Organ Utilization Rates from Non-Ideal Donors for Solid Organ Transplant in the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Logistic Regression Modeling
2.3. Data Analysis
3. Results
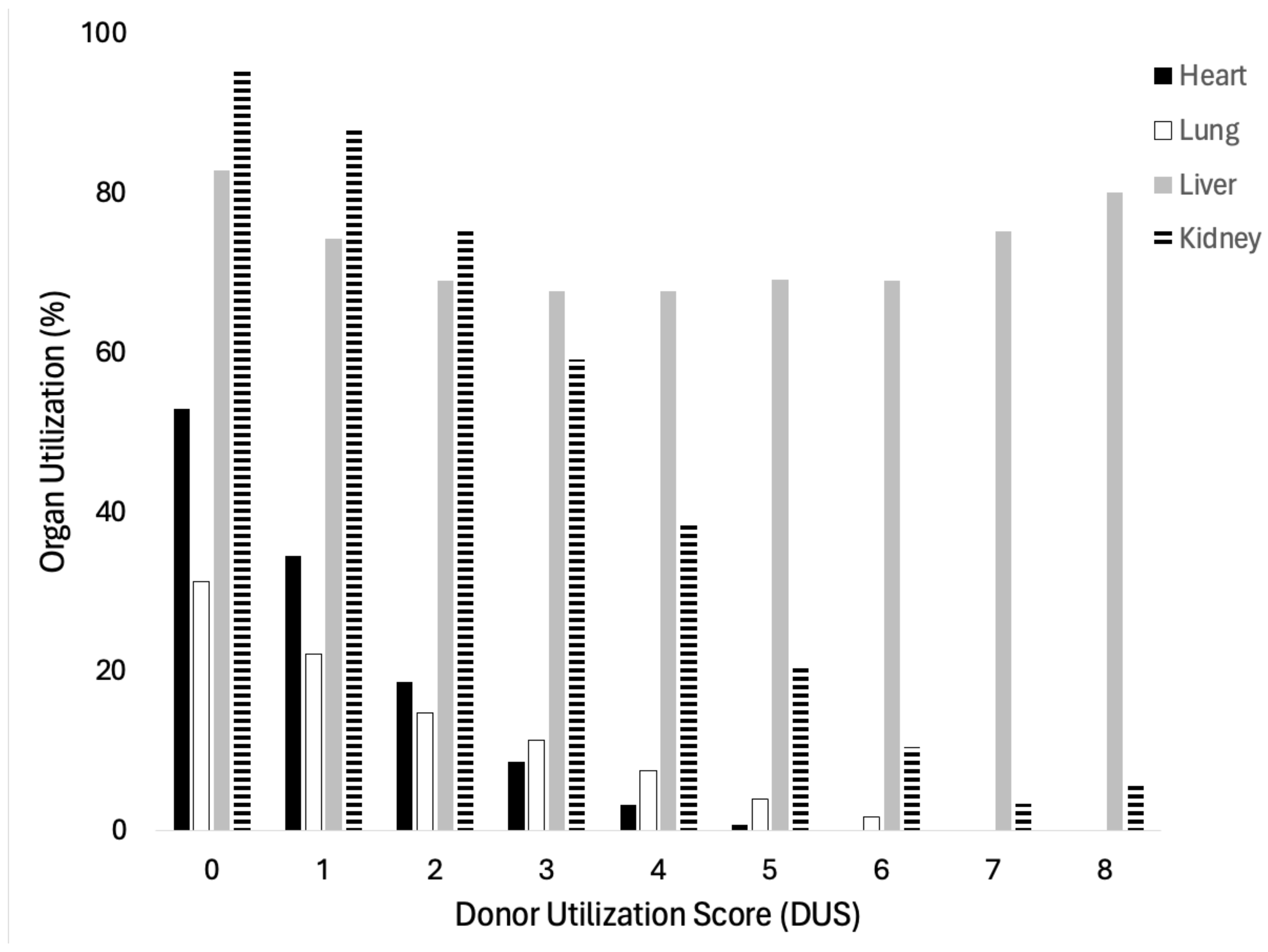
3.1. Validation of Donor Utilization Score
3.2. Population Characteristics
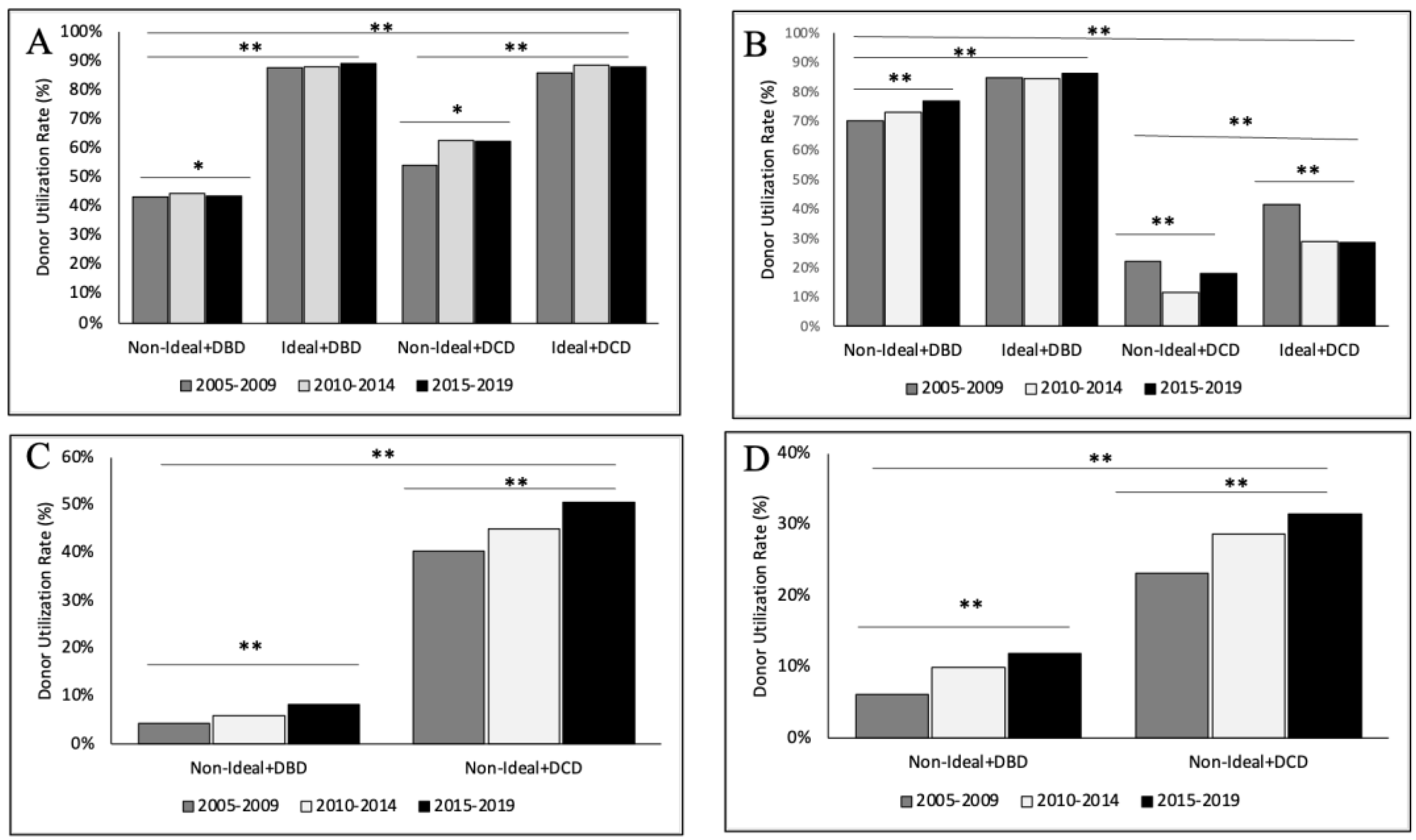
3.3. Trends in Non-Ideal Donors by Era
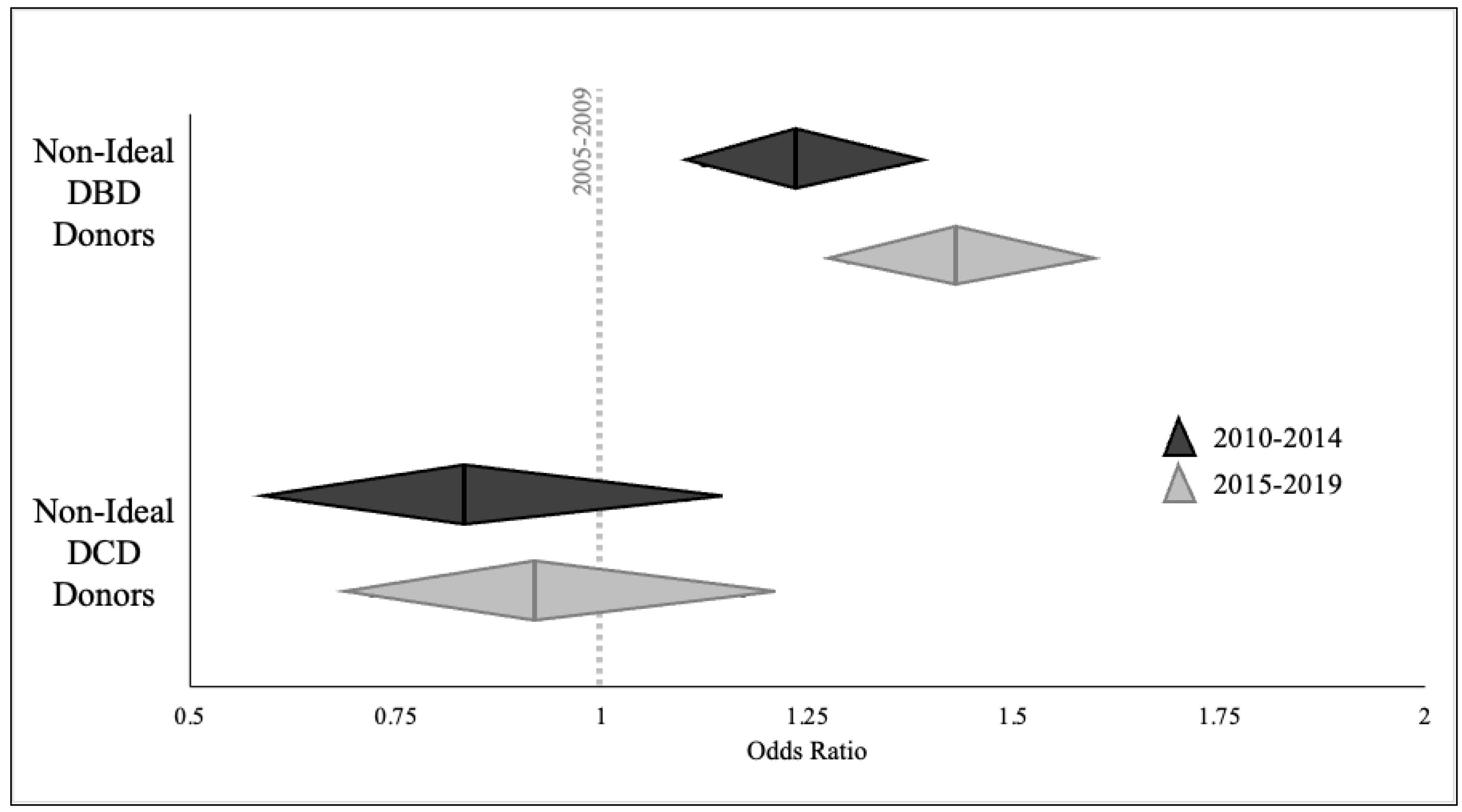
3.4. Likelihood of Non-Ideal Donor Utilization
3.5. Organ-Specific Donor Utilization—Kidney
3.6. Organ-Specific Donor Utilization—Liver
3.7. Organ-Specific Donor Utilization—Heart
3.8. Organ-Specific Donor Utilization—Lung
4. Discussion
4.1. Definitions of Marginal and Non-Ideal Donors
4.2. Organ-Specific Donor Utilization
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Metzger, R.A.; Delmonico, F.L.; Feng, S.; Port, F.K.; Wynn, J.J.; Merion, R.M. Expanded criteria donors for kidney transplantation. Am. J. Transpl. 2003, 3 (Suppl. 4), 114–125. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Schaubel, D.E.; Guidinger, M.K.; Andreoni, K.A.; Wolfe, R.A.; Merion, R.M.; Port, F.K.; Sung, R.S. A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 2009, 88, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Hsu, C.Y.; Johansen, K.L. The enlarging body of evidence: Obesity and chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 1501–1502. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Goodrich, N.P.; Bragg-Gresham, J.L.; Dykstra, D.M.; Punch, J.D.; DebRoy, M.A.; Greenstein, S.M.; Merion, R.M. Characteristics associated with liver graft failure: The concept of a donor risk index. Am. J. Transpl. 2006, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Dunson, J.; Kanwal, F.; Galvan, N.T.N.; Vierling, J.M.; O’Mahony, C.; Goss, J.A.; Rana, A. Trends in Outcomes for Marginal Allografts in Liver Transplant. JAMA Surg. 2020, 155, 926–932. [Google Scholar] [CrossRef]
- Attia, M.; Silva, M.A.; Mirza, D.F. The marginal liver donor—An update. Transpl. Int. 2008, 21, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Navidad, A.; Caballero, F. Extended criteria for organ acceptance. Strategies for achieving organ safety and for increasing organ pool. Clin. Transpl. 2003, 17, 308–324. [Google Scholar] [CrossRef]
- Velidedeoglu, E.; Desai, N.M.; Campos, L.; Olthoff, K.M.; Shaked, A.; Nunes, F.; Zeldin, G.; Stewart, C.; Blumberg, E.; Abrams, J.; et al. The outcome of liver grafts procured from hepatitis C-positive donors. Transplantation 2002, 73, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003, 9, 651–663. [Google Scholar] [CrossRef]
- Weiss, E.S.; Allen, J.G.; Kilic, A.; Russell, S.D.; Baumgartner, W.A.; Conte, J.V.; Shah, A.S. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J. Heart Lung Transpl. 2012, 31, 266–273. [Google Scholar] [CrossRef]
