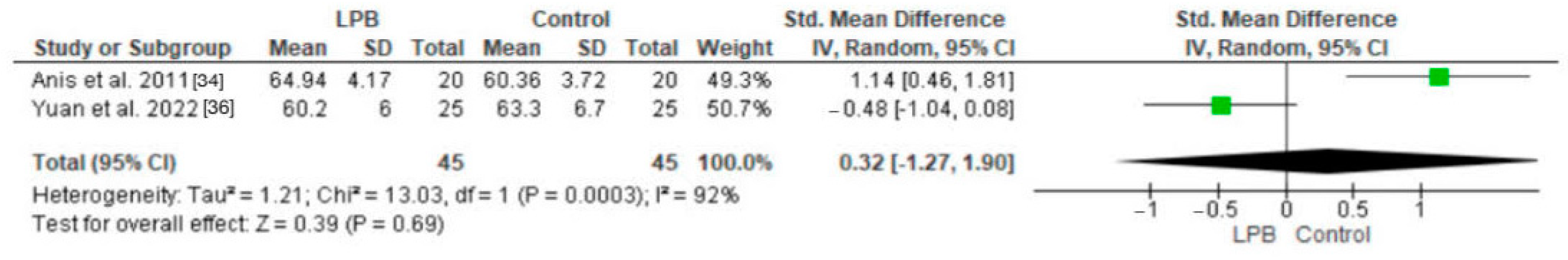
3.2. Pain Scores
3.3. Opioid Use
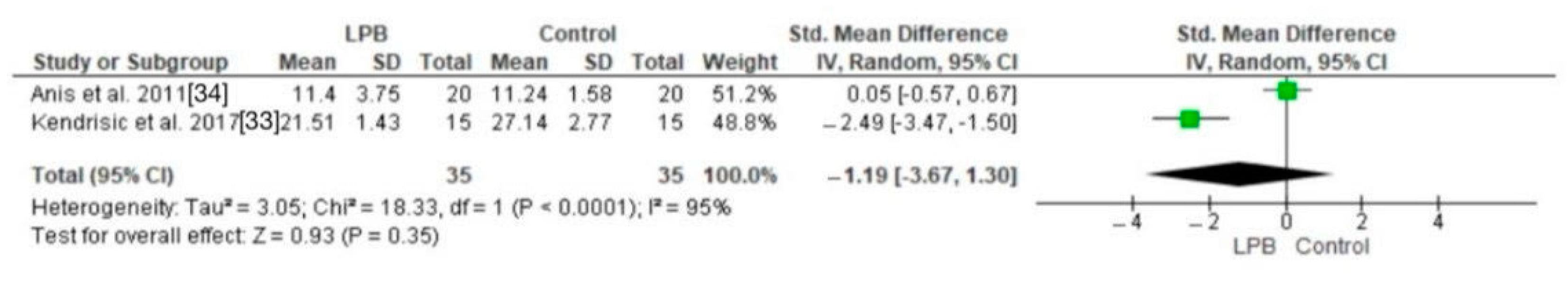
3.4. Cortisol Levels
3.5. Adverse Effects
3.6. Patient Satisfaction
3.7. Heart Rate Parameters and Blood Pressure
3.8. Quality of Recovery
3.9. Length of Hospitalization
4. Discussion
5. Clinical Implications
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Khalil, H.; Shajrawi, A.; Henker, R. Predictors of severe postoperative pain after orthopedic surgery in the immediate postoperative period. Int. J. Orthop. Trauma Nurs. 2021, 43, 100864. [Google Scholar] [CrossRef]
- Trasolini, N.A.; McKnight, B.M.; Dorr, L.D. The Opioid Crisis and the Orthopedic Surgeon. J. Arthroplast. 2018, 33, 3379–3382.e1. [Google Scholar] [CrossRef]
- Amiri, H.R.; Safari, S.; Makarem, J.; Rahimi, M.; Jahanshahi, B. Comparison of Combined Femoral Nerve Block and Spinal Anesthesia with Lumbar Plexus Block for Postoperative Analgesia in Intertrochanteric Fracture Surgery. Anesthesiol. Pain Med. 2012, 2, 32–35. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Farahini, H.; Faiz, S.H.-R.; Mokarami, F.; Safari, S. Pain Management for Total Knee Arthroplasty: Single-Injection Femoral Nerve Block versus Local Infiltration Analgesia. Iran. Red Crescent Med. J. 2014, 16, e13247. [Google Scholar] [CrossRef]
- Winnie, A.P.; Ramamurthy, S.; Durrani, Z. The inguinal paravascular technic of lumbar plexus anesthesia: The “3-in-1 block”. Anesth. Analg. 1973, 52, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ekstein, M.P.; Weinbroum, A.A. Immediate Postoperative Pain in Orthopedic Patients Is More Intense and Requires More Analgesia than in Post-Laparotomy Patients. Pain Med. 2011, 12, 308–313. [Google Scholar] [CrossRef]
- Morrison, S.R.; Magaziner, J.; McLaughlin, M.A.; Orosz, G.; Silberzweig, S.B.; Koval, K.J.; Siu, A.L. The impact of post-operative pain on outcomes following hip fracture. Pain 2003, 103, 303–311. [Google Scholar] [CrossRef]
- Bano, G.; Dianin, M.; Biz, C.; Bedogni, M.; Alessi, A.; Bordignon, A.; Bizzotto, M.; Berizzi, A.; Ruggieri, P.; Manzato, E.; et al. Efficacy of an interdisciplinary pathway in a first level trauma center orthopaedic unit: A prospective study of a cohort of elderly patients with hip fractures. Arch. Gerontol. Geriatr. 2020, 86, 103957. [Google Scholar] [CrossRef] [PubMed]
- Amiri, H.R.; Zamani, M.M.; Safari, S. Lumbar Plexus Block for Management of Hip Surgeries. Anesthesiol. Pain Med. 2014, 4, e19407. [Google Scholar] [CrossRef]
- Gan, T.J. Poorly controlled postoperative pain: Prevalence, consequences, and prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Niraj, G.; Kelkar, A.; Kaushik, V.; Tang, Y.; Fleet, D.; Tait, F.; Mcmillan, T.; Rathinam, S. Audit of postoperative pain management after open thoracotomy and the incidence of chronic postthoracotomy pain in more than 500 patients at a tertiary center. J. Clin. Anesth. 2017, 36, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Naqibuddin, M.; Rowlingson, A.J.; Lietman, S.A.; Jermyn, R.M.; Fleisher, L.A. The Effect of Pain on Health-Related Quality of Life in the Immediate Postoperative Period. Anesth. Analg. 