Three-Dimensional Printing in Breast Reconstruction: Current and Promising Applications
Abstract
1. Introduction
2. Current Technologies of 3D Bioprinting
3. Preoperative Applications
3.1. Surgical Planning
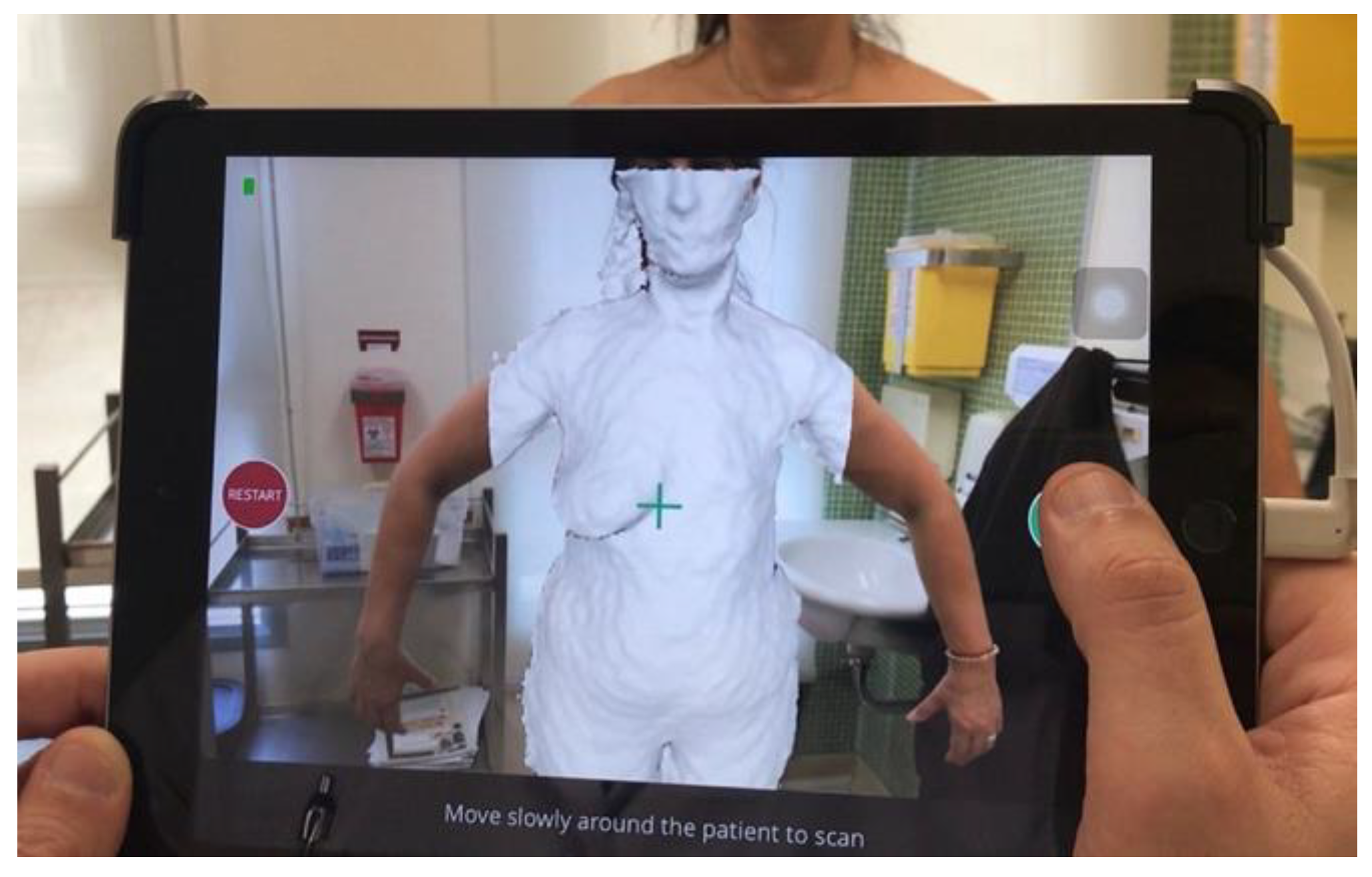
3.2. Three-Dimensional Breast Volume Measurement
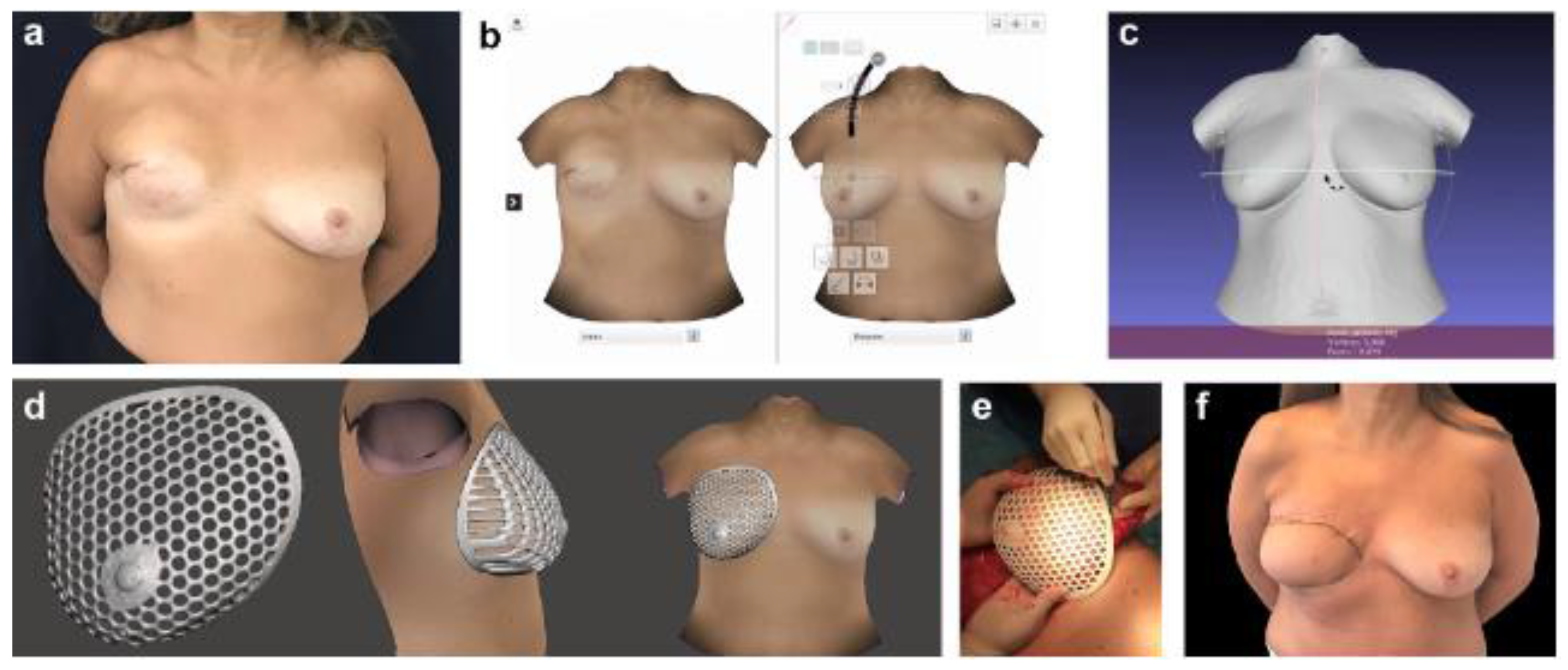
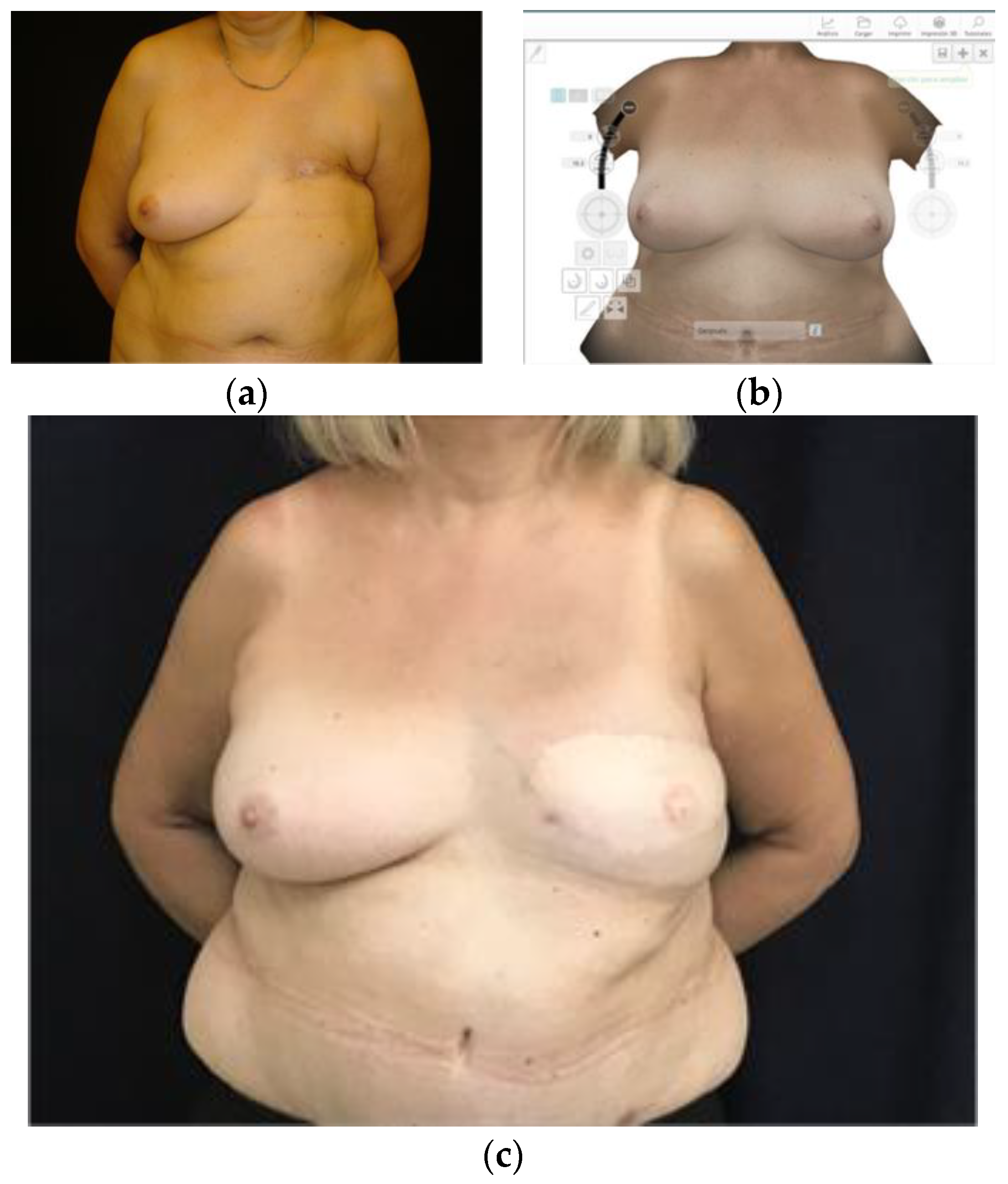
3.3. Planning Prosthetic Reconstruction
3.4. Planning Autologous Reconstruction
4. Intraoperative Applications
4.1. Implants Customization
4.2. Flap Modeling with Scaffolds
4.3. Tissue Engineering Based on 3D Printed Scaffolds
4.3.1. Features of the Scaffold Structure
4.3.2. Scaffolding Biomaterials
5. Postoperative Applications
Objective Assessment of Outcome
6. Three-Dimensional Printing as an Educational Tool
7. Current Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Plasticsurgery.org. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2022/plastic-surgery-statistics-report-2022.pdf (accessed on 21 February 2024).
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef]
- Miseré, R.M.L.; Joosen, M.E.M.; Claassens, E.L.; de Grzymala, A.A.P.; Heuts, E.M.; van der Hulst, R.R.W.J. Patient-reported outcomes following bilateral prophylactic mastectomy and immediate breast reconstruction: Comparing implant-based with autologous breast reconstruction. Eur. J. Plast. Surg. 2022, 45, 763–769. [Google Scholar] [CrossRef]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Women’s Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. Implant-Based Breast Reconstruction: Hot Topics, Controversies, and New Directions. Plast. Reconstr. Surg. 2019, 143, 404e–416e. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.M.; Martins, P. A review of bioengineering techniques applied to breast tissue: Mechanical properties, tissue engineering and finite element analysis. Front. Bioeng. Biotechnol. 2023, 11, 1161815. [Google Scholar] [CrossRef]
- Mu, X.; Zhang, J.; Jiang, Y. 3D Printing in Breast Reconstruction: From Bench to Bed. Front. Surg. 2021, 8, 641370. [Google Scholar] [CrossRef]
- Crook, J.M. (Ed.) 3D Bioprinting: Principles and Protocols; Springer: Greer, SC, USA, 2020; Volume 2140. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Di Rosa, L. (Ed.) 3D Printing in Plastic Reconstructive and Aesthetic Surgery: A Guide for Clinical Practice; Springer International Publishing: Greer, SC, USA, 2022; pp. 1–13. [Google Scholar] [CrossRef]
- Alawi, S.A.; Matschke, J.; Muallah, D.; Gelinksy, M.; Dragu, A. 3D bioprinting in plastic and reconstructive surgery: Current concepts, progress, and clinical application. Eur. J. Plast. Surg. 2023, 46, 833–843. [Google Scholar] [CrossRef]
- O’Connell, R.L.; Stevens, R.J.G.; Harris, P.A.; Rusby, J.E. Review of three-dimensional (3D) surface imaging for oncoplastic, reconstructive and aesthetic breast surgery. Breast 2015, 24, 331–342. [Google Scholar] [CrossRef]
- Kovacs, L.; Eder, M.; Hollweck, R.; Zimmermann, A.; Settles, M.; Schneider, A.; Endlich, M.; Mueller, A.; Schwenzer-Zimmerer, K.; Papadopulos, N.A.; et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast 2007, 16, 137–145. [Google Scholar] [CrossRef]