- Houyel, L.; Petit, J.; Nottin, R.; Duffet, J.P.; Mace, L.; Neveux, J.Y. Adult heart transplantation: Adverse role of chronic alcoholism in donors on early graft function. J. Heart Lung Transpl. 1992, 11, 1184–1187. [Google Scholar]
- Fudim, M.; Davis, M.E.; Jenkins, C.; Brown, C.L.; Wigger, M.A.; Stulak, J.M.; Maltais, S.; Haglund, N. Marginal Donor Use in Patients Undergoing Heart Transplantation With Left Ventricular Assist Device Explantation. Ann. Thorac. Surg. 2015, 100, 2117–2125, discussion 2125–2116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.E.; Blok, J.J.; Putter, H.; Adam, R.; Burroughs, A.K.; Rahmel, A.O.; Porte, R.J.; Rogiers, X.; Ringers, J.; for the European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am. J. Transpl. 2012, 12, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Feray, C.; Audureau, E.; Azoulay, D.; Antoine, C.; Daures, J.P.; Landais, P. A Donor Quality Index for liver transplantation: Development, internal and external validation. Sci. Rep. 2018, 8, 9871. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Levvey, B.J.; Whitford, H.; Griffiths, A.P.; Kotsimbos, T.; Williams, T.J.; Snell, G.I. Feasibility and utility of a lung donor score: Correlation with early post-transplant outcomes. Ann. Thorac. Surg. 2007, 83, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.M.; van der Bij, W.; Van Raemdonck, D.; de Vries, E.; Rahmel, A.; Laufer, G.; De Pauw, M.; Meiser, B. Defining an extended criteria donor lung: An empirical approach based on the Eurotransplant experience. Transpl. Int. 2011, 24, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Loor, G.; Radosevich, D.M.; Kelly, R.F.; Cich, I.; Grabowski, T.S.; Lyon, C.; Michael Morrow, J.; Bender, E.M.; Billings, J.; Hertz, M. The University of Minnesota Donor Lung Quality Index: A Consensus-Based Scoring Application Improves Donor Lung Use. Ann. Thorac. Surg. 2016, 102, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Sanchez, P.G.; Evans, C.F.; Wang, Y.; Kon, Z.N.; Rajagopal, K.; Iacono, A.T.; Gammie, J.S.; Griffith, B.P.; Pham, S.M. The use of extended criteria donors decreases one-year survival in high-risk lung recipients: A review of the United Network of Organ Sharing Database. J. Thorac. Cardiovasc. Surg. 2016, 152, 891–898.e892. [Google Scholar] [CrossRef]
- Whited, W.M.; Trivedi, J.R.; van Berkel, V.H.; Fox, M.P. Objective Donor Scoring System for Lung Transplantation. Ann. Thorac. Surg. 2019, 107, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Ehrsam, J.P.; Held, U.; Opitz, I.; Inci, I. A new lung donor score to predict short and long-term survival in lung transplantation. J. Thorac. Dis. 2020, 12, 5485–5494. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.M.; De Pauw, M.; de Vries, E.; Rahmel, A.; Meiser, B.; Laufer, G.; Zuckermann, A. Donor scoring system for heart transplantation and the impact on patient survival. J. Heart Lung Transpl. 2012, 31, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W.; Zola, J.C. Expanding the donor pool: Use of marginal donors for solid organ transplantation. Clin. Transpl. 1996, 10, 1–19. [Google Scholar]
