Real-World Outcomes of a Rhythm Control Strategy for Atrial Fibrillation Patients with Reduced Left Ventricular Ejection Fraction (<50%)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Patient Follow-Up
2.3. Echocardiographic Examination
2.4. Study Outcomes
2.5. Statistical Analysis
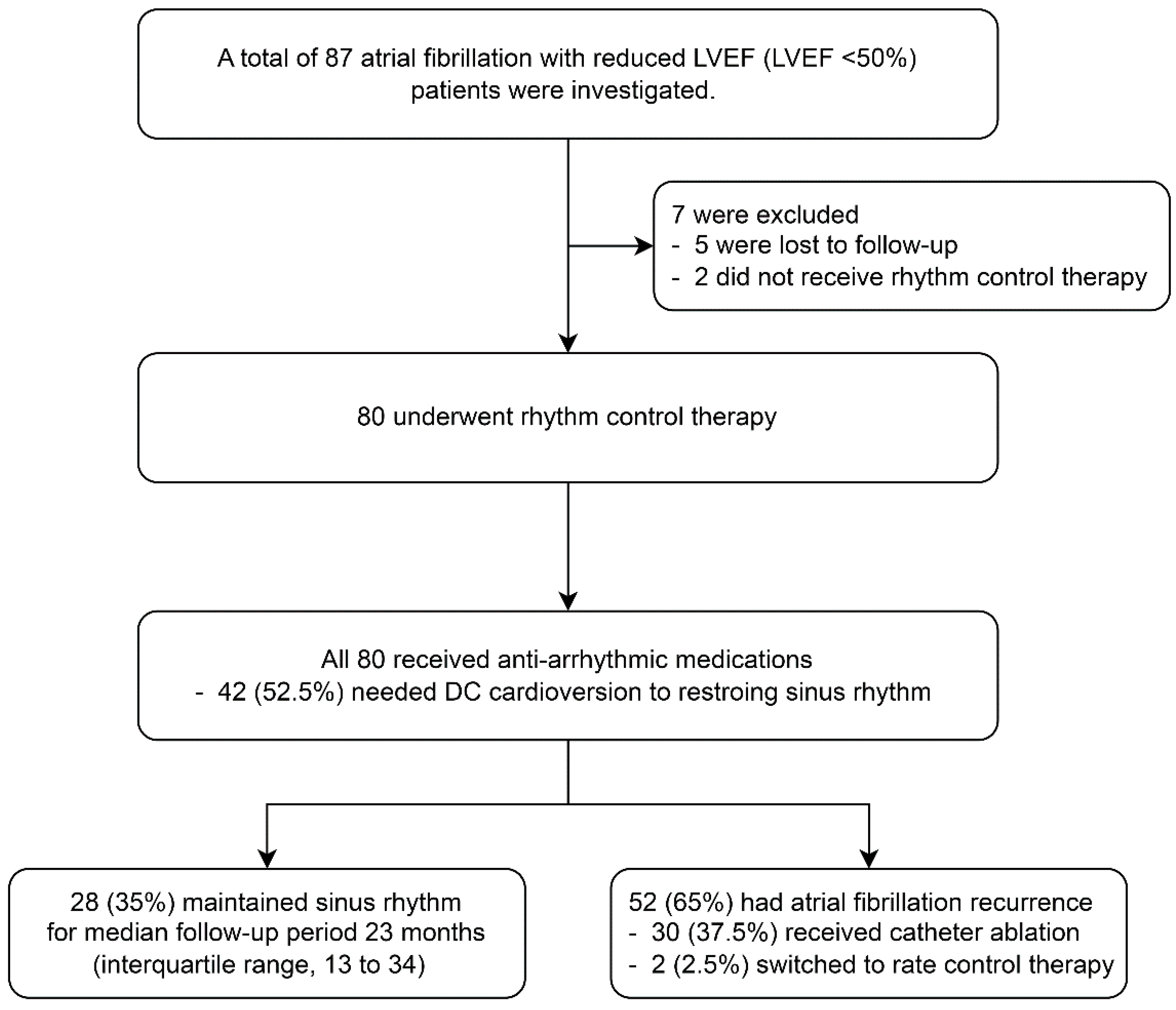
3. Results
3.1. Baseline Characteristics and Outcome of Rhythm Control
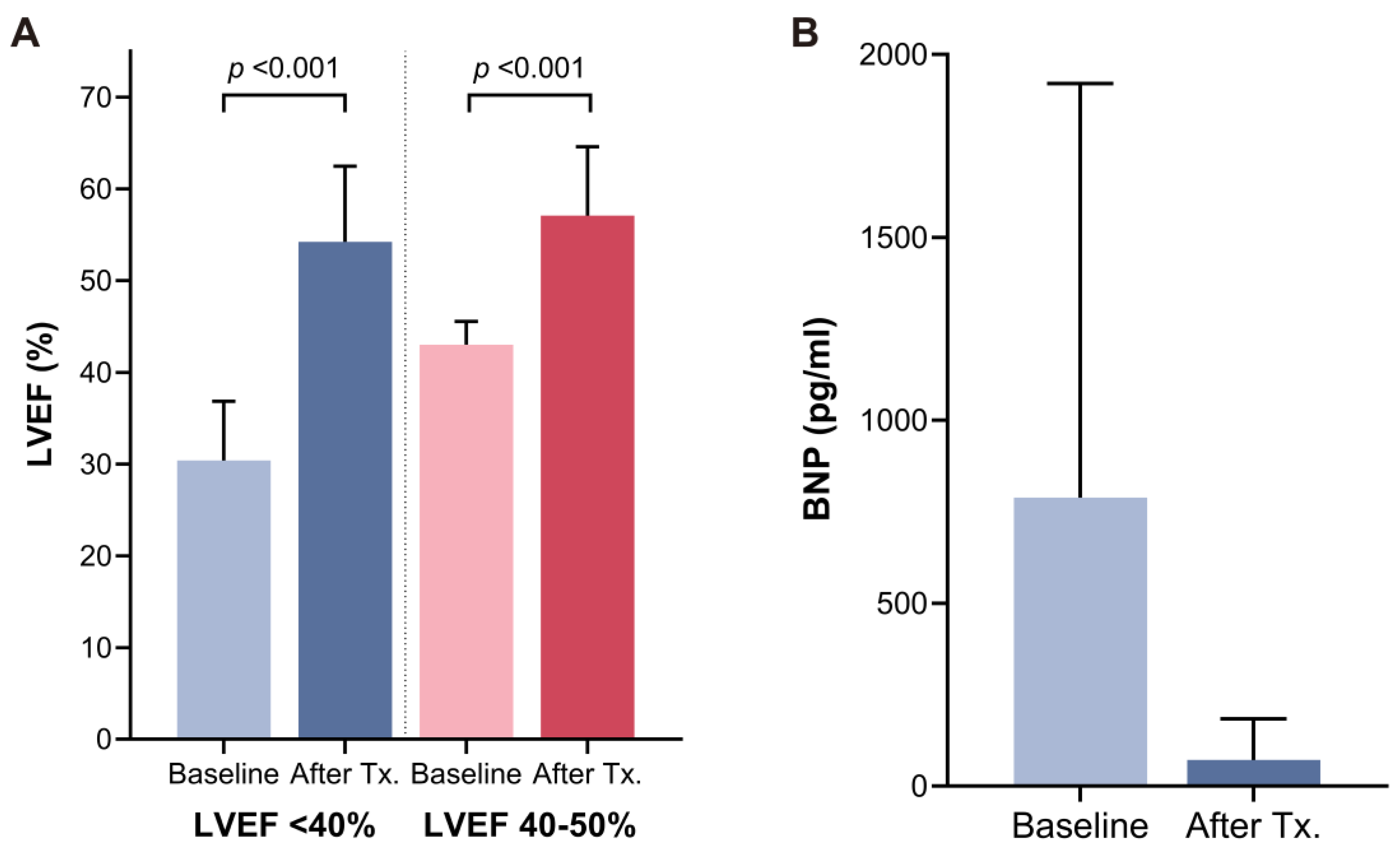
3.2. Improvement in Echocardiographic Parameters and Brain Natriuretic Peptide
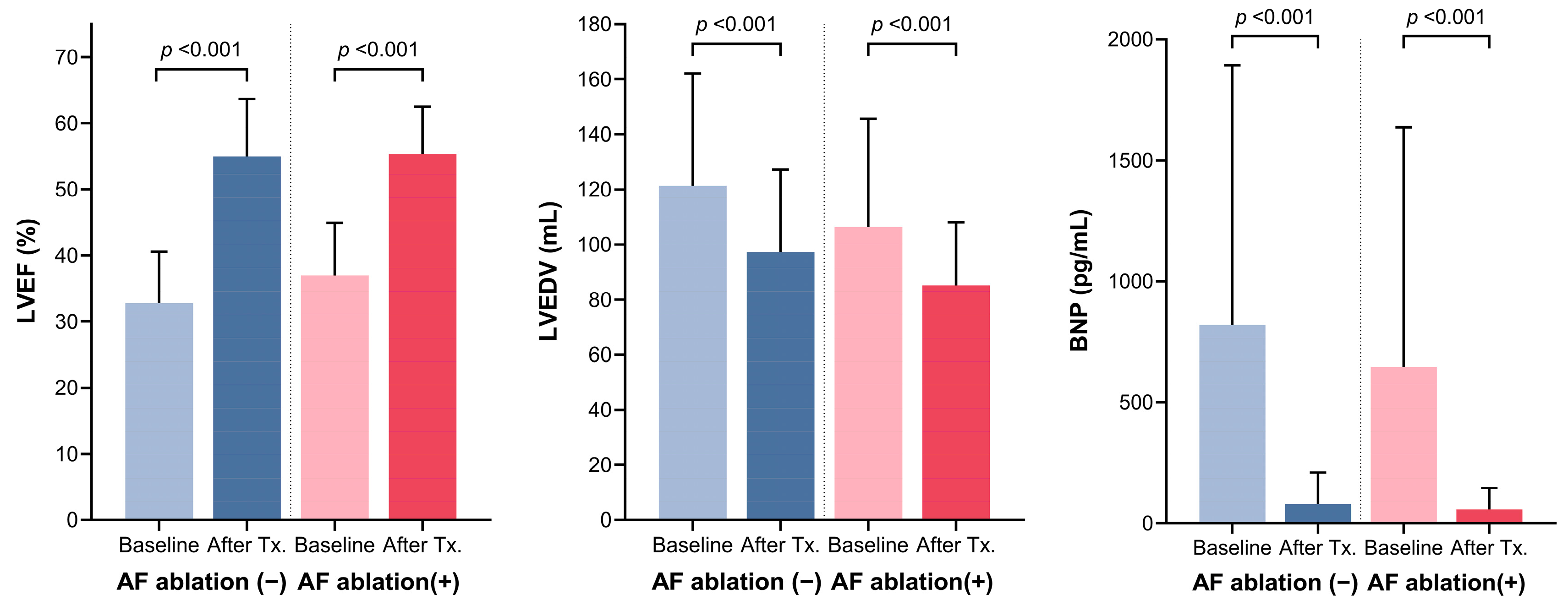
3.3. Comparison of Baseline Characteristics and Outcome of Rhythm Control between Patients with or without Catheter Ablation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AADs | anti-arrhythmic drugs |
| AF | atrial fibrillation |
| BNP | brain natriuretic peptide |
| GDMT | guideline-directed medical therapy |
| HF | heart failure |
| LA | left atrium |
| LVEF | left ventricular ejection fraction |
References
- Johnson, D.L.; Day, J.D.; Mahapatra, S.; Bunch, T.J. Adverse Outcomes from Atrial Fibrillation;Mechanisms, Risks, and Insights Learned from Therapeutic Options. J. Atr. Fibrillation 2012, 4, 477. [Google Scholar]
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370. [Google Scholar] [CrossRef]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; Mcmanus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef]
- Verma, A.; Kalman, J.M.; Callans, D.J. Treatment of Patients with Atrial Fibrillation and Heart Failure With Reduced Ejection Fraction. Circulation 2017, 135, 1547–1563. [Google Scholar] [CrossRef]
- Maisel, W.H.; Stevenson, L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003, 91, 2D–8D. [Google Scholar] [CrossRef]