- Ahcan, U.; Bracun, D.; Zivec, K.; Pavlic, R.; Butala, P. The use of 3D laser imaging and a new breast replica cast as a method to optimize autologous breast reconstruction after mastectomy. Breast 2012, 21, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Yano, K.; Hata, Y.; Nishibayashi, A.; Hosokawa, K. DIEP Flap Breast Reconstruction Using 3-dimensional Surface Imaging and a Printed Mold. Plast. Reconstr. Surg.—Glob. Open 2015, 3, e316. [Google Scholar] [CrossRef] [PubMed]
- Hummelink, S.; Verhulst, A.C.; Maal, T.J.J.; Ulrich, D.J.O. Applications and limitations of using patient-specific 3D printed molds in autologous breast reconstruction. Eur. J. Plast. Surg. 2018, 41, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Wesselius, T.S.; Verhulst, A.C.; Xi, T.; Ulrich, D.J.O.; Maal, T.J.J. Effect of skin tone on the accuracy of hybrid and passive stereophotogrammetry. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Ciechomski, P.d.H.; Constantinescu, M.; Garcia, J.; Olariu, R.; Dindoyal, I.; Le Huu, S.; Reyes, M. Development and implementation of a web-enabled 3D consultation tool for breast augmentation surgery based on 3D-image reconstruction of 2D pictures. J. Med. Internet Res. 2012, 14, e21. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.F. The Use of a 3D Simulator Software and 3D Printed Biomodels to Aid Autologous Breast Reconstruction. Aesthetic Plast. Surg. 2020, 44, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-Dimensional Printing in Plastic and Reconstructive Surgery: A Systematic Review. Ann. Plast. Surg. 2016, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Cevik, J.; Seth, I.; Hunter-Smith, D.J.; Rozen, W.M. A History of Innovation: Tracing the Evolution of Imaging Modalities for the Preoperative Planning of Microsurgical Breast Reconstruction. J. Clin. Med. 2023, 12, 5246. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Assessment and planning for oncoplastic and reconstructive breast surgery: A review and a proposed chart. Eur. J. Plast. Surg. 2016, 39, 321–330. [Google Scholar] [CrossRef]
- Hammond, D.C.; Kim, K.; Bageris, M.H.; Chaudhry, A. Use of Three-Dimensional Imaging to Assess the Effectiveness of Volume as a Critical Variable in Breast Implant Selection. Plast. Reconstr. Surg. 2022, 149, 70–79. [Google Scholar] [CrossRef]
- Gouveia, P.F.; Oliveira, H.P.; Monteiro, J.P.; Teixeira, J.F.; Silva, N.L.; Pinto, D.; Mavioso, C.; Anacleto, J.; Martinho, M.; Duarte, I.; et al. 3D Breast Volume Estimation. Eur. Surg. Res. 2021, 63, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Markovic, A.; Pessoa SG de, P.; Leite, J.A.D.; de Alcântara, F.S.; Collaço, B.G.; Ariel de Lima, D. Assessment of Three Breast Volume Measurement Techniques: Single Marking, MRI and Crisalix 3D Software®. Aesthetic Plast. Surg. 2023, 47, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Koban, K.C.; Etzel, L.; Li, Z.; Pazos, M.; Schönecker, S.; Belka, C.; Giunta, R.E.; Schenck, T.L.; Corradini, S. Three-dimensional surface imaging in breast cancer: A new tool for clinical studies? Radiat. Oncol. 2020, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Y.; Lin, X.-Y.; Wang, Y.; Zhuang, Z.-M.; Zhong, X.-C.; Zhang, T.; Li, Y.; Tan, W.-Q. Three-dimensional scanning for breast plastic and reconstructive surgery: An updated review. Eur. J. Plast. Surg. 2024, 47, 15. [Google Scholar] [CrossRef]