- Gastaca, M. Extended criteria donors in liver transplantation: Adapting donor quality and recipient. Transpl. Proc. 2009, 41, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Atluri, P. Expanded donor selection criteria can increase organ utilization. J. Heart Lung Transpl. 2018, 37, 427. [Google Scholar] [CrossRef]
- Adjei, M.A.; Wisel, S.A.; Kim, I.K.; Steggerda, J.A. Drug Overdose and Cardiovascular Deaths Among Deceased Organ Donors: Implications for Donor Utilization and Data Reporting. Ann. Transpl. 2023, 28, e940255. [Google Scholar] [CrossRef] [PubMed]
- Adjei, M.; Wisel, S.A.; Yang, J.D.; Nissen, N.N.; Kim, I.K.; Steggerda, J.A. Implications of drug intoxication on donor utilization and outcomes in liver transplantation. Clin. Transpl. 2024, 38, e15276. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K. OPTN/SRTR 2020 Annual Data Report: Introduction. Am J Transpl. 2022, 22 (Suppl. 2), 11–20. [Google Scholar] [CrossRef]
- Stewart, D.E.; Garcia, V.C.; Rosendale, J.D.; Klassen, D.K.; Carrico, B.J. Diagnosing the Decades-Long Rise in the Deceased Donor Kidney Discard Rate in the United States. Transplantation 2017, 101, 575–587. [Google Scholar] [CrossRef]
- Husain, S.A.; King, K.L.; Pastan, S.; Patzer, R.E.; Cohen, D.J.; Radhakrishnan, J.; Mohan, S. Association Between Declined Offers of Deceased Donor Kidney Allograft and Outcomes in Kidney Transplant Candidates. JAMA Netw. Open 2019, 2, e1910312. [Google Scholar] [CrossRef]
- Montero, N.; Toapanta, N.; Pallares, N.; Crespo, M.; Diekmann, F.; Guirado, L.; Esteban, R.; Codina, S.; Melilli, E.; Buxeda, A.; et al. Deciphering transplant outcomes of expanded kidney allografts donated after controlled circulatory death in the current transplant era. A call for caution. Transpl. Int. 2021, 34, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am. J. Transpl. 2019, 19, 425–433. [Google Scholar] [CrossRef]
- Massie, A.B.; Luo, X.; Chow, E.K.; Alejo, J.L.; Desai, N.M.; Segev, D.L. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am. J. Transpl. 2014, 14, 2310–2316. [Google Scholar] [CrossRef]
- Steggerda, J.A.; Ladner, D.P.; Kim, I.K.; Wisel, S.A.; Borja-Cacho, D. A retrospective evaluation of changing health characteristics amongst deceased organ donors in the United States. Transpl. Proc. 2023, 55, 251–262. [Google Scholar] [CrossRef]