- Chua, W.; Purmah, Y.; Cardoso, V.R.; Gkoutos, G.V.; Tull, S.P.; Neculau, G.; Thomas, M.R.; Kotecha, D.; Lip, G.Y.H.; Kirchhof, P.; et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur. Heart J. 2019, 40, 1268–1276. [Google Scholar] [CrossRef]
- Mamas, M.A.; Caldwell, J.C.; Chacko, S.; Garratt, C.J.; Fath-Ordoubadi, F.; Neyses, L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 676–683. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal Relations of Atrial Fibrillation and Congestive Heart Failure and Their Joint Influence on Mortality. Circulation 2003, 107, 2920–2925. [Google Scholar] [CrossRef]
- Talajic, M.; Khairy, P.; Levesque, S.; Connolly, S.J.; Dorian, P.; Dubuc, M.; Guerra, P.G.; Hohnloser, S.H.; Lee, K.L.; Macle, L.; et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J. Am. Coll. Cardiol. 2010, 55, 1796–1802. [Google Scholar] [CrossRef]
- Hagens, V.E.; Crijns, H.J.; Van Veldhuisen, D.J.; Van Den Berg, M.P.; Rienstra, M.; Ranchor, A.V.; Bosker, H.A.; Kamp, O.; Tijssen, J.G.; Veeger, N.J.; et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: Results from the RAte Control versus Electrical cardioversion (RACE) study. Am. Heart J. 2005, 149, 1106–1111. [Google Scholar] [CrossRef]
- Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure. N. Engl. J. Med. 2008, 358, 2667–2677. [Google Scholar] [CrossRef]
- Rillig, A.; Magnussen, C.; Ozga, A.-K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients with Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Sohns, C.; Fox, H.; Marrouche, N.F.; Crijns, H.; Costard-Jaeckle, A.; Bergau, L.; Hindricks, G.; Dagres, N.; Sossalla, S.; Schramm, R.; et al. Catheter Ablation in End-Stage Heart Failure with Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1380–1389. [Google Scholar] [CrossRef]