- Koban, K.C.; Härtnagl, F.; Titze, V.; Schenck, T.L.; Giunta, R.E. Chances and limitations of a low-cost mobile 3D scanner for breast imaging in comparison to an established 3D photogrammetric system. J. Plast. Reconstr. Aesthetic. Surg. 2018, 71, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.S.; Plotsker, E.L.B.; Rubenstein, R.; Mehrara, E.; Haglich, K.; Zoghbi, Y.; Mehrara, B.J.; Nelson, J.A. Three-Dimensional Surface Analysis for Preoperative Prediction of Breast Volume: A Validation Study. Plast. Reconstr. Surg. 2023, 152, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Colwell, A.S.; Taylor, E.M. Recent Advances in Implant-Based Breast Reconstruction. Plast. Reconstr. Surg. 2020, 145, 421e–432e. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, A.; Maxwell, G.P. Implant selection in the setting of prepectoral breast reconstruction. Gland Surg. 2019, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Chou, Y.Y.; Liu, H.H.; Dai, N.T.; Tzeng, Y.S.; Chen, S.G. Is 3-Dimensional Scanning Really Helpful in Implant-Based Breast Reconstruction?: A Prospective Study. Ann. Plast. Surg. 2022, 88 (1s Suppl. 1), S85–S91. [Google Scholar] [CrossRef]
- Tebbetts, J.B. A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics. Plast. Reconstr. Surg. 2002, 109, 1396–1409; discussion 1410–1415. [Google Scholar] [CrossRef]
- Hudson, D.A. Factors determining shape and symmetry in immediate breast reconstruction. Ann. Plast. Surg. 2004, 52, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Westreich, M. Anthropomorphic breast measurement: Protocol and results in 50 women with aesthetically perfect breasts and clinical application. Plast. Reconstr. Surg. 1997, 100, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, L.J.; Kirkland, S.A. Breast volume determination in breast hypertrophy: An accurate method using two anthropomorphic measurements. Plast. Reconstr. Surg. 2006, 118, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Kusano, T.; Sato, N.; Yoshimoto, S. Estimating Implant Volume and Mastectomy-Specimen Volume by Measuring Breast Volume With a 3-Dimensional Scanner. Ann. Plast. Surg. 2017, 79, 79–81. [Google Scholar] [CrossRef]
- Pöhlmann, S.T.L.; Harkness, E.; Taylor, C.J.; Gandhi, A.; Astley, S.M. Preoperative implant selection for unilateral breast reconstruction using 3D imaging with the Microsoft Kinect sensor. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1059–1067. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.W.; Woo, K.J. Prediction of the Ideal Implant Size Using 3-Dimensional Healthy Breast Volume in Unilateral Direct-to-Implant Breast Reconstruction. Medicina 2020, 56, 498. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Mahoney, M.H.; Soon, G.; Pinchuk, B.; Somogyi, R. Predictive value of 3D imaging to guide implant selection in immediate breast reconstruction. J. Plast. Reconstr. Aesthetic. Open 2022, 31, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Karp, N.S.; Small, K.; Unger, J.; Rudolph, L.; Pritchard, A.; Choi, M. Three-dimensional imaging provides valuable clinical data to aid in unilateral tissue expander-implant breast reconstruction. Breast J. 2008, 14, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Szychta, P.; Raine, C.; Butterworth, M.; Stewart, K.; Witmanowski, H.; Zadrozny, M.; Rykala, J. Preoperative implant selection for two stage breast reconstruction with 3D imaging. Comput. Biol. Med. 2014, 44, 136–143. [Google Scholar] [CrossRef]
- Ma, J.X.; Xia, Y.C.; Li, B.; Zhao, H.M.; Lei, Y.T. Unilateral Tissue Expander/Implant Two-Stage Breast Reconstruction with the Assistance of Three-Dimensional Surface Imaging. Aesthetic Plast. Surg. 2020, 44, 60–69. [Google Scholar] [CrossRef]
- Tepper, O.M.; Small, K.; Rudolph, L.; Choi, M.; Karp, N. Virtual 3-dimensional modeling as a valuable adjunct to aesthetic and reconstructive breast surgery. Am. J. Surg. 2006, 192, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Santanelli, F.; Longo, B.; Cagli, B.; Pugliese, P.; Sorotos, M.; Paolini, G. Predictive and protective factors for partial necrosis in DIEP flap breast reconstruction: Does nulliparity bias flap viability? Ann. Plast. Surg. 2015, 74, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sorotos, M.; Firmani, G.; Schiavone, L.; Ricci, A.; Santanelli di Pompeo, F. Effects of DIEP flap-based breast reconstruction on respiratory function. J. Plast. Reconstr. Aesthetic Surg. 2023, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yueh, J.H.; Slavin, S.A.; Adesiyun, T.; Nyame, T.T.; Gautam, S.; Morris, D.J.; Tobias, A.M.; Lee, B.T. Patient satisfaction in postmastectomy breast reconstruction: A comparative evaluation of DIEP, TRAM, latissimus flap, and implant techniques. Plast. Reconstr. Surg. 2010, 125, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Toyserkani, N.M.; Jørgensen, M.G.; Tabatabaeifar, S.; Damsgaard, T.; Sørensen, J.A. Autologous versus implant-based breast reconstruction: A systematic review and meta-analysis of Breast-Q patient-reported outcomes. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y.; Momen, B.; Galdino, G.; Manson, P.N. Breast Reconstruction with the free TRAM or DIEP flap: Patient selection, choice of flap, and outcome. Plast. Reconstr. Surg. 2002, 110, 466–475; discussion 476–477. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.; Colakoglu, S.; Bhoopalam, M.; Ibrahim, A.; Mathes, D.; Kaoutzanis, C.; Mureau, M.; Reddy, S. Perforator Characteristics and Impact on Postoperative Outcomes in DIEP Flap Breast Reconstruction: A Systematic Review and Meta-Analysis. J. Reconstr. Microsurg. 2023, 39, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Rozen, W.M.; Anavekar, N.S.; Ashton, M.W.; Stella, D.L.; Grinsell, D.; Bloom, R.J.; Taylor, G.I. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008, 28, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Schaverien, M.V.; Ludman, C.N.; Neil-Dwyer, J.; Perks, G.B.; Akhtar, N.; Rodrigues, J.N.; Benetatos, K.; Raurell, A.; Rasheed, T.; McCulley, S.J. Contrast-enhanced magnetic resonance angiography for preoperative imaging in DIEP flap breast reconstruction. Plast. Reconstr. Surg. 2011, 128, 56–62. [Google Scholar] [CrossRef]