- Halazun, K.J.; Quillin, R.C.; Rosenblatt, R.; Bongu, A.; Griesemer, A.D.; Kato, T.; Smith, C.; Michelassi, F.; Guarrera, J.V.; Samstein, B.; et al. Expanding the Margins: High Volume Utilization of Marginal Liver Grafts Among >2000 Liver Transplants at a Single Institution. Ann. Surg. 2017, 266, 441–449. [Google Scholar] [CrossRef]
- Haugen, C.E.; Holscher, C.M.; Luo, X.; Bowring, M.G.; Orandi, B.J.; Thomas, A.G.; Garonzik-Wang, J.; Massie, A.B.; Philosophe, B.; McAdams-DeMarco, M.; et al. Assessment of Trends in Transplantation of Liver Grafts From Older Donors and Outcomes in Recipients of Liver Grafts From Older Donors, 2003–2016. JAMA Surg. 2019, 154, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; de Wilde, R.F.; Polak, W.G.; JNM, I.J. The effect of donor body mass index on graft function in liver transplantation: A systematic review. Transpl. Rev. 2020, 34, 100571. [Google Scholar] [CrossRef] [PubMed]
- Bruggenwirth, I.M.A.; van Reeven, M.; Vasiliauskaite, I.; van der Helm, D.; van Hoek, B.; Schaapherder, A.F.; Alwayn, I.P.J.; van den Berg, A.P.; de Meijer, V.E.; Darwish Murad, S.; et al. Donor diabetes mellitus is a risk factor for diminished outcome after liver transplantation: A nationwide retrospective cohort study. Transpl. Int. 2021, 34, 110–117. [Google Scholar] [CrossRef]
- Zheng, J.; Xiang, J.; Zhou, J.; Li, Z.; Hu, Z.; Lo, C.M.; Wang, W. Liver grafts for transplantation from donors with diabetes: An analysis of the Scientific Registry of Transplant Recipients database. PLoS ONE 2014, 9, e98104. [Google Scholar] [CrossRef]
- Saberi, B.; Hamilton, J.P.; Durand, C.M.; Li, Z.; Philosophe, B.; Cameron, A.M.; Sulkowski, M.S.; Gurakar, A. Utilization of hepatitis C virus RNA-positive donor liver for transplant to hepatitis C virus RNA-negative recipient. Liver Transpl. 2018, 24, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.S.; Hamilton, J.P.; Gurakar, A.; Urrunaga, N.H.; Ma, M.; Glorioso, J.; King, E.; Toman, L.P.; Wesson, R.; Garonzik-Wang, J.; et al. Hepatitis C-positive donor liver transplantation for hepatitis C seronegative recipients. Transpl. Infect. Dis. 2019, 21, e13194. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Argo, C.K.; Nguyen, D.T.; McBride, M.A.; Kumer, S.C.; Schmitt, T.M.; Pruett, T.L. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: A US National Transplant Registry analysis. Transpl. Int. 2010, 23, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Punch, J.D.; Hayes, D.H.; LaPorte, F.B.; McBride, V.; Seely, M.S. Organ donation and utilization in the United States, 1996-2005. Am J Transpl. 2007, 7, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Meers, C.; Van Raemdonck, D.; Verleden, G.M.; Coosemans, W.; Decaluwe, H.; De Leyn, P.; Nafteux, P.; Lerut, T. