The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review
Abstract
:1. Introduction
2. Methods
3. Mechanisms of Action of OSA (Summarized in Figure 1)
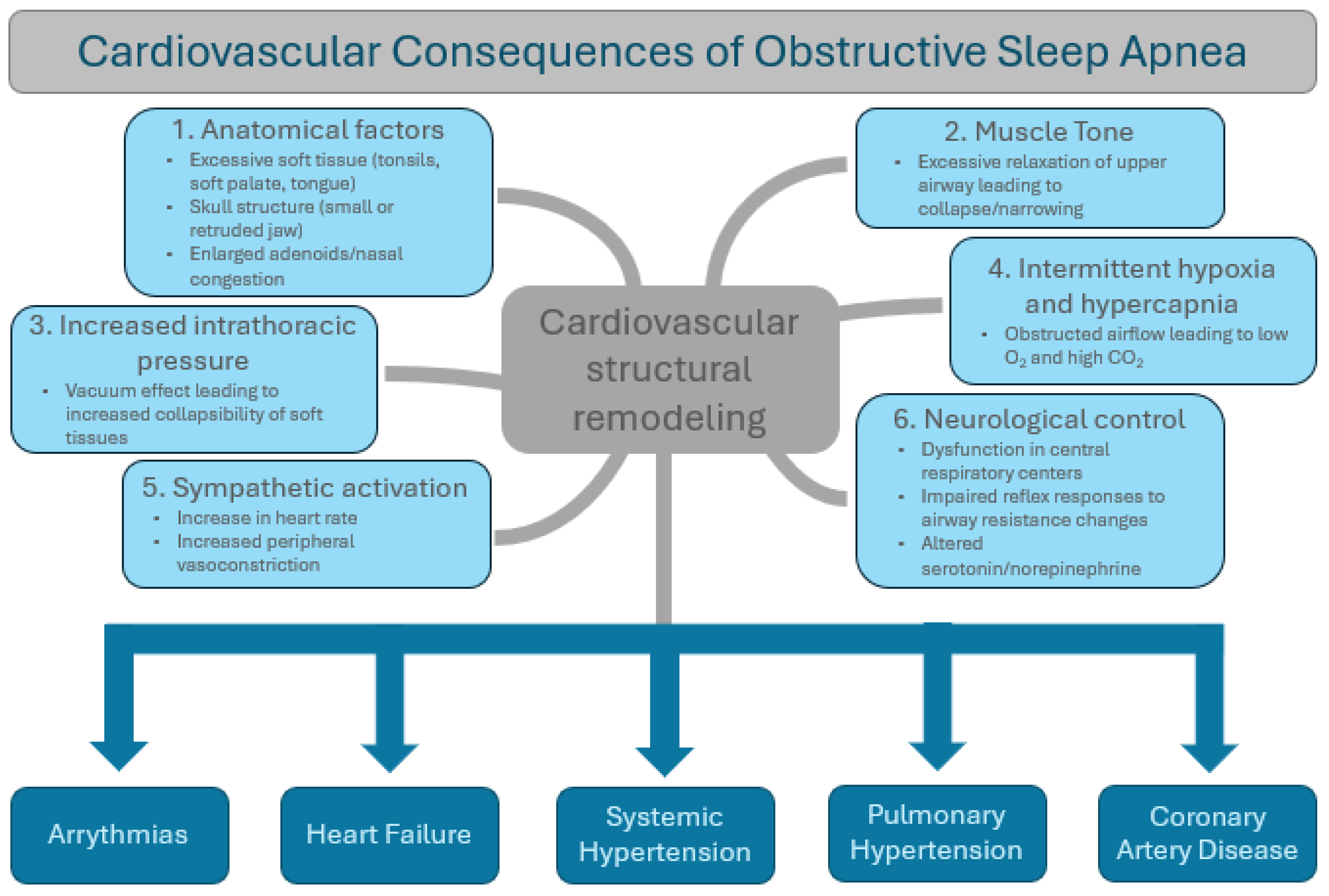
3.1. Anatomical Factors
3.2. Muscle Tone
3.3. Negative Intrathoracic Pressure
3.4. Intermittent Hypoxia and Hypercapnia
3.5. Sympathetic Activation
3.6. Neurological Control of Upper Airway
4. Diagnosis
4.1. Clinical History and Physical Examination
4.2. Sleep Questionnaires
4.3. Polysomnography (PSG)
4.4. Home Sleep Apnea Testing (HSAT)
4.5. Apnea–Hypopnea Index (AHI)
- Mild OSA: AHI 5–14 events per hour
- Moderate OSA: AHI 15–29 events per hour
- Severe OSA: AHI ≥ 30 events per hour
5. Prevalence of Cardiovascular Effects
5.1. Cardiac Arrhythmias
- Atrial fibrillation: OSA is a well-established independent risk factor for the development of atrial fibrillation (AF). The prevalence of AF in the general population is roughly 1–2% [28]. In patients with OSA, the prevalence is approximately 5% [29]. Growing evidence also implicates a concomitant prevalence of AF of 21–74% in patients with OSA, which may suggest that OSA may play a role in AF pathogenesis and development and may also trigger the persistence of AF [30,31]. Numerous clinical trials and meta-analyses have confirmed the strong association between OSA and AF, including one meta-analysis performed in 2018 consisting of 12 studies and nearly 20,000 patients [31]. OSA severity correlates with a higher incidence of AF and may predict AF recurrence after cardioversion and/or with ablation procedures. Furthermore, standard antiarrhythmic therapies in patients with severe OSA are much less likely to be successful compared to patients without OSA [32]. The presence of OSA is associated with an increased incidence of AF in patients with heart failure, coronary artery disease, and hypertrophic cardiomyopathy as well [33,34].
- Sinus arrest: The prevalence of sinus arrest in patients with OSA has been documented to be as high as 11%, with the majority of cases lasting between 2.5 and 12 s [35]. Other studies using polysomnography have documented sinus arrests lasting greater than 2 s in 4% of healthy study participants without known cardiac abnormalities or OSA [36]. According to the European multicenter polysomnographic study, 58% of patients with implanted pacemakers for sinus node dysfunction had symptomatic OSA [37]. The pathophysiologic mechanisms underlying OSA likely create an environment conducive to sinus arrest. The long-term structural heart changes occurring in the left atrium are also likely involved. Despite this, OSA has also been shown to cause nocturnal bradyarrhythmias and sinus arrest in the acute nocturnal setting in the absence of cardiac conduction disease [35,38].