- Chae, M.P.; Hunter-Smith, D.J.; Rozen, W.M. Comparative analysis of fluorescent angiography, computed tomographic angiography and magnetic resonance angiography for planning autologous breast reconstruction. Gland Surg. 2015, 4, 164–178. [Google Scholar] [CrossRef]
- DeFazio, M.V.; Arribas, E.M.; Ahmad, F.I.; Le-Petross, H.T.; Liu, J.; Chu, C.K.; Santiago, L.; Clemens, M.W. Application of Three-Dimensional Printed Vascular Modeling as a Perioperative Guide to Perforator Mapping and Pedicle Dissection during Abdominal Flap Harvest for Breast Reconstruction. J. Reconstr. Microsurg. 2020, 36, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Sotsuka, Y.; Matsuda, K.; Fujita, K.; Fujiwara, T.; Kakibuchi, M. A Perforator Model as an Aid to Elevate Deep Inferior Epigastric Perforator Flap. Plast. Reconstr. Surg. Glob. Open 2015, 3, e462. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Byrne, N.; Karunanithy, N.; Farhadi, J. 3D printing provides unrivalled bespoke teaching tools for autologous free flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.P.; Hunter-Smith, D.J.; Rostek, M.; Smith, J.A.; Rozen, W.M. Enhanced Preoperative Deep Inferior Epigastric Artery Perforator Flap Planning with a 3D-Printed Perforasome Template: Technique and Case Report. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1644. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.M.; Wu, R.T.; Mittermiller, P.A.; Gifford, K.; Momeni, A. 3-DIEPrinting: 3D-printed Models to Assist the Intramuscular Dissection in Abdominally Based Microsurgical Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2222. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.A.; Deptula, P.L.; Inchauste, S.M.; Zelones, J.T.; Walters, S.; Gifford, K.; LeCastillo, C.; Napel, S.; Fleischmann, D.; Nguyen, D.H. The utility of three-dimensional models in complex microsurgical reconstruction. Arch. Plast. Surg. 2020, 47, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Kaise, H.; Ojima, Y.; Ayabe, N.; Kato, D.; Kojima, M.; Ishikawa, T.; Matsumura, H. Volume Prediction of Extended Latissimus Dorsi Musculocutaneous Flap for Breast Reconstruction Using a Computed Tomography Volume-Rendering Technique with an X-ray Contrast Thread Marking. Aesthetic Plast. Surg. 2023, 47, 1335–1342. [Google Scholar] [CrossRef]
- Sbitany, H. Breast Reconstruction. Surg. Clin. N. Am. 2018, 98, 845–857. [Google Scholar] [CrossRef]
- Gerstle, T.L.; Ibrahim, A.M.S.; Kim, P.S.; Lee, B.T.; Lin, S.J. A plastic surgery application in evolution: Three-dimensional printing. Plast. Reconstr. Surg. 2014, 133, 446–451. [Google Scholar] [CrossRef]