- Moysidis, D.V.; Kartas, A.; Samaras, A.; Papazoglou, A.S.; Akrivos, E.; Vouloagkas, I.; Papanastasiou, A.; Vrana, E.; Baroutidou, A.; Botis, M.; et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure across the left ventricular ejection fraction spectrum. Hellenic J. Cardiol. 2022, 66, 32–40. [Google Scholar] [CrossRef]
- Shelton, R.J.; Clark, A.L.; Goode, K.; Rigby, A.S.; Houghton, T.; Kaye, G.C.; Cleland, J.G. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart 2009, 95, 924–930. [Google Scholar] [CrossRef]
- Hunter, R.J.; Berriman, T.J.; Diab, I.; Kamdar, R.; Richmond, L.; Baker, V.; Goromonzi, F.; Sawhney, V.; Duncan, E.; Page, S.P.; et al. A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure (The CAMTAF Trial). Circ. Arrhythmia Electrophysiol. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2024, 83, 109–279. [Google Scholar] [CrossRef]
- Arabia, G.; Bellicini, M.G.; Cersosimo, A.; Memo, M.; Mazzarotto, F.; Inciardi, R.M.; Cerini, M.; Chen, L.Y.; Aboelhassan, M.; Benzoni, P.; et al. Ion channel dysfunction and fibrosis in atrial fibrillation: Two sides of the same coin. Pacing Clin. Electrophysiol. 2024, 47, 417–428. [Google Scholar] [CrossRef]
- Deedwania, P.C.; Singh, B.N.; Ellenbogen, K.; Fisher, S.; Fletcher, R.; Singh, S.N. Spontaneous Conversion and Maintenance of Sinus Rhythm by Amiodarone in Patients with Heart Failure and Atrial Fibrillation. Circulation 1998, 98, 2574–2579. [Google Scholar] [CrossRef]