- Bradyarrhythmias: These occur in up to 18% of patients with OSA [35]. The most frequently observed bradyarrhythmias are sinus bradycardia, sinus arrest leading to bradycardia (described above), and atrioventricular (AV) nodal block (predominantly grade II and III). In comparison, the prevalence of nocturnal bradyarrhythmias in healthy adults is roughly 3% [39]. Several studies have reported the incidence of grade II and III AV nodal block to occur in about 10% of patients with OSA, compared with 1% in the healthy elderly population [35,39,40]. In the European multicenter polysomnography study, 68% of patients treated with a pacemaker for atrioventricular block had minimally symptomatic OSA, and 27% fulfilled the criteria for severe OSA. Similarly to other arrhythmias associated with OSA, the incidence of bradycardia arrhythmias in patients with OSA is likely correlated with the degree of severity. Significantly higher incidences of bradyarrhythmias have been observed in OSA patients with higher AHI [37,40,41].
- Ventricular repolarization abnormalities and arrhythmias: QTc interval dispersion and prolongation and ventricular arrhythmias are seen more frequently in patients with OSA compared to the general population [10]. In particular, the QTc interval has been shown to be prolonged during the onset of apnea (482 +/− 34 msec) with the return to baseline during apnea and the post-apnea hyperventilation period [42]. Both monomorphic and polymorphic ventricular tachycardias (VTs) have been reported in higher incidence in patients with OSA [43]. In patients with co-existing heart failure and OSA, OSA was found to be an independent risk factor for ventricular arrhythmias [44]. Furthermore, patients with OSA undergoing catheter ablation for ventricular arrhythmias are associated with a higher rate of recurrence when compared to the general population [45]. The Sleep Heart Health Study, a population study involving 566 patients, showed an increased prevalence of complex ventricular ectopy (ventricular bigeminy, trigeminy, and quadrigeminy) when compared to the general population [29].
5.2. Heart Failure
5.3. Systemic Hypertension
5.4. Pulmonary Hypertension (PH)
5.5. Coronary Artery Disease (CAD)
5.6. Stroke
| Cardiovascular Condition | Prevalence/Incidence among OSA Patients | Prevalence/Incidence in General Population | Pathophysiology |
|---|---|---|---|
Cardiac arrhythmias
| 30–50% [27] | 1.5–5% [62] |
|
Heart failure
| 11–37% [63] | 4.4% [65] |
|
| Systemic hypertension | 59% [53] | 21% [53] |
|
| Pulmonary hypertension | 27–30% [66] | 13–28% [67] |
|
Vascular disease
| 16.6% [63] | 13% [63] |
|
6. Mechanism of Action of Cardiovascular Effects
6.1. Cardiac Arrhythmias
- Bradycardia: Upper airway obstruction elicits the “diving reflex”, which manifests physiologically as increased sympathetic vasoconstriction to muscles and viscera in order to maintain tissue perfusion. The lack of lung expansion from upper airway obstruction prevents the stretching of vagolytic fibers in the lungs, causing a rise in blood pressure and vagally induced reflex bradycardia [9]. This frequently occurs during apneic episodes. Additionally, the decrease in intrathoracic pressure during apnea leads to a transient increase in venous return to the heart and filling of the right atrium. The baroreceptors sensed this increase in cardiac preload, leading to increased parasympathetic afferent activity and reduced heart rate [32,72]. As bradycardia can be observed in healthy individuals during sleep, the bradycardia is generally more pronounced and significant in individuals with OSA during apneic episodes [73].
- Heart Rate Variability: In prolonged states of hypoxemia and hypercapnia, cardiac output usually increases in a compensatory fashion to maintain tissue perfusion. As such, the heart rate may increase in patients with OSA after an apneic episode. As the apnea episode ends and the individual partially or fully awakens to resume breathing, there is often a surge in sympathetic nervous system activity. This arousal response can lead to further increases in heart rate, contributing to fluctuations in heart rate variability throughout the night. Additionally, when parasympathetic tone predominates, heart rate slows, and bradycardia is observed, as described above. The converse reaction occurs when a sympathetic tone predominates and causes the heart rate to rise. The autonomic nervous system therefore controls heart rate variability during sleep in patients with OSA [32].
- First Degree Heart Block and Sinus Arrest: Prolonged PR interval and prolonged sinus arrest occur in patients with OSA to a greater degree than in the general population [32]. Similarly to the mechanisms of underlying bradycardia in OSA patients, these rhythm variations occur primarily because of the autonomic effects associated with prolonged apnea, oxygen desaturation, changes in cardiac hemodynamics, and variations in intrathoracic pressure [32,72].
- Premature Ventricular Complexes (PVCs): Patients with OSA have been shown to experience a higher percentage of ventricular ectopy, including frequent PVCs and significant PVC burden during level 3 polysomnography compared to the general population [74]. This includes increased incidence of bigeminy and trigeminy [74]. Increased incidence of ventricular ectopy in patients with OSA is likely due to metabolic abnormalities, prolonged hypoxia, and mechanical stress, leading to cardiac remodeling. These remodeling changes may include fibrosis, hypertrophy, and alterations in the electrophysiological properties of cardiac myocytes, which increase the susceptibility to PVCs and other arrhythmias.
- Atrial Fibrillation (AF): Intermittent hypoxia due to obstruction during apnea, sympathetic activation, and left atrial remodeling all contribute to the pathogenesis of AF by promoting atrial remodeling, fibrosis, and electrical instability [75]. Additionally, OSA may impair the effectiveness of treatments for AF, which will be further discussed below.
- Atrioventricular (AV) Block: While OSA itself may not directly cause heart block, the physiological disturbances associated with OSA can create an environment conducive to the development, or exacerbation, of AV block, primarily Mobitz type 2 second-degree block [36]. Chronic exposure to OSA-related stressors can lead to structural and functional changes in the cardiovascular system [7]. These changes may include fibrosis, hypertrophy, and alterations in the architecture of the cardiac conduction system [76]. Over time, cardiac remodeling can disrupt normal electrical conduction and increase the susceptibility to heart block. Individuals with OSA who experience symptoms suggestive of heart block during waking hours, such as dizziness, fainting, or palpitations, should undergo appropriate cardiac evaluation to determine the need for a permanent pacemaker.