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transpl. Int. 2010, 23, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Zych, B.; Garcia Saez, D.; Sabashnikov, A.; De Robertis, F.; Amrani, M.; Bahrami, T.; Mohite, P.N.; Patil, N.P.; Weymann, A.; Popov, A.F.; et al. Lung transplantation from donors outside standard acceptability criteria—Are they really marginal? Transpl. Int. 2014, 27, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Bonser, R.S.; Taylor, R.; Collett, D.; Thomas, H.L.; Dark, J.H.; Neuberger, J.; On Behalf of the Cardiothoracic Advisory Group to NHS Blood and Transplant and the Association of Lung Transplant Physicians (UK). Effect of donor smoking on survival after lung transplantation: A cohort study of a prospective registry. Lancet 2012, 380, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Jayarajan, S.; Komaroff, E.; Horai, T.; Brann, S.; Cordova, F.; Criner, G.; Guy, T.S.; Toyoda, Y. Double-lung transplantation can be safely performed using donors with heavy smoking history. Ann. Thorac. Surg. 2013, 95, 1912–1917, discussion 1917–1918. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D., Jr.; Hsich, E.; Sadavarte, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report-2020; focus on deceased donor characteristics. J. Heart Lung Transpl. 2020, 39, 1003–1015. [Google Scholar] [CrossRef]
- Roig, E.; Almenar, L.; Crespo-Leiro, M.; Segovia, J.; Mirabet, S.; Delgado, J.; Perez-Villa, F.; Luis Lambert, J.; Teresa Blasco, M.; Muniz, J.; et al. Heart transplantation using allografts from older donors: Multicenter study results. J. Heart Lung Transpl. 2015, 34, 790–796. [Google Scholar] [CrossRef]
- Huckaby, L.V.; Hickey, G.; Sultan, I.; Kilic, A. Trends in the utilization of marginal donors for orthotopic heart transplantation. J. Card. Surg. 2021, 36, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Halpern, A.L.; Helmkamp, L.J.; Roberts, S.H.; Houk, A.K.; Osho, A.; Okoh, A.K.; Meguid, R.A.; Seese, L.; Weyant, M.J.; et al. Geographic and temporal patterns of growth in the utilization of donation after circulatory death donors for lung transplantation in the United States. J. Heart Lung Transpl. 2020, 39, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Ingemansson, R.; Eyjolfsson, A.; Mared, L.; Pierre, L.; Algotsson, L.; Ekmehag, B.; Gustafsson, R.; Johnsson, P.; Koul, B.; Lindstedt, S.; et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann. Thorac. Surg. 2009, 87, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Collaboration, C.T.; et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Steggerda, J.A.; Kim, I.K.; Malinoski, D.; Klein, A.S.; Bloom, M.B. Regional Variation in Utilization and Outcomes of Liver Allografts From Donors With High Body Mass Index and Graft Macrosteatosis: A Role for Liver Biopsy. Transplantation 2019, 103, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Chiles, M.C.; Patzer, R.E.; Pastan, S.O.; Husain, S.A.; Carpenter, D.J.; Dube, G.K.; Crew, R.J.; Ratner, L.E.; Cohen, D.J. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018, 94, 187–198. [Google Scholar] [CrossRef]