- Cleversey, C.; Robinson, M.; Willerth, S.M. 3D Printing Breast Tissue Models: A Review of Past Work and Directions for Future Work. Micromachines 2019, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Lim, T.H.; Choi, H.S.; Kim, M.S.; Lee, S.J.; Kim, S.H.; Park, C.H. 3D Printing and NIR Fluorescence Imaging Techniques for the Fabrication of Implants. Materials 2020, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Chavoin, J.-P.; Grolleau, J.-L.; Moreno, B.; Brunello, J.; André, A.; Dahan, M.; Garrido, I.; Chaput, B. Correction of Pectus Excavatum by Custom-Made Silicone Implants: Contribution of Computer-Aided Design Reconstruction. A 20-Year Experience and 401 Cases. Plast. Reconstr. Surg. 2016, 137, 860e–871e. [Google Scholar] [CrossRef] [PubMed]
- Chavoin, J.; Facchini, F.; Martinot-Duquennoy, V.; Duteille, F.; Herlin, C.; Le Pimpec-Barthes, F.; Assouad, J.; Chevallier, B.; Tiffet, O.; Brouchet, L.; et al. [Congenital thoracic deformities and 3D custom-made implants. New classification based on a series of 789 treated cases]. Ann. Chir. Plast. Esthétique 2022, 67, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chavoin, J.P.; Taizou, M.; Moreno, B.; Leyx, P.; Grolleau, J.L.; Chaput, B. Correcting Poland Syndrome with a Custom-Made Silicone Implant: Contribution of Three-Dimensional Computer-Aided Design Reconstruction. Plast. Reconstr. Surg. 2018, 142, 109e–119e. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Q.; Huang, M.; Zhang, M.; Meng, H.-M.; Hou, L. Computer-Assisted 3-Dimensional Printing Technology for Immediate Breast Reconstruction after Breast-Conserving Surgery. Chin. J. Breast Dis. 2018, 12, 12–16. [Google Scholar] [CrossRef]
- Chen, K.; Feng, C.-J.; Ma, H.; Hsiao, F.-Y.; Tseng, L.-M.; Tsai, Y.-F.; Lin, Y.-S.; Huang, L.-Y.; Yu, W.-C.; Perng, C.-K. Preoperative breast volume evaluation of one-stage immediate breast reconstruction using three-dimensional surface imaging and a printed mold. J. Chin. Med. Assoc. 2019, 82, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Yano, K.; Taminato, M.; Nomori, M.; Hosokawa, K. DIEP Flap Breast Reconstruction in Patients with Breast Ptosis: 2-Stage Reconstruction Using 3-Dimensional Surface Imaging and a Printed Mold. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1511. [Google Scholar] [CrossRef] [PubMed]
- Gelati, C.; Lozano Miralles, M.E.; Morselli, P.G.; Fabbri, E.; Cipriani, R. Deep Inferior Epigastric Perforator Breast Reconstruction With Computer-Aided Design/Computer-Aided Manufacturing Sizers. Ann. Plast. Surg. 2020, 84, 24–29. [Google Scholar] [CrossRef]
- Erdim, M.; Tezel, E.; Numanoglu, A.; Sav, A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: An experimental study. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1210–1214. [Google Scholar] [CrossRef]