- Ventricular Tachycardia (VT): The interplay of sympathetic activation, intermittent hypoxia, cardiac remodeling, myocardial ischemia, electrolyte imbalance, autonomic dysfunction, and underlying cardiovascular disease creates a conducive environment for the initiation and perpetuation of ventricular tachycardia in individuals with OSA. Chronic OSA-related changes in ventilation and gas exchange may lead to electrolyte imbalances, promoting QTc prolongation and predisposition to the development of polymorphic ventricular tachycardia [42,77]. Specifically, hypokalemia and hypomagnesemia have been associated with chronic untreated OSA [42,78]. Significant cardiac chamber remodeling also occurs due to prolonged, untreated OSA. Unregulated fluxes in parasympathetic and sympathetic activity, fluctuations in intrathoracic pressure, and increased respiratory effort impose mechanical stress on the heart. The repetitive stretching of the myocardium leads to myocardial remodeling and scarring, which in turn contributes to electrical system remodeling and increases the risk of monomorphic and polymorphic VT [79].
- Sudden Cardiac Death: As a result of prolonged exposure to hypoxia, autonomic dysfunction, and hemodynamic stresses caused by OSA, patients may have a heightened risk of sudden cardiac death, both during nocturnal apneic episodes and during waking hours [80]. Increased intrathoracic pressure causes increased ventricular afterload, which leads to increased myocardial demand, subendocardial ischemia, hypertrophy and fibrosis, and predisposition to malignant arrhythmias. As mentioned previously, hypoxia and electrolyte abnormalities also cause prolonged QTc and an increased propensity for arrhythmogenicity [80,81]. Each of these factors may contribute to the higher incidence of sudden cardiac death in patients with OSA compared to the general population.
6.2. Hemodynamic Consequences
- Systemic Hypertension: Intermittent hypoxia and hypercapnia due to recurrent apnea cause elevated sympathetic nervous system response and lead to catecholamine release and increased vascular tone, resulting in systemic hypertension. The degree of hypertension is likely related to the severity of the OSA [15]. OSA also negatively affects sleep efficiency, which is known to be correlated with risk factors for hypertension, including endothelial dysfunction, arterial stiffness, and increased sympathetic activity [32]. Furthermore, obesity is often co-existent with OSA and is also associated with systemic hypertension, though studies have shown that OSA is related to hypertension when adjusting for body mass index [32]. The renin–angiotensin system (RAS) is known to be activated by obesity and has also been shown to be activated by OSA [82].
- Right Ventricular (RV) Failure: Numerous overlapping pathophysiologic mechanisms are responsible for the development and progression of RV failure in patients with OSA. During inspiration through a narrowed, occluded upper airway, increasingly negative intrathoracic pressure results in blood being drawn into the thorax, augmenting RV preload [9]. Simultaneously, apnea-induced hypoxia causes pulmonary vasoconstriction. Known as the von Euler–Liljesand mechanism, hypoxic pulmonary vasoconstriction causes pulmonary hypertension and increased RV afterload, which causes right heart failure over time [83]. Intermittent hypoxia can also cause oxygen-free-radical production. Reactive oxygen species are known to activate inflammatory pathways that impair vascular endothelial function and can lead to impaired pulmonary vascular vasodilation [84]. Furthermore, OSA causes left ventricular failure (described below) and worsens right ventricular failure.
- Left Ventricular (LV) Failure: Similar to right ventricular failure, the mechanisms leading to LV failure in OSA are overlapping and multifactorial. Hypertension, RAS activation, obesity, intermittent hypoxia and hypercapnia, and autonomic dysregulation all contribute to LV remodeling. Hypoxic pulmonary vasoconstriction, as described previously, leads to increased RV pressure which causes RV distension, leading to a leftward shift of the interventricular septum during both systole and diastole, which impedes LV filling and results in reduced stroke volume [85]. Intermittent hypoxia and hypercapnia also elicit fluctuations in sympathetic–parasympathetic activity. During sympathetically mediated systemic increases in vascular tone, vasoconstriction occurs, resulting in increased LV afterload [86]. Increased afterload leads to reduced stroke volume and increased end-systolic volume, leading to maladaptive responses by the left ventricle over time, causing concentric hypertrophy and reduced chamber diameter. LV hypertrophy reduces chamber size, causing heart failure with preserved ejection fraction (HFpEF), which eventually deteriorates into heart failure with reduced ejection fraction (HFrEF) [87]. The RAS system is also activated by enhanced sympathetic activity, which causes increased angiotensin II, aldosterone, and subsequent fluid retention, further exacerbating heart failure. Finally, negative intrathoracic pressure also leads to increased atrial remodeling (facilitating atrial fibrillation), elevated LV end-diastolic pressure, increased afterload, and increased cardiac workload leading to increased myocardial oxygen demand [49].
- Pulmonary Hypertension: As previously mentioned, negative intrathoracic pressure leads to hypoxic pulmonary vasoconstriction, which causes increased RV afterload. Pulmonary vasoconstriction also raises pulmonary artery pressure in the short- and long term. Alveolar hypoxia also causes endothelial remodeling via the production of reactive oxygen species and other vasoactive substances such as serotonin, endothelin, and Rho-kinase. Nitric oxide scavenging via reactive oxygen species also occurs, leading to a reduction in vasodilation. OSA also leads to pulmonary hypertension via hydrostatic mechanisms due to increases in left atrial pressure causing pulmonary venous hypertension and pulmonary interstitial edema. Each of these pathways causes pulmonary vascular remodeling via the proliferation of smooth muscle cells and asymmetric neointimal hyperplasia [66,88].
- Myocardial Ischemia: Oscillations in blood pressure and cardiac output during apneic episodes lead to increased myocardial oxygen consumption, therapy facilitating type 2 myocardial infarction via supply/demand mismatch [89]. Elevated oxidative stress from hypoxia results in the production of reactive oxygen species, which leads to coronary artery remodeling over time. Abnormal peroxidation of lipids due to increased oxidative burden leads to increased concentration of low-density lipoprotein (LDL), which in turn promotes the formation of coronary arterial vulnerable plaques [90]. Furthermore, prolonged oxidative stress results in the production of oxygen-free radicals and inflammatory mediators. Chronic inflammation, which occurs via oxidative stress and increased vascular tone, ultimately results in atherosclerosis and coronary artery disease over the long term, which further predisposes patients to acute coronary syndrome [91].