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Trivedi, C.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistoclakis, S.; Dello Russo, A.; et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted Device. Circulation 2016, 133, 1637–1644. [Google Scholar] [CrossRef]
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021, 143, 1377–1390. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail. Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.H.; Ryu, K.-H. Tachycardia induced Cardiomyopathy. Korean Circ. J. 2019, 49, 808. [Google Scholar] [CrossRef]
- Clark, D.M.; Plumb, V.J.; Epstein, A.E.; Kay, G.N. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J. Am. Coll. Cardiol. 1997, 30, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.-H.; Khammy, O.; Byrne, M.; Amirahmadi, F.; Foster, A.; Li, G.; Zhang, L.; Remedios, C.D.; Chen, C.; Kaye, D.M. Irregular Rhythm Adversely Influences Calcium Handling in Ventricular Myocardium. Circ. Heart Fail. 2012, 5, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, A.; Duenas, A.; Liggett, M.S.; Dimond, E.G. Contribution of Atrial Systole to the Cardiac Function at a Fixed and at a Variable Ventricular Rate. Am. J. Cardiol. 1965, 16, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Chen, L.Y.; Chung, M.K.; Cornwell, W.K.; Furie, K.L.; Lakkireddy, D.R.; Marrouche, N.F.; Natale, A.; Olshansky, B.; Joglar, J.A. Managing Atrial Fibrillation in Patients with Heart Failure and Reduced Ejection Fraction: A Scientific Statement from the American Heart Association. Circ. Arrhythmia Electrophysiol. 2021, 14, e000078. [Google Scholar] [CrossRef] [PubMed]