- Al Sufyani, M.A.; Al Hargan, A.H.; Al Shammari, N.A.; Al Sufyani, M.A. Autologous Fat Transfer for Breast Augmentation: A Review. Dermatol. Surg. 2016, 42, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P. Breast Silicone Gel Implants versus Autologous Fat Grafting: Biomaterials and Bioactive Materials in Comparison. J. Clin. Med. 2021, 10, 3310. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P. Tuberous Breast, Deformities, and Asymmetries: A Retrospective Analysis Comparing Fat Grafting Versus Mastopexy and Breast Implants. Aesthetic Plast. Surg. 2023, 47, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Janzekovic, J.; Wagels, M.; Hutmacher, D.W. Breast reconstruction using scaffold-based tissue engineering. In Breast Reconstruction: Modern and Promising Surgical Techniques; Chapter 29; Mayer, H.F., Ed.; Springer: Cham, Switzerland, 2020; pp. 279–290. [Google Scholar] [CrossRef]
- Cheng, M.; Heald, A.; Wagels, M.; Ung, O.; Hutmacher, D. Scaffold-guide breast tissue engineering: The future of breast implants. Australas. J. Plast. Surg. 2023, 6, 71282. [Google Scholar] [CrossRef]
- Chae, M.P.; Hunter-Smith, D.J.; Murphy, S.V.; Findlay, M.W. 15-3D bioprinting adipose tissue for breast reconstruction. In 3D Bioprinting for Reconstructive Surgery; Thomas, D.J., Jessop, Z.M., Whitaker, I.S., Eds.; Woodhead Publishing: Southton, UK, 2018; pp. 305–353. [Google Scholar] [CrossRef]
- Donnely, E.; Griffin, M.; Butler, P.E. Breast Reconstruction with a Tissue Engineering and Regenerative Medicine Approach (Systematic Review). Ann. Biomed. Eng. 2020, 48, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Omidi, E.; Fuetterer, L.; Reza Mousavi, S.; Armstrong, R.C.; Flynn, L.E.; Samani, A. Characterization and assessment of hyperelastic and elastic properties of decellularized human adipose tissues. J. Biomech. 2014, 47, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, N.; Potter, S.; Kerin, M.; Lowery, A. Recent Advances and Future Directions in Postmastectomy Breast Reconstruction. Clin. Breast Cancer 2018, 18, e571–e585. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Chhaya, M.P.; Balmayor, E.R.; Hutmacher, D.W.; Schantz, J.T. Transformation of Breast Reconstruction via Additive Biomanufacturing. Sci. Rep. 2016, 6, 28030. [Google Scholar] [CrossRef]
- Mohseni, M.; Bas, O.; Castro, N.; Schmutz, B.; Hutmacher, D. Additive Biomanufacturing of Scaffolds for Breast Reconstruction. Addit. Manuf. 2019, 30, 100845. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Arevalo, M.d.P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Visscher, L.E.; Cheng, M.; Chhaya, M.; Hintz, M.L.; Schantz, J.-T.; Tran, P.; Ung, O.; Wong, C.; Hutmacher, D.W. Breast Augmentation and Reconstruction from a Regenerative Medicine Point of View: State of the Art and Future Perspectives. Tissue Eng. Part B Rev. 2017, 23, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Quanta Medical. First-in-Human Study of MATTISSE® Tissue Engineering Chamber in Adult Female after Total Mastectomy for Breast Cancer in Immediate or Delayed 2-Stage Tissue Expander Reconstruction or Conversion From Implant-Based to Autologous Reconstruction; Quanta Medical: Strasbourg, France, 2024. Available online: https://clinicaltrials.gov/study/NCT05460780 (accessed on 31 December 2023).
- 2023_05_09_BellaSeno-Trial-Results.pdf. 2024. Available online: https://www.bellaseno.com/wp-content/uploads/2023/05/2023_05_09_BellaSeno-Trial-Results.pdf (accessed on 2 March 2024).