6.3. Vascular Disease
- Coronary Artery Disease (CAD): Untreated OSA is associated with a significantly elevated risk of CAD [9]. Untreated OSA is characterized by chronic low-grade inflammation, with elevated levels of inflammatory markers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-alpha). Systemic inflammation promotes endothelial dysfunction, oxidative stress, atherosclerosis, and subsequent acute coronary syndrome. The development of CAD via chronic inflammation occurs via redox reactions involving oxidized LDL leading to atheroma formation. As previously described, oxidative stress results in free radical production and subsequent recruitment of inflammatory cells, including neutrophils and monocytes [92,93]. Prolonged exposure to these inflammatory mediators results in endothelial dysfunction and unstable plaque formation. Hypertension, which is highly associated with OSA, also causes CAD via increased vessel stiffness and reduced vascular compliance. These factors, when cumulatively accounted for, contribute to a significant risk in the development of CAD [94].
- Cerebral Vascular Disease and Stroke: OSA may be an independent risk factor for ischemic stroke [95]. In OSA patients, significant contributing factors include cerebrovascular disease, atrial fibrillation, and sleep fragmentation. Elevated oxidative stress results in endothelial dysfunction, inflammation, and vascular hardening, thus predisposing patients to cerebral ischemia. Cerebral vasculatures are most susceptible to plaque development and ischemia [95]. Atrial fibrillation is also a significant risk factor for ischemic stroke, which is well-documented. Atrial fibrillation in OSA patients is also more difficult to control than in the general population, which leads to an enhanced risk of atrial-fibrillation-induced clot formation (most commonly in the left atrial appendage) and embolization of the brain. OSA also disrupts normal sleep architecture, including rapid eye movement (REM) sleep, which plays a crucial role in cerebral hemodynamics and neurovascular regulation. Fragmented REM sleep may impair cerebral blood flow regulation and increase the risk of stroke [95,96].
- Peripheral Arterial Disease: Similar mechanisms leading to cerebrovascular disease and coronary artery disease also contribute to the development of peripheral arterial disease in patients with OSA. These mechanisms are marked by repetitive occurrences of hypoxemia and hypercapnia, which promote highly inflammatory environments and oxidative stress, thereby leading to endothelial dysfunction and vascular remodeling [97].
7. Treatment Mechanisms and Evidence
7.1. CPAP Therapy—How It Works
- Maintaining Airway Patency: The continuous flow of pressurized air delivered by the CPAP machine creates a pneumatic splint that keeps the soft tissues of the upper airway—from the back of the throat to the nasal passages—open and prevents them from collapsing or obstructing the flow of air [98]. By maintaining airway patency, CPAP therapy effectively eliminates or reduces apnea episodes and hypopneas, restoring normal breathing patterns during sleep.
- Preventing Airway Collapse: Individuals with OSA experience repeated episodes of airway collapse or narrowing during sleep, leading to breathing pauses and disruptions in oxygen supply to the body. CPAP therapy counteracts this by delivering constant positive pressure to the airway, preventing it from collapsing or narrowing even during the relaxation of the throat muscles [99].
- Improving Oxygenation: CPAP therapy helps improve oxygenation by ensuring a continuous flow of oxygen-rich air into the lungs throughout the night. By preventing episodes of hypoxemia (low oxygen levels in the blood) associated with OSA, CPAP therapy reduces the strain on the cardiovascular system and minimizes the risk of cardiovascular complications.
- Alleviating Symptoms: CPAP therapy effectively alleviates the symptoms of OSA, including excessive daytime sleepiness, fatigue, morning headaches, and cognitive impairment. By increasing restorative sleep and reducing sleep fragmentation, CPAP therapy helps individuals with OSA feel more refreshed and alert during the day, improving overall quality of life.
7.2. Oral Appliances Used in the Treatment of Sleep Apnea
- MAD consists of an upper and lower dental tray connected by adjustable metal or plastic hinges. The appliance is custom-made to fit the individual’s teeth and oral anatomy. By adjusting the hinges, the lower jaw can be gradually advanced relative to the upper jaw, which helps open and stabilize the upper airway, thereby preventing obstruction and increasing airway patency [102]. Physiologically, these devices work to enlarge the oropharynx and velopharynx during sleep and activate stretch receptors, reducing airway collapse. While effective for mild to moderate OSA, it is ineffective in severe cases. Negative side effects are also reported, including jaw pain, tenderness, and hypersalivation [103].
- Palate lift devices: These devices are designed to lift and stabilize the soft palate, thereby enlarging the upper airway and reducing the risk of airway collapse during sleep. They consist of a custom-fitted acrylic or silicone appliance that is inserted into the mouth and positioned against the palate. The device incorporates a mechanism, such as a spring-loaded component or an adjustable screw, that applies upward pressure to the soft palate, lifting it and preventing its collapse during sleep [104].
- Tongue retention devices: These devices are relatively older and becoming outdated and obsolete due to newer innovations in MADs [105]. Tongue retention devices primarily focus on retaining the tongue in a forward position to prevent its collapse and upper airway obstruction during sleep.
7.3. Surgical Treatment of Sleep Apnea
- Uvulopalatopharyngoplasty (UPPP)
- 2.