- Moysidis, D.V.; Kartas, A.; Samaras, A.; Papazoglou, A.S.; Patsiou, V.; Bekiaridou, A.; Baroutidou, A.; Tsagkaris, C.; Karagiannidis, E.; Daios, S.; et al. Prescription Rates and Prognostic Implications of Optimally Targeted Guideline-Directed Medical Treatment in Heart Failure and Atrial Fibrillation: Insights From The MISOAC-AF Trial. J. Cardiovasc. Pharmacol. 2023, 81, 203–211. [Google Scholar] [CrossRef]




| N = 80 | |
|---|---|
| Age, years | 63.6 ± 10.9 |
| Male sex, n (%) | 65 (81.3) |
| Body mass index, kg/m2 | 25.4 ± 3.4 |
| Atrial fibrillation type | |
| Paroxysmal | 7 (8.8) |
| Persistent | 58 (72.5) |
| Longstanding persistent | 15 (18.8) |
| AF duration, days | 273 ± 606 |
| Hemoglobin, g/dL | 14.6 ± 1.7 |
| Creatinine, mg/dL | 1.04 ± 0.58 |
| BNP, pg/mL | 789 ± 1133 |
| Hypertension, n (%) | 42 (52.5) |
| Diabetes, n (%) | 25 (31.3) |
| Coronary artery disease, n (%) | 5 (6.3) |
| Myocardial infarction, n (%) | 1 (1.3) |
| PCI, n (%) | 4 (5.0) |
| Stroke/TIA, n (%) | 6 (7.5) |
| CHA2DS2VASc score, mean | 2.4 ± 1.8 |
| LVEF, % | 34.3 ± 8.1 |
| LVEF < 40%, n (%) | 55 (68.8) |
| LVEF 40–50%, n (%) | 25 (31.3) |
| LA diameter, cm | 4.3 ± 0.6 |
| LA volume index, mL/m2 | 41.8 ± 14.7 |
| LVEDV, mL | 115.6 ± 40.7 |
| LVEDVI, mL/m2 | 62.4 ± 23.3 |
| LVESV, mL | 77.9 ± 34.0 |
| LVESVI, mL/m2 | 41.9 ± 18.9 |
| Mitral regurgitation grade | |
| None, n (%) | 13 (16.3) |
| Minimal, n (%) | 28 (35.0) |
| Mild, n (%) | 25 (31.3) |
| Moderate, n (%) | 14 (17.5) |
| Severe, n (%) | 0 |
| Tricuspid regurgitation grade | |
| None, n (%) | 11 (13.8) |
| Minimal, n (%) | 34 (42.5) |
| Mild, n (%) | 25 (31.3) |
| Moderate, n (%) | 5 (6.3) |
| Severe, n (%) | 5 (6.3) |
| Beta-blocker, n (%) | 74 (92.5) |
| ACEi/ARB, n (%) | 64 (80.0) |
| MRA, n (%) | 57 (71.3) |
| Digoxin, n (%) | 24 (30.0) |
| Antiarrhythmic drugs, n (%) | 80 (100.0) |