- Yu, K.; King, K.; Husain, S.A.; Dube, G.K.; Stevens, J.S.; Ratner, L.E.; Cooper, M.; Parikh, C.R.; Mohan, S. Kidney nonprocurement in solid organ donors in the United States. Am. J. Transpl. 2020, 20, 3413–3425. [Google Scholar] [CrossRef]

| Scoring System | Variables Evaluated | |
|---|---|---|
| Kidney | Extended Criteria Donor (ECD) [14] | Age, history of HTN, cerebrovascular accident as COD, terminal SCr >1.5 mg/dL |
| Kidney Donor Risk Index (KDRI) [2] | Age, ethnicity, SCr, HTN, DM, COD, height, DCD, weight, donor type, HCV status, HLA mismatch, CIT, en bloc or double kidney transplant | |
| Liver | Donor Risk Index (DRI) [4] | Age, COD, race, DCD, whole or split graft, height, CIT, location of organs |
| Eurotransplant-DRI [15] | Age, COD, serum GGT, DCD, whole or split graft, rescue allocation, CIT | |
| Donor Quality Index (DQI) [16] | Age, COD, ICU stay, lowest MDRD creatinine clearance, whole or split graft | |
| Lung | Oto-Donor Score [17] | Age, smoking history, CXR findings, secretions, PaO2/FiO2 |
| Eurotransplant Score [18] | Age, general and smoking history, CXR findings, bronchoscopy findings, PaO2/FiO2 ratio | |
| Minnesota-Donor-Lung Quality Index [19] | Donor age, recipient age, CIT, preexisting lung disease, smoking history, ABG values, procurement complexity, HLA matching, size mismatch, lung allocation score; risk of pneumonia, aspiration, pulmonary edema, pulmonary malignancy, donor transmitted disease, extrapulmonary malignancy and contusions | |
| Maryland-UNOS-data donor score [20] | Age, DM, smoking history, race | |
| Louisville-UNOS-data donor score [21] | Age, DM, smoking history, race | |
| Zurich Donor Score [22] | Age, smoking history, DM, significant pulmonary infection, PaO2/FiO2 | |
| Heart | Heart Donor Score [23] | Age, COD, compromised history, HTN, cardiac arrest, LVEF, valve function, LVH, coronary angiography, serum sodium, vasopressor support (e.g., norepinephrine, dopamine, dobutamine) |
| Heart Donor Risk Index [10] | CIT, age, race, blood urea nitrogen/creatinine ratio |
| Donor Utilization Score (DUS) | |
|---|---|
| Variable | Point(s) |
| Age | |
| >70 years | 3 |
| 50–69 years | 1 |
| <50 years | 0 |
| BMI > 30 | 1 |
| Diabetes | 1 |
| Hypertension | 1 |
| Prior MI | 1 |
| HCV-positive | 1 |
| Terminal SCr > 1.5 | 1 |
| DUS | Total Donors | Any Organs Donated | Multi-Organ Donor | Organs Donated (Mean ± SD) |
|---|---|---|---|---|
| 0 | 41,769 | 41,310 (98.9%) | 35,981 (86.2%) | 4.17 ± 1.66 |
| 1 | 33,980 | 32,859 (96.7%) | 25,025 (73.7%) | 3.26 ± 1.57 |
| 2 | 24,503 | 22,764 (92.9%) | 14,578 (59.5%) | 2.54 ± 1.43 |
| 3 | 16,872 | 14,966 (88.7%) | 7654 (45.4%) | 2.02 ± 1.32 |
| 4 | 9372 | 7779 (83.0%) | 2621 (28.0%) | 1.49 ± 1.15 |