- Hypoglossal Nerve Stimulation
7.4. Lifestyle Modifications
7.5. Treatment Outcomes
7.5.1. Atrial Fibrillation
7.5.2. Cardiac Remodeling and Heart Failure
7.5.3. Systemic Hypertension
7.5.4. Pulmonary Hypertension
7.6. Future Directions
8. Conclusions
Funding
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Hader, C.; Schroeder, A.; Hinz, M.; Micklefield, G.H.; Rasche, K. Sleep disordered breathing in the elderly: Comparison of women and men. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 56 (Suppl. S4), 85–91. [Google Scholar]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M.; et al. Predictors of Sleep-Disordered Breathing in Community-Dwelling Adults: The Sleep Heart Health Study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef]
- Glasser, M.; Bailey, N.; McMillan, A.; Goff, E.; Morrell, M.J. Sleep apnoea in older people. Breathe 2011, 7, 248–256. [Google Scholar] [CrossRef]
- Mashaqi, S.; Gozal, D. The impact of obstructive sleep apnea and PAP therapy on all-cause and cardiovascular mortality based on age and gender—A literature review. Respir. Investig. 2020, 58, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, P.; Tak, T. Obstructive sleep apnea and cardiovascular disease. Cardiol. Rev. 2011, 19, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet Lond. Engl. 2009, 373, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. J. Am. Heart Assoc. 2019, 8, e010440. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Chen, H.-C.; Liu, S.Y.-C.; Lee, L.-A.; Lin, C.-Y.; Chang, G.-H.; Tsai, Y.-T.; Lee, Y.-C.; Hsu, C.-M.; Li, H.-Y. Holistic care for obstructive sleep apnea (OSA) with an emphasis on restoring nasal breathing: A review and perspective. J. Chin. Med. Assoc. JCMA 2022, 85, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Su, S.; Liang, J.; Jiang, Y.; Shu, Y.; Ding, L. Analysis of the Risk Factors Associated with Obstructive Sleep Apnea Syndrome in Chinese Children. Front. Pediatr. 2022, 10, 900216. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Pasirstein, M.; Pierson, R.; Mackley, A.; Hachadoorian, R.; Arens, R.; Maislin, G.; Pack, A.I. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am. J. Respir. Crit. Care Med. 2003, 168, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A. Craniofacial abnormalities in obstructive sleep apnoea: Implications for treatment. Respirology 1996, 1, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; White, D.P. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir. Physiol. Neurobiol. 2008, 160, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol and the risk of sleep apnoea: A systematic review and meta-analysis. Sleep Med. 2018, 42, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, L.; Chen, X.; Kelly, B.C.; Qi, C.; Pan, C.; Yang, M.; Hao, W.; Liu, T.; Tang, J. Sleep quality in cigarette smokers and nonsmokers: Findings from the general population in central China. BMC Public Health 2019, 19, 808. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Trakada, G.; Chrousos, G.P.; Pejovic, S.; Vgontzas, A.N. Sleep Apnea and its Association with the Stress System, Inflammation, Insulin Resistance and Visceral Obesity. Sleep Med. Clin. 2007, 2, 251–261. [Google Scholar] [CrossRef]
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous Excitatory Drive Modulating Respiratory Muscle Activity across Sleep–Wake States. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chen, P.-Y.; Chuang, L.-P.; Chen, N.-H.; Tu, Y.-K.; Hsieh, Y.-J.; Wang, Y.-C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Nurgul, Y. A simple and validated test for detecting patients with OSA: STOP-BANG questionnaire. Ann. Card. Anaesth. 2021, 24, 313–314. [Google Scholar] [PubMed]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Weber, R.P.; Arvanitis, M.; Stine, A.; Lux, L.; Harris, R.P. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 415–433. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Hersi, A.S. Obstructive sleep apnea and cardiac arrhythmias. Ann. Thorac. Med. 2010, 5, 10–17. [Google Scholar] [CrossRef]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in AdultsNational Implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of Nocturnal Arrhythmias with Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, X.; Huang, B.; Zhou, X.; Wang, M.; Zhou, L.; Meng, G.; Wang, Y.; Wang, Z.; Deng, J.; et al. Atrial Fibrillation in Acute Obstructive Sleep Apnea: Autonomic Nervous Mechanism and Modulation. J. Am. Heart Assoc. 2017, 6, e006264. [Google Scholar] [CrossRef]
- Youssef, I.; Kamran, H.; Yacoub, M.; Patel, N.; Goulbourne, C.; Kumar, S.; Kane, J.; Hoffner, H.; Salifu, M.; McFarlane, S.I. Obstructive Sleep Apnea as a Risk Factor for Atrial Fibrillation: A Meta-Analysis. J. Sleep Disord. Ther. 2018, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Gupta, S.S.; Sabharwal, N.; Meghrajani, V.; Sharma, S.; Kamholz, S.; Kupfer, Y. A comprehensive review of obstructive sleep apnea. Sleep Sci. 2021, 14, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.P.; Drager, L.F.; Genta, P.R.; Amaro, A.C.S.; Antunes, M.O.; Matsumoto, A.Y.; Arteaga, E.; Mady, C.; Lorenzi-Filho, G. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest 2010, 137, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.; Pierucci, N.; Piro, A.; Trivigno, S.; Chimenti, C.; Galardo, G.; Miraldi, F.; Vizza, C.D. Incidence and Determinants of Spontaneous Cardioversion of Early Onset Symptomatic Atrial Fibrillation. Available online: https://www.mdpi.com/1648-9144/58/11/1513 (accessed on 19 May 2024).