- Isogai, N.; Sai, K.; Kamiishi, H.; Watatani, M.; Inui, H.; Shiozaki, H. Quantitative analysis of the reconstructed breast using a 3-dimensional laser light scanner. Ann. Plast. Surg. 2006, 56, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Losken, A.; Seify, H.; Denson, D.D.; Paredes, A.A.; Carlson, G.W. Validating three-dimensional imaging of the breast. Ann. Plast. Surg. 2005, 54, 471–476; discussion 477–478. [Google Scholar] [CrossRef] [PubMed]
- Kraft, C.T.; Rendon, J.L.; Koutz, C.A.; Miller, M.J. Novel 3-Dimensional Imaging Analysis of the Ryan Procedure for Inframammary Fold Elevation in the Reconstruction of the Revised Breast. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2287. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Weiherer, M.; Schiltz, D.; Seitz, S.; Lotter, L.; Anker, A.; Palm, C.; Prantl, L.; Brébant, V. A Novel Method of Outcome Assessment in Breast Reconstruction Surgery: Comparison of Autologous and Alloplastic Techniques Using Three-Dimensional Surface Imaging. Aesthetic Plast. Surg. 2020, 44, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Henseler, H.; Smith, J.; Bowman, A.; Khambay, B.S.; Ju, X.; Ayoub, A.; Ray, A.K. Objective evaluation of the latissimus dorsi flap for breast reconstruction using three-dimensional imaging. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Wilting, F.N.H.; Hameeteman, M.; Tielemans, H.J.P.; Ulrich, D.J.O.; Hummelink, S. Three-dimensional evaluation of breast volume changes following autologous free flap breast reconstruction over six months. Breast 2020, 50, 85–94. [Google Scholar] [CrossRef]
- Bai, L.; Sandelin, K.; Wickman, M.; Arver, B.; Lundström, O.M.P.; Johansson, H.S.; Brandberg, Y.P. Patient-reported Outcomes and 3-dimensional Surface Imaging after Risk-reducing Mastectomy and Immediate Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3561. [Google Scholar] [CrossRef]
- Papavasiliou, T.; Ubong, S.; Khajuria, A.; Chatzimichail, S.; Chan, J.C.Y. 3D Printed Chest Wall: A Tool for Advanced Microsurgical Training Simulating Depth and Limited View. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3817. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Lau, J.H.; Lee, J.; Yen, C.C. A First Reported Adjustable Breast Volume Simulator for Teaching Oncoplastic Surgery Marking: Adjustable Breast Oncoplastic Surgery Simulator. Surg. Innov. 2022, 29, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Sokol, E.S.; Miller, D.H.; Breggia, A.; Spencer, K.C.; Arendt, L.M.; Gupta, P.B. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Moroni, S.; Casettari, L.; Lamprou, D.A. 3D and 4D Printing in the Fight against Breast Cancer. Biosensors 2022, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.P.; Shabab, T.; Shafiee, A.; Peiffer, Q.C.; Fox, K.; Tran, N.; Dargaville, T.R.; Hutmacher, D.W.; Tran, P.A. 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Biofabrication 2019, 11, 035014. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Regondi, S. Artificial Intelligence-Empowered 3D and 4D Printing Technologies toward Smarter Biomedical Materials and Approaches. Polymers 2022, 14, 2794. [Google Scholar] [CrossRef]
- Seth, I.; Bulloch, G.; Joseph, K.; Hunter-Smith, D.J.; Rozen, W.M. Use of Artificial Intelligence in the Advancement of Breast Surgery and Implications for Breast Reconstruction: A Narrative Review. J. Clin. Med. 2023, 12, 5143. [Google Scholar] [CrossRef]

| Limiting Factor | Description |
|---|---|
| Breast base | It does not detect the boundary between the breast and the chest wall, so it is necessary to simulate the latter with software from the surrounding chest wall. |
| High BMI 1 | Difficult to precisely define the lateral border of the breast. |
| Severe breast ptosis | Difficult to locate the submammary fold. |
| Movement and skin color | Postural variations, respiratory movements during scanning and patient skin tone may affect measurements. |
| Applications | Description |
|---|---|
| Preoperative Applications | |
|
|
|
|
|
|
|
|
| Intraoperative Applications | |
|
|
|
|
|
|
| Postoperative Applications | |
|
|
| Educational Applications | |
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, H.F.; Coloccini, A.; Viñas, J.F. Three-Dimensional Printing in Breast Reconstruction: Current and Promising Applications. J. Clin. Med. 2024, 13, 3278. https://doi.org/10.3390/jcm13113278
Mayer HF, Coloccini A, Viñas JF. Three-Dimensional Printing in Breast Reconstruction: Current and Promising Applications. Journal of Clinical Medicine. 2024; 13(11):3278. https://doi.org/10.3390/jcm13113278
Chicago/Turabian StyleMayer, Horacio F., Alejandro Coloccini, and José F. Viñas. 2024. "Three-Dimensional Printing in Breast Reconstruction: Current and Promising Applications" Journal of Clinical Medicine 13, no. 11: 3278. https://doi.org/10.3390/jcm13113278
APA StyleMayer, H. F., Coloccini, A., & Viñas, J. F. (2024). Three-Dimensional Printing in Breast Reconstruction: Current and Promising Applications. Journal of Clinical Medicine, 13(11), 3278. https://doi.org/10.3390/jcm13113278