| 5 | 4023 | 3118 (77.5%) | 586 (14.6%) | 1.10 ± 0.92 |
| 6 | 1062 | 784 (73.8%) | 83 (7.8%) | 0.90 ± 0.72 |
| 7 | 279 | 214 (76.7%) | 5 (1.8%) | 0.80 ± 0.50 |
| 8 | 26 | 21 (80.8%) | 0 (0.0%) | 0.81 ± 0.40 |
| Donor Characteristics | All Donors (n = 132,465) | Non-Ideal Donors (n = 32,710) | Ideal Donors (n = 99,755) | p Values |
|---|---|---|---|---|
| Age, years (median, IQR) | 42 (26–54) | 56 (51–64) | 35 (22–48) | <0.001 |
| Gender, female (n, %) | 53,475 (40.4%) | 14,484 (44.3%) | 38,991 (39.1%) | <0.001 |
| BMI, kg/m2 | 26.5 (22.8–31.1) | 31.4 (26.7–35.9) | 25.3 (22.1–29.0) | <0.001 |
| BMI ≥30 kg/m2 | 39,415 (29.8%) | 19,841 (60.7%) | 19,574 (19.6%) | <0.001 |
| Ethnicity | <0.001 | |||
| White | 88,010 (66.4%) | 20,497 (62.7%) | 67,513 (67.7%) | |
| Black | 21,767 (16.4%) | 7216 (22.1%) | 14,551 (14.6%) | |
| Hispanic | 18,474 (14.0%) | 4022 (12.3%) | 14,452 (14.5%) | |
| Asian | 3200 (2.4%) | 805 (2.5%) | 2395 (2.4%) | |
| Other | 1014 (0.8%) | 170 (0.5%) | 844 (0.9%) | |
| Blood Type | <0.001 | |||
| O | 63,511 (48.0%) | 15,941 (48.7%) | 47,570 (47.7%) | |
| A | 48,760 (36.8%) | 11,826 (36.2%) | 36,934 (37.0%) | |
| B | 15,658 (11.8%) | 3914 (12.0%) | 11,744 (11.8%) | |
| AB | 4529 (3.4%) | 1028 (3.1%) | 3501 (3.5%) | |
| DCD Donor | 19,248 (14.5%) | 3214 (9.8%) | 16,034 (16.1%) | <0.001 |
| Cause of Death | <0.001 | |||
| Anoxia | 41,477 (31.3%) | 9973 (30.5%) | 31,504 (31.6%) | |
| Trauma | 42,103 (31.8%) | 3580 (10.9%) | 38,523 (38.6%) | |
| CVA/Stroke | 44,822 (33.8%) | 18,495 (56.5%) | 26,327 (26.4%) | |
| Other | 4063 (3.1%) | 662 (2.0%) | 3401 (3.4%) | |
| Hx of Hypertension | 46,501 (35.3%) | 28,704 (87.8%) | 17,797 (17.8%) | <0.001 |
| Hx of Diabetes | 15,558 (11.8%) | 13,141 (40.2%) | 2417 (2.4%) | <0.001 |
| Prior MI | 5162 (3.9%) | 4198 (12.8%) | 964 (1.0%) | <0.001 |
| HCV-Positive | 7463 (5.6%) | 2804 (8.6%) | 4659 (4.7%) | <0.001 |
| Heavy Alcohol Use | 22,943 (17.3%) | 5482 (16.8%) | 17,461 (17.5%) | 0.002 |
| Cigarette Smoker a | 31,687 (23.9%) | 11,242 (34.4%) | 20,445 (20.5%) | <0.001 |
| Any Drug Use | 52,686 (39.8%) | 9578 (29.3%) | 43,106 (43.2%) | <0.001 |
| Serum Creatinine | 1.0 (0.7–1.6) | 1.7 (1.0–3.3) | 1.0 (0.7–1.6) | <0.001 |
| Serum AST | 47 (28–91) | 41 (26–81) | 49 (29–95) | <0.001 |
| Serum ALT | 38 (22–76) | 33 (20–62) | 39 (23–80) | <0.001 |
| Total Bilirubin | 0.7 (0.4–1.1) | 0.7 (0.4–1.1) | 0.7 (0.4–1.1) | <0.001 |
| INR | 1.3 (1.1–1.4) | 1.3 (1.1–1.4) | 1.3 (1.1–1.4) | 0.071 |
| Serum Sodium | 147 (142–153) | 147 (142–153) | 147 (141–153) | 0.101 |
| 2005–2009 (n = 8562) | 2010–2014 (n = 8398) | 2015–2019 (10,894) | p-Values | |
|---|---|---|---|---|
| Organ Donor (n, %) | 8093 (83.0%) | 8367 (85.8%) | 11,383 (86.2%) | <0.001 |
| Multi-Organ Donor | 3185 (32.7%) | 3441 (35.3%) | 4827 (36.6%) | <0.001 |