- Guilleminault, C.; Connolly, S.J.; Winkle, R.A. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am. J. Cardiol. 1983, 52, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gula, L.J.; Krahn, A.D.; Skanes, A.C.; Yee, R.; Klein, G.J. Clinical relevance of arrhythmias during sleep: Guidance for clinicians. Heart 2004, 90, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Garrigue, S.; Pépin, J.-L.; Defaye, P.; Murgatroyd, F.; Poezevara, Y.; Clémenty, J.; Lévy, P. High Prevalence of Sleep Apnea Syndrome in Patients with Long-Term Pacing. Circulation 2007, 115, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Zwillich, C.; Devlin, T.; White, D.; Douglas, N.; Weil, J.; Martin, R. Bradycardia during sleep apnea. Characteristics and mechanism. J. Clin. Investig. 1982, 69, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L.; Kennedy, H.L. Cardiac Arrhythmias in a Healthy Elderly Population: Detection by 24-hour Ambulatory Electrocardiography. Chest 1982, 81, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.F.; Koehler, U.; Stammnitz, A.; Peter, J.H. Heart block in patients with sleep apnoea. Thorax 1998, 53, S29–S32. [Google Scholar] [CrossRef]
- Olmetti, F.; La Rovere, M.T.; Robbi, E.; Taurino, A.E.; Fanfulla, F. Nocturnal cardiac arrhythmia in patients with obstructive sleep apnea. Sleep Med. 2008, 9, 475–480. [Google Scholar] [CrossRef]
- Gillis, A.M.; Stoohs, R.; Guilleminault, C. Changes in the QT interval during obstructive sleep apnea. Sleep 1991, 14, 346–350. [Google Scholar] [CrossRef]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2013, 41, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Westerheide, N.; Prinz, C.; Hossain, M.S.; Vogt, J.; Langer, C.; Horstkotte, D.; Oldenburg, O. Cheyne–Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur. Heart J. 2011, 32, 61–74. [Google Scholar] [CrossRef]
- Koshino, Y.; Satoh, M.; Katayose, Y.; Kuroki, K.; Sekiguchi, Y.; Yamasaki, H.; Yoshida, K.; Yasuda, K.; Tanigawa, T.; Kuga, K.; et al. Sleep apnea and ventricular arrhythmias: Clinical outcome, electrophysiologic characteristics, and follow-up after catheter ablation. J. Cardiol. 2010, 55, 211–216. [Google Scholar] [CrossRef]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef]
- Milleron, O.; Pillière, R.; Foucher, A.; de Roquefeuil, F.; Aegerter, P.; Jondeau, G.; Raffestin, B.G.; Dubourg, O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: A long-term follow-up study. Eur. Heart J. 2004, 25, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, K.; Campbell, A.; Yee, B.; Richards, M.; O’Meeghan, T.; Weatherall, M.; Neill, A. Sleep-Disordered Breathing Occurs Frequently in Stable Outpatients with Congestive Heart Failure. Chest 2005, 128, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S. Sleep disorders in systolic heart failure: A prospective study of 100 male patients. The final report. Int. J. Cardiol. 2006, 106, 21–28. [Google Scholar] [CrossRef]
- Wang, H.; Parker, J.D.; Newton, G.E.; Floras, J.S.; Mak, S.; Chiu, K.-L.; Ruttanaumpawan, P.; Tomlinson, G.; Bradley, T.D. Influence of Obstructive Sleep Apnea on Mortality in Patients with Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 1625–1631. [Google Scholar] [CrossRef]
- Lugaresi, E.; Cirignotta, F.; Coccagna, G.; Piana, C. Some epidemiological data on snoring and cardiocirculatory disturbances. Sleep 1980, 3, 221–224. [Google Scholar] [CrossRef]
- Fletcher, E.C.; DeBehnke, R.D.; Lovoi, M.S.; Gorin, A.B. Undiagnosed sleep apnea in patients with essential hypertension. Ann. Intern. Med. 1985, 103, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Young, T.B.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’Agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000, 283, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Jilwan, F.N.; Escourrou, P.; Garcia, G.; Jaïs, X.; Humbert, M.; Roisman, G. High occurrence of hypoxemic sleep respiratory disorders in precapillary pulmonary hypertension and mechanisms. Chest 2013, 143, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mooe, T.; Rabben, T.; Wiklund, U.; Franklin, K.A.; Eriksson, P. Sleep-Disordered Breathing in Men with Coronary Artery Disease. Chest 1996, 109, 659–663. [Google Scholar] [CrossRef]
- Yumino, D.; Tsurumi, Y.; Takagi, A.; Suzuki, K.; Kasanuki, H. Impact of Obstructive Sleep Apnea on Clinical and Angiographic Outcomes Following Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2007, 99, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Hedner, J.; Kraiczi, H.; Löth, S. Respiratory Disturbance Index: An independent predictor of mortality in coronary artery disease. Am. J. Respir. Crit. Care Med. 2000, 162, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Good, D.C.; Henkle, J.Q.; Gelber, D.; Welsh, J.; Verhulst, S. Sleep-Disordered Breathing and Poor Functional Outcome After Stroke. Stroke 1996, 27, 252–259. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep Apnea and 20-Year Follow-Up for All-Cause Mortality, Stroke, and Cancer Incidence and Mortality in the Busselton Health Study Cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef]
- Kaneko, Y.; Hajek, V.E.; Zivanovic, V.; Raboud, J.; Bradley, T.D. Relationship of Sleep Apnea to Functional Capacity and Length of Hospitalization Following Stroke. Sleep 2003, 26, 293–297. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Desai, D.S.; Hajouli, S. Arrhythmias. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Herrscher, T.E.; Akre, H.; Øverland, B.; Sandvik, L.; Westheim, A.S. High Prevalence of Sleep Apnea in Heart Failure Outpatients: Even in Patients with Preserved Systolic Function. J. Card. Fail. 2011, 17, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef]
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary hypertension in obstructive sleep apnea: Is it clinically significant? A critical analysis of the association and pathophysiology. Pulm. Circ. 2015, 5, 220–227. [Google Scholar] [CrossRef]
- Rich, S.; Chomka, E.; Hasara, L.; Hart, K.; Drizd, T.; Joo, E.; Levy, P.S. The Prevalence of Pulmonary Hypertension in the United States: Adult Population Estimates Obtained from Measurements of Chest Roentgenograms from the NHANES II Survey. Chest 1989, 96, 236–241. [Google Scholar] [CrossRef]
- Peker, Y.; Kraiczi, H.; Hedner, J.; Löth, S.; Johansson, A.; Bende, M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur. Respir. J. 1999, 14, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Wang, B.; Fan, J.; Que, B.; Ai, H.; Wang, X.; Nie, S. Obstructive sleep apnea is associated with the long-term prognosis of patients in acute coronary syndromes with prior myocardial infarction: Insights from OSA-ACS study. Sleep Med. 2023, 112, 141–148. [Google Scholar] [CrossRef]