2003, 97, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Wagstaff, K.; Sanghera, S.; Kerry, R. Postoperative pain following primary lower limb arthroplasty and enhanced recovery pathway. Ann. R. Coll. Surg. Engl. 2014, 96, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Corbett, M.S.; Higgins, J.P.T.; Woolacott, N.F. Assessing baseline imbalance in randomised trials: Implications for the Cochrane risk of bias tool. Res. Synth. Methods 2014, 5, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Clarke, V.; Braun, V. Thematic analysis. J. Posit. Psychol. 2017, 12, 297–298. [Google Scholar] [CrossRef]
- Badiola, I.; Liu, J.; Huang, S.; Kelly, J.D.; Elkassabany, N. A comparison of the fascia iliaca block to the lumbar plexus block in providing analgesia following arthroscopic hip surgery: A randomized controlled clinical trial. J. Clin. Anesth. 2018, 49, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Bravo, D.; Layera, S.; Aliste, J.; Jara, A.; Fernandez, D.; Barrientos, C.; Wulf, R.; Munoz, G.; Finlayson, R.J.; Tran, D.Q. Lumbar plexus block versus suprainguinal fascia iliaca block for total hip arthroplasty: A single-blinded, randomized trial. J. Clin. Anesth. 2020, 66, 109907. [Google Scholar] [CrossRef] [PubMed]
- Goytizolo, E.A.; Stundner, O.; Rúa, S.H.; Marcello, D.; Buschiazzo, V.; Vaz, A.M.; Memtsoudis, S.G. The Effect of Regional Analgesia on Vascular Tone in Hip Arthroplasty Patients. HSS J. 2016, 12, 125–131. [Google Scholar] [CrossRef]
- Gutierrez, J.J.P.; Ben-David, B.; Rest, C.; Grajales, M.T.; Khetarpal, S.K. Quadratus lumborum block type 3 versus lumbar plexus block in hip replacement surgery: A randomized, prospective, non-inferiority study. Reg. Anesth. Pain Med. 2021, 46, 111–117. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Mariano, E.R.; Madison, S.J.; Loland, V.J.; Sandhu, N.S.; Suresh, P.J.; Bishop, M.L.; Kim, T.E.; Donohue, M.C.; Kulidjian, A.A.; et al. Continuous Femoral versus Posterior Lumbar Plexus Nerve Blocks for Analgesia after Hip Arthroplasty. Anesth. Analg. 2011, 113, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Wolla, C.D.; Wolf, B.J.; Hay, E.; Babb, S.; Wilson, S.H. Comparison of lateral quadratus lumborum and lumbar plexus blocks for postoperative analgesia following total hip arthroplasty: A randomized clinical trial. Reg. Anesth. Pain Med. 2022, 47, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Kendrisic, M.; Surbatovic, M.; Djordjevic, D.; Jevdjic, J. Surgical stress response following hip arthroplasty regarding choice of anesthesia and postoperative analgesia. Vojn. Pregl. 2017, 74, 1162–1169. [Google Scholar] [CrossRef]
- Scanaliato, J.P.; Christensen, D.; Polmear, M.M.; Salfiti, C.; Gaspar, P.S.; Wolff, A.B. Prospective Single-Blinded Randomized Controlled Trial Comparing Pericapsular Injection versus Lumbar Plexus Peripheral Nerve Block for Hip Arthroscopy. Am. J. Sports Med. 2020, 48, 2740–2746. [Google Scholar] [CrossRef]
- Siddiqui, Z.I.; Cepeda, M.S.; Denman, W.; Schumann, R.; Carr, D.B. Continuous lumbar plexus block provides improved analgesia with fewer side effects compared with systemic opioids after hip arthroplasty: A randomized controlled trial. Reg. Anesth. Pain Med. 2007, 32, 393–398. [Google Scholar] [CrossRef]