| Organs per Donor (median, IQR) | 1 (1–3) | 1 (1–3) | 1 (1–3) | <0.001 |
| Kidneys | <0.001 | |||
| Transplanted | 4302 (44.1%) | 4482 (46.0%) | 6104 (46.2%) | |
| Recovered—Not Transplanted | 2933 (30.1%) | 2858 (29.3%) | 4183 (31.7%) | |
| Not Recovered | 2516 (25.8%) | 2415 (24.8%) | 2917 (22.1%) | |
| Liver | <0.001 | |||
| Transplanted | 6517 (66.8%) | 6657 (68.2%) | 9059 (69.8%) | |
| Recovered—Not Transplanted | 1548 (15.9%) | 1302 (13.4%) | 1434 (18.9%) | |
| Not Recovered | 1686 (17.3%) | 1796 (18.4%) | 12,711 (20.5%) | |
| Heart | 0.006 | |||
| Transplanted | 390 (4.0%) | 523 (5.4%) | 936 (7.1%) | |
| Recovered—Not Transplanted | 11 (0.1%) | 7 (0.1%) | 15 (0.1%) | |
| Not Recovered | 9350 (95.9%) | 9225 (94.6%) | 12,253 (92.8%) | |
| Lungs | <0.001 | |||
| Transplanted | 569 (5.8%) | 902 (9.3%) | 1392 (10.5%) | |
| Recovered—Not Transplanted | 11 (0.1%) | 33 (0.3%) | 101 (0.8%) | |
| Not Recovered | 9171 (94.1%) | 8820 (90.4%) | 11,711 (88.7%) |
| DBD Donors | DCD Donors | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Kidney | ||||||
| 2005–2009 | Reference | 0.002 | Reference | 0.61 | ||
| 2010–2014 | 0.882 | 0.822–0.946 | 1.150 | 0.896–1.475 | ||
| 2015–2019 | 0.938 | 0.876–1.004 | 1.176 | 0.949–1.458 | ||
| Liver | ||||||
| 2005–2009 | Reference | <0.001 | Reference | <0.001 | ||
| 2010–2014 | 1.211 | 1.131–1.297 | 0.441 | 0.319–0.609 | ||
| 2015–2019 | 1.511 | 1.411–1.618 | 0.752 | 0.583–0.968 | ||
| Heart | ||||||
| 2005–2009 | Reference | <0.001 | ||||
| 2010–2014 | 1.143 | 0.987–1.324 | ||||
| 2015–2019 | 1.623 | 1.415–1.862 | ||||
| Lung | ||||||
| 2005–2009 | Reference | <0.001 | ||||
| 2010–2014 | 1.615 | 1.436–1.818 | ||||
| 2015–2019 | 2.251 | 2.011–2.520 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisel, S.A.; Borja-Cacho, D.; Megna, D.; Adjei, M.; Kim, I.K.; Steggerda, J.A. Organ Utilization Rates from Non-Ideal Donors for Solid Organ Transplant in the United States. J. Clin. Med. 2024, 13, 3271. https://doi.org/10.3390/jcm13113271
Wisel SA, Borja-Cacho D, Megna D, Adjei M, Kim IK, Steggerda JA. Organ Utilization Rates from Non-Ideal Donors for Solid Organ Transplant in the United States. Journal of Clinical Medicine. 2024; 13(11):3271. https://doi.org/10.3390/jcm13113271
Chicago/Turabian StyleWisel, Steven A., Daniel Borja-Cacho, Dominick Megna, Michie Adjei, Irene K. Kim, and Justin A. Steggerda. 2024. "Organ Utilization Rates from Non-Ideal Donors for Solid Organ Transplant in the United States" Journal of Clinical Medicine 13, no. 11: 3271. https://doi.org/10.3390/jcm13113271
APA StyleWisel, S. A., Borja-Cacho, D., Megna, D., Adjei, M., Kim, I. K., & Steggerda, J. A. (2024). Organ Utilization Rates from Non-Ideal Donors for Solid Organ Transplant in the United States. Journal of Clinical Medicine, 13(11), 3271. https://doi.org/10.3390/jcm13113271