- Miller, W.P. Cardiac arrhythmias and conduction disturbances in the sleep apnea syndrome. Prevalence and significance. Am. J. Med. 1982, 73, 317–321. [Google Scholar] [CrossRef]
- Flemons, W.W.; Remmers, J.E.; Gillis, A.M. Sleep apnea and cardiac arrhythmias. Is there a relationship? Am. Rev. Respir. Dis. 1993, 148, 618–621. [Google Scholar] [CrossRef]
- Hirani, R.; Smiley, A. A Scoping Review of Sleep Apnea: Where Do We Stand? Life 2023, 13, 387. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Lorenzi-Filho, G. Cardiac rhythm disorders in obstructive sleep apnea. J. Thorac. Dis. 2018, 10, S4221–S4230. [Google Scholar] [CrossRef] [PubMed]
- Shepard, J.W.; Garrison, M.W.; Grither, D.A.; Dolan, G.F. Relationship of ventricular ectopy to oxyhemoglobin desaturation in patients with obstructive sleep apnea. Chest 1985, 88, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Marulanda-Londoño, E.; Chaturvedi, S. The Interplay between Obstructive Sleep Apnea and Atrial Fibrillation. Front. Neurol. 2017, 8, 668. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Camen, G.; Clarenbach, C.F.; Stöwhas, A.-C.; Rossi, V.A.; Sievi, N.A.; Stradling, J.R.; Kohler, M. The effects of simulated obstructive apnea and hypopnea on arrhythmic potential in healthy subjects. Eur. J. Appl. Physiol. 2013, 113, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Al Wadee, Z.; Ooi, S.L.; Pak, S.C. Serum Magnesium Levels in Patients with Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 2273. [Google Scholar] [CrossRef]
- Saeed, S.; Romarheim, A.; Solheim, E.; Bjorvatn, B.; Lehmann, S. Cardiovascular remodeling in obstructive sleep apnea: Focus on arterial stiffness, left ventricular geometry and atrial fibrillation. Expert Rev. Cardiovasc. Ther. 2022, 20, 455–464. [Google Scholar] [CrossRef]
- Gami, A.S.; Howard, D.E.; Olson, E.J.; Somers, V.K. Day-night pattern of sudden death in obstructive sleep apnea. N. Engl. J. Med. 2005, 352, 1206–1214. [Google Scholar] [CrossRef]
- Blackwell, J.N.; Walker, M.; Stafford, P.; Estrada, S.; Adabag, S.; Kwon, Y. Sleep Apnea and Sudden Cardiac Death. Circ. Rep. 2019, 1, 568–574. [Google Scholar] [CrossRef]
- Hanly, P.J.; Ahmed, S.; Fjell, C.D.; Handley, G.B.; Sola, D.; Nicholl, D.; Zalucky, A. Urine biomarkers of renal renin–angiotensin system activity: Exploratory analysis in humans with and without obstructive sleep apnea. Physiol. Rep. 2020, 8, e14376. [Google Scholar] [CrossRef]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2016, 47, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005, 112, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Stoohs, R.; Guilleminault, C. Cardiovascular changes associated with obstructive sleep apnea syndrome. J. Appl. Physiol. 1992, 72, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.L.; Brooks, D.; Kozar, L.F.; Tse, S.; Phillipson, E.A. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J. Appl. Physiol. 1995, 79, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Borowski, A.G.; Popović, Z.B.; Greenberg, N.L.; Martin, D.S.; Bungo, M.W.; Levine, B.D.; Thomas, J.D. Effect of Gravitational Gradients on Cardiac Filling and Performance. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2017, 30, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Sullivan, C.C.; Chu, D.; Cho, A.J.; Kido, M.; Wolf, P.L.; Yuan, J.X.-J.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Signaling molecules in nonfamilial pulmonary hypertension. N. Engl. J. Med. 2003, 348, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Barcelo, A.; Miralles, C.; Barbe, F.; Vila, M.; Pons, S.; Agusti, A.G. Abnormal lipid peroxidation in patients with sleep apnoea. Eur. Respir. J. 2000, 16, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Kraiczi, H.; Caidahl, K.; Samuelsson, A.; Peker, Y.; Hedner, J. Impairment of Vascular Endothelial Function and Left Ventricular Filling: Association with the Severity of Apnea-Induced Hypoxemia During Sleep. Chest 2001, 119, 1085–1091. [Google Scholar] [CrossRef]
- Li, R.C.; Haribabu, B.; Mathis, S.P.; Kim, J.; Gozal, D. Leukotriene B4 Receptor-1 Mediates Intermittent Hypoxia-induced Atherogenesis. Am. J. Respir. Crit. Care Med. 2011, 184, 124–131. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Wang, Y.; Duggan, R.C.; Harshan Vardhan, S.; Tan, H.-L.; Molero Ramirez, H.; Khalyfa, A.; Bhattacharjee, R.; Bandla, H.P.R.; Gozal, D. Nitric oxide production by monocytes in children with OSA and endothelial dysfunction. Clin. Sci. 2014, 127, 323–330. [Google Scholar] [CrossRef]
- Torres-Alba, F.D.; Gemma, D.; Armada-Romero, E.; Rey-Blas, J.R.; López-de-Sá, E.; López-Sendon, J.L. Obstructive Sleep Apnea and Coronary Artery Disease: From Pathophysiology to Clinical Implications. Pulm. Med. 2013, 2013, 768064. [Google Scholar] [CrossRef]
- Jehan, S.; Farag, M.; Zizi, F.; Pandi-Perumal, S.R.; Chung, A.; Truong, A.; Tello, D.; McFarlane, S.I. Obstructive sleep apnea and stroke. Sleep Med. Disord. Int. J. 2018, 2, 120–125. [Google Scholar]
- Koo, D.L.; Nam, H.; Thomas, R.J.; Yun, C.-H. Sleep Disturbances as a Risk Factor for Stroke. J. Stroke 2018, 20, 12–32. [Google Scholar] [CrossRef]
- AlSheikh, S. Relationship between Peripheral Arterial Diseases and Obstructive Sleep Apnea: A Systematic Review. Cureus 2023, 15, e35550. [Google Scholar] [CrossRef]
- Owens, R.L.; Malhotra, A.; Eckert, D.J.; White, D.P.; Jordan, A.S. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J. Appl. Physiol. 2010, 108, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Squier, S.B.; Patil, S.P.; Schneider, H.; Kirkness, J.P.; Smith, P.L.; Schwartz, A.R. Effect of end-expiratory lung volume on upper airway collapsibility in sleeping men and women. J. Appl. Physiol. 2010, 109, 977–985. [Google Scholar] [CrossRef]
- Kanagala, R.; Murali, N.S.; Friedman, P.A.; Ammash, N.M.; Gersh, B.J.; Ballman, K.V.; Shamsuzzaman, A.S.M.; Somers, V.K. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003, 107, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Bradley, T.D.; Spaak, J.; Ryan, C.M.; Kubo, T.; Kaneko, Y.; Floras, J.S. Inhibition of Awake Sympathetic Nerve Activity of Heart Failure Patients with Obstructive Sleep Apnea by Nocturnal Continuous Positive Airway Pressure. J. Am. Coll. Cardiol. 2005, 45, 2008–2011. [Google Scholar] [CrossRef]