- Sharma, H.; Mitra, S.; Singh, J.; Gupta, S.; Garg, S. A Randomized Study Comparing the Efficacy of Ultrasound Guided Lumbar Plexus Block and Epidural Anesthesia for Postoperative Analgesia in Patients Undergoing Total Hip Replacement. Asian J. Anesthesiol. 2020, 58, 131–137. [Google Scholar] [PubMed]
- Stevens, R.D.; Van Gessel, E.; Flory, N.; Fournier, R.; Gamulin, Z. Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology 2000, 93, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Marino, J.; Russo, J.; Kenny, M.; Herenstein, R.; Livote, E.; E Chelly, J. Continuous Lumbar Plexus Block for Postoperative Pain Control after Total Hip Arthroplasty. J. Bone Jt. Surg. 2009, 91, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Diwan, S.; Lonikar, A.; Dongre, H.; Sancheti, P.; Panchawagh, S. Comparative study lumbar plexus block and lumbar erector spinae plane block for postoperative pain relief after proximal femoral nail for proximal femoral fractures. Saudi J. Anaesth. 2023, 17, 147–154. [Google Scholar] [CrossRef]
- Wardhan, R.; Auroux, A.-S.M.; Ben-David, B.; Chelly, J.E. Is L2 paravertebral block comparable to lumbar plexus block for postoperative analgesia after total hip arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 1475–1481. [Google Scholar] [CrossRef]
- YaDeau, J.T.; Tedore, T.; Goytizolo, E.A.; Kim, D.H.; Green, D.S.T.; Westrick, A.; Fan, R.; Rade, M.C.; Ranawat, A.S.; Coleman, S.H.; et al. Lumbar plexus blockade reduces pain after hip arthroscopy: A prospective randomized controlled trial. Anesth. Analg. 2012, 115, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Amundson, A.W.; Abdel, M.P.; Sviggum, H.P.; Mabry, T.M.; Mantilla, C.B.; Schroeder, D.R.; Pagnano, M.W.; Kopp, S.L. Continuous Posterior Lumbar Plexus Nerve Block versus Periarticular Injection with Ropivacaine or Liposomal Bupivacaine for Total Hip Arthroplasty. J. Bone Jt. Surg. 2017, 99, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Kendrisic, M.; Surbatovic, M.; Djordjevic, D.; Trifunovic, B.; Jevdjic, J. Analgesic efficacy and safety of four different anesthesia/postoperative analgesia protocols in patients following total hip arthroplasty. Vojn. Pregl. 2017, 74, 814–820. [Google Scholar] [CrossRef]
- Anis, S.; El Moaty, N.A.; Youssef, A.; Ramzy, R.; Hassan, R. Lumbar plexus block as a method of postoperative analgesia after hip surgery. Egypt. J. Anaesth. 2011, 27, 127–133. [Google Scholar] [CrossRef]
- Yang, R.; Liu, R.-H.; Xu, J.-N.; Xu, G.-H.; Jin, X.-B.; Xiao, R.; Mei, B. Effects of Different Local Analgesic Techniques on Postoperative Quality of Life and Pain in Patients Undergoing Total Hip Arthroplasty under General Anesthesia: A Randomized Controlled Trial. J. Pain Res. 2021, 14, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, C.; Zhang, Y.; Wang, G. Comparative efficacy analysis of ultrasound-guided quadratus lumborum block and lumbar plexus block in hip arthroscopy: A pilot prospective randomized controlled trial. J. Hip Preserv. Surg. 2022, 9, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Small, C.; Laycock, H. Acute postoperative pain management. Br. J. Surg. 2020, 107, E70–E80. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; Briggs, A.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; de Bekker-Grob, E.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BJOG 2022, 129, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Hewlett-Smith, N.; Pope, R.; Furness, J.; Simas, V.; Hing, W. Prognostic factors for inpatient functional recovery following total hip and knee arthroplasty: A systematic review. Acta Orthop. 2020, 91, 313–318. [Google Scholar] [CrossRef]