- Jayesh, S.R.; Bhat, W.M. Mandibular advancement device for obstructive sleep apnea: An overview. J. Pharm. Bioallied Sci. 2015, 7, S223. [Google Scholar] [CrossRef]
- Alessandri-Bonetti, A.; Bortolotti, F.; Moreno-Hay, I.; Michelotti, A.; Cordaro, M.; Alessandri-Bonetti, G.; Okeson, J.P. Effects of mandibular advancement device for obstructive sleep apnea on temporomandibular disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 48, 101211. [Google Scholar] [CrossRef] [PubMed]
- Mickelson, S.A. Oral Appliances for Snoring and Obstructive Sleep Apnea. Otolaryngol. Clin. N. Am. 2020, 53, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Fernandez-Salvador, C.; Giambo, J.; Nesbitt, B.; Liu, S.Y.-C.; Capasso, R.; Kushida, C.A.; Camacho, M. Tongue retaining devices for obstructive sleep apnea: A systematic review and meta-analysis. Am. J. Otolaryngol. 2017, 38, 272–278. [Google Scholar] [CrossRef]
- MacKay, S.G.; Lewis, R.; McEvoy, D.; Joosten, S.; Holt, N.R. Surgical management of obstructive sleep apnoea: A position statement of the Australasian Sleep Association*. Respirology 2020, 25, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Browaldh, N.; Nerfeldt, P.; Lysdahl, M.; Bring, J.; Friberg, D. SKUP3 randomised controlled trial: Polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax 2013, 68, 846–853. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yin, G.; Zhan, S.; Xu, J.; Cao, X.; Li, J.; Ye, J. Long-term Efficacy of Uvulopalatopharyngoplasty among Adult Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2019, 161, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Watach, A.J.; Hwang, D.; Sawyer, A.M. Personalized and Patient-Centered Strategies to Improve Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Patient Prefer. Adherence 2021, 15, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Neupane, P.; Sigua, N.L. Upper Airway Stimulation/Hypoglossal Nerve Stimulator. Am. J. Respir. Crit. Care Med. 2020, 202, P23–P24. [Google Scholar] [CrossRef] [PubMed]
- Mashaqi, S.; Patel, S.I.; Combs, D.; Estep, L.; Helmick, S.; Machamer, J.; Parthasarathy, S. The Hypoglossal Nerve Stimulation as a Novel Therapy for Treating Obstructive Sleep Apnea-A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 1642. [Google Scholar] [CrossRef]
- Woodson, B.T.; Strohl, K.P.; Soose, R.J.; Gillespie, M.B.; Maurer, J.T.; de Vries, N.; Padhya, T.A.; Badr, M.S.; Lin, H.-S.; Vanderveken, O.M.; et al. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2018, 159, 194–202. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions between obesity and obstructive sleep apnea: Implications for treatment. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Fritscher, L.G.; Canani, S.; Mottin, C.C.; Fritscher, C.C.; Berleze, D.; Chapman, K.; Chatkin, J.M. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respir. Int. Rev. Thorac. Dis. 2007, 74, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Hock, L.M.; Bowman, T.J. Higher Prevalence of Smoking in Patients Diagnosed as Having Obstructive Sleep Apnea. Sleep Breath. 2001, 05, 167–172. [Google Scholar] [CrossRef]
- Gottlieb, D.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review | Otolaryngology | JAMA | JAMA Network. Available online: https://jamanetwork-com.ezproxy.library.unlv.edu/journals/jama/fullarticle/2764461 (accessed on 19 May 2024).
- Vural, M.G.; Cetin, S.; Firat, H.; Akdemir, R.; Yeter, E. Impact of continuous positive airway pressure therapy on left atrial function in patients with obstructive sleep apnoea: Assessment by conventional and two-dimensional speckle-tracking echocardiography. Acta Cardiol. 2014, 69, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Naruse, Y.; Tada, H.; Satoh, M.; Yanagihara, M.; Tsuneoka, H.; Hirata, Y.; Ito, Y.; Kuroki, K.; Machino, T.; Yamasaki, H.; et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: Clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013, 10, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Mediano, O.; Martínez, I.; Villamor, J. Obstructive Sleep Apnea Syndrome Affects Left Ventricular Diastolic Function. Circulation 2005, 112, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Shivalkar, B.; Van de Heyning, C.; Kerremans, M.; Rinkevich, D.; Verbraecken, J.; De Backer, W.; Vrints, C. Obstructive sleep apnea syndrome: More insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J. Am. Coll. Cardiol. 2006, 47, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S. Effects of Continuous Positive Airway Pressure on Sleep Apnea and Ventricular Irritability in Patients with Heart Failure. Circulation 2000, 101, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Montesi, S.B.; Edwards, B.A.; Malhotra, A.; Bakker, J.P. The Effect of Continuous Positive Airway Pressure Treatment on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 587–596. [Google Scholar] [CrossRef]
- Goyal, M.; Johnson, J. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120–124. [Google Scholar]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-H.; Tan, A.; Lee, C.-H. Management of hypertension in obstructive sleep apnea. Am. J. Prev. Cardiol. 2023, 13, 100475. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Martínez, I.; Villamor, J. Pulmonary hypertension in obstructive sleep apnoea: Effects of continuous positive airway pressure: A randomized, controlled cross-over study. Eur. Heart J. 2006, 27, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
| Apnea–Hypopnea Index (AHI) Score (Apnea or Hypopnea Episodes/Hour) | OSA Severity |
|---|---|
| <5 | Normal (no sleep apnea) |
| 5–15 | Mild sleep apnea |
| 15–30 | Moderate sleep apnea |
| >30 | Severe sleep apnea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiCaro, M.V.; Lei, K.; Yee, B.; Tak, T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. J. Clin. Med. 2024, 13, 3223. https://doi.org/10.3390/jcm13113223
DiCaro MV, Lei K, Yee B, Tak T. The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. Journal of Clinical Medicine. 2024; 13(11):3223. https://doi.org/10.3390/jcm13113223
Chicago/Turabian StyleDiCaro, Michael V., KaChon Lei, Brianna Yee, and Tahir Tak. 2024. "The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review" Journal of Clinical Medicine 13, no. 11: 3223. https://doi.org/10.3390/jcm13113223
APA StyleDiCaro, M. V., Lei, K., Yee, B., & Tak, T. (2024). The Effects of Obstructive Sleep Apnea on the Cardiovascular System: A Comprehensive Review. Journal of Clinical Medicine, 13(11), 3223. https://doi.org/10.3390/jcm13113223