- Lee, B.H.; Wu, C.L. Educating Patients Regarding Pain Management and Safe Opioid Use after Surgery: A Narrative Review. Anesth. Analg. 2020, 130, 574–581. [Google Scholar] [CrossRef]
- Hyland, S.J.; Brockhaus, K.K.; Vincent, W.R.; Spence, N.Z.; Lucki, M.M.; Howkins, M.J.; Cleary, R.K. Perioperative Pain Management and Opioid Stewardship: A Practical Guide. Healthcare 2021, 9, 333. [Google Scholar] [CrossRef] [PubMed]





| Study | Sample Size | Age | Study Duration | Country | Type of Surgery\Technique | Anesthetic | Outcome Measures |
|---|---|---|---|---|---|---|---|
| Anis et al. (2011) [34] | 60 | 18–60 | Unspecified | Egypt | Hip surgery; posterior LPB | 15 mL bupivacaine 0.5% Clonidine 2.5 lg/mL | Pain scores (VAS) |
| Badiola et al. (2018) [17] | 50 | >18 | 13 months | USA | Hip arthroscopic surgery; FIB vs. LPB | 30 mL of 0.25% bupivacaine with 1:200,000 epinephrine | Pain scores |
| Bravo et al. (2020) [18] | 60 | 18–80 | Unspecified | Chile | Total hip arthroplasty; SIFIB vs. LPB | Ultrasound-guided lumbar plexus block 40 mL of levobupivacaine 0.25% with epinephrine 5 μg/mL | Pain scores, opioid consumption |
| Goytizolo et al. (2016) [19] | 92 | 60–100 | Unspecified | USA | Total hip arthroplasty; LPB | 35 mL of 0.5% Bupivacaine 5 mg of IV midazolam 60 mg of 1.5% mepivacaine 2% lidocaine in 3 mL IV propofol 2–4 mg/kg | Pain scores |
| Gutierrez et al. (2020) [20] | 46 | 19–90 | 11 months | USA | Hip arthroplasty; QLB vs. LPB | Hyperbaric 0.75% bupivacaine (10.5–12 mg) Propofol infusion at 50–100 mcg/kg/ min ketamine 20 mg bolus | Pain score, quadriceps strength, and opioid consumption |
| Ilfeld et al. (2011) [21] | 50 | ≥18 | 10 months | USA | Total hip arthroplasty; Continuous FNB vs. continuous LPB | Perineural ropivacaine, 0.2% (basal 6 mL/h, bolus 4 mL, 30 min lockout) | Pain scores |
| Johnson et al. (2017) [32] | 159 | >18 | 15 months | USA | Total hip arthroplasty; PNB | Bupivacaine 0.5% with 1:200,000 epinephrine 30 mL bolus Infusion of bupivacaine 0.2% | Pain scores |
| Kelly et al. (2022) [22] | 103 | >18 | 11 months | USA | Total hip arthroplasty; PNB | Ropivacaine (20 mL, 0.5%) | Opioid consumption |
| Kendrisic et al. (2017) [33] | 60 | 59 ± 9 | Unspecified | Serbia | Total hip arthroplasty; LPB vs. epidural analgesia | 20 mL levobupivacaine 0.25% | Pain scores (VAS) at rest and on moving |
| Marino et al. (2009) [28] | 225 | 18–80 | 30 months | USA | Total hip arthroplasty; Continous LPB vs. FIB | Bolus of 0.6 mL/kg of 0.5% ropivacaine | VAS pain scores |
| Kendrisic et al. (2017) [23] | 60 | Unspecified | Unspecified | Serbia | Surgical stress response following hip arthroplasty regarding choice of anesthesia and post-operative analgesia | 20 mL 0.25% levobupivacaine | Serum cortisol, insulin, glucose, CRP, incidence of hypotension |
| Diwan et al. (2023) [29] | 70 | ≥65 | 12 months | India | Proximal femoral nail for proximal femoral fractures; LESPB vs. LPB | 0.2% ropivacaine and 30 mcg clonidine | Pain scores |
| Scanaliato et al. (2020) [24] | 64 | 17–49 | 6 months | USA | Hip arthroscopy; Pericapsular injections vs. LPB | 40 mL 0.375% ropivacaine | NRS pain scores |
| Sharma et al. (2020) [26] | 50 | 20–80 | Unspecified | India | Total hip replacement; LPB vs. EA | 15 mL 0.5% ropivacaine | NRS pain scores |
| Siddiqui et al. (2007) [25] | 34 | 18–80 | Unspecified | USA | Hip arthroscopy; LPB vs. systemic opioids | 20 cc 0.25% bupivacaine | Perioperative opioid requirement |
| Stevens et al. (2000) [27] | 60 | 66 ± 10 | Unspecified | Switzerland | Total hip arthroplasty; LPB | 0.4 mg/kg 0.5% bupivacaine | Pain reduction |
| Wardhan et al. (2014) [30] | 60 | 18–75 | 29 months | USA | Total hip arthroplasty; LPB vs. L2 PVB | 15 mL 0.5% ropivacaine | Post-operative opioid consumption and post-operative pain scores |
| YaDeau et al. (2012) [31] | 60 | 35 | Unspecified | Switzerland | Total hip arthroplasty; LPB | 0.4 mL/kg 0.5% bupivacaine | Pain scores at rest and morphine consumption |
| Yang et al. (2021) [35] | 167 | 40–80 | 10 months | China | Elective total hip arthroplasty; LSPB | 0.5% ropivacaine | QoL scores, Mobility, Self-Care, Usual Activities, Pain/Discomfort, and Anxiety/Depression. |
| Yuan et al. (2022) [36] | 50 | 18–60 | 6 months | China | Hip arthroscopic surgery; QLB vs. LPB | 0.4 mL/kg ropivacaine | VAS pain scores |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMutiri, W.A.; AlMajed, E.; Alneghaimshi, M.M.; AlAwadh, A.; AlSarhan, R.; AlShebel, M.N.; AlMatrody, R.A.M.; Hadaddi, R.; AlTamimi, R.; Bin Salamah, R.; et al. Efficacy of Continuous Lumbar Plexus Blockade in Managing Post-Operative Pain after Hip or Femur Orthopedic Surgeries: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3194. https://doi.org/10.3390/jcm13113194
AlMutiri WA, AlMajed E, Alneghaimshi MM, AlAwadh A, AlSarhan R, AlShebel MN, AlMatrody RAM, Hadaddi R, AlTamimi R, Bin Salamah R, et al. Efficacy of Continuous Lumbar Plexus Blockade in Managing Post-Operative Pain after Hip or Femur Orthopedic Surgeries: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(11):3194. https://doi.org/10.3390/jcm13113194
Chicago/Turabian StyleAlMutiri, Wijdan A., Ebtesam AlMajed, Muath M. Alneghaimshi, Afnan AlAwadh, Reem AlSarhan, Malak N. AlShebel, Rayan Abdullah M. AlMatrody, Rafa Hadaddi, Reem AlTamimi, Rawan Bin Salamah, and et al. 2024. "Efficacy of Continuous Lumbar Plexus Blockade in Managing Post-Operative Pain after Hip or Femur Orthopedic Surgeries: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 11: 3194. https://doi.org/10.3390/jcm13113194
APA StyleAlMutiri, W. A., AlMajed, E., Alneghaimshi, M. M., AlAwadh, A., AlSarhan, R., AlShebel, M. N., AlMatrody, R. A. M., Hadaddi, R., AlTamimi, R., Bin Salamah, R., AlZelfawi, L. A., AlBatati, S. K., AlHarthi, A., AlMazroa, G., & AlHossan, A. M. (2024). Efficacy of Continuous Lumbar Plexus Blockade in Managing Post-Operative Pain after Hip or Femur Orthopedic Surgeries: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(11), 3194. https://doi.org/10.3390/jcm13113194