| Flecainide, n (%) | 18 (22.5) |
| Propafenone, n (%) | 3 (3.8) |
| Amiodarone, n (%) | 53 (66.3) |
| Dronedarone, n (%) | 1 (1.3) |
| Sotalol | 5 (6.3) |
| AF Ablation (−), N = 50 | AF Ablation (+), N = 30 | p-Value | |
|---|---|---|---|
| Age, years | 64.6 ± 11.6 | 62.0 ± 9.4 | 0.292 |
| Male sex, n (%) | 39 (78.0) | 26 (86.7) | 0.336 |
| Body mass index, kg/m2 | 25.0 ± 3.7 | 26.1 ± 2.8 | 0.167 |
| Atrial fibrillation type | 0.356 | ||
| Paroxysmal | 5 (10.0) | 2 (6.7) | |
| Persistent | 38 (76.0) | 20 (66.7) | |
| Longstanding persistent | 7 (14.0) | 8 (26.7) | |
| AF duration, days | 212 ± 556 | 374 ± 679 | 0.250 |
| Hemoglobin, g/dL | 14.3 ± 1.8 | 15.1 ± 1.6 | 0.056 |
| Creatinine, mg/dL | 1.1 ± 0.7 | 1.0 ± 0.2 | 0.510 |
| BNP, pg/mL | 464 ± 426 | 238 ± 237 | 0.003 |
| Hypertension, n (%) | 27 (54.0) | 15 (50.0) | 0.729 |
| Diabetes, n (%) | 17 (34.0) | 8 (26.7) | 0.493 |
| Coronary artery disease, n (%) | 2 | 3 | |
| Myocardial infarction, n (%) | 0 | 1 (3.3) | 0.375 |
| PCI, n (%) | 2 (4.0) | 2 (6.7) | 0.628 |
| Stroke/TIA, n (%) | 6 (12.0) | 0 | 0.079 |
| CHA2DS2VASc score, mean | 2.6 ± 2.0 | 2.1 ± 1.3 | 0.169 |
| LVEF, % | 32.8 ± 7.8 | 36.9 ± 8.0 | 0.025 |
| LVEF < 40%, n (%) | 40 (80.0) | 15 (50.0) | 0.005 |
| LVEF 40–50%, n (%) | 10 (20.0) | 15 (50.0) | |
| LA diameter, cm | 4.6 ± 0.7 | 4.6 ± 0.5 | 0.812 |
| LA volume index, mL/m2 | 51.8 ± 15.4 | 49.0 ± 14.8 | 0.452 |
| LVEDV, mL | 121.3 ± 40.8 | 106.4 ± 39.4 | 0.115 |
| LVEDVI, mL/m2 | 65.2 ± 25.1 | 57.6 ± 19.4 | 0.161 |
| LVESV, mL | 83.5 ± 34.0 | 68.9 ± 32.8 | 0.065 |
| LVESVI, mL/m2 | 44.7 ± 19.8 | 37.3 ± 16.6 | 0.087 |
| Mitral regurgitation grade | |||
| None, n (%) | 8 (16.0) | 5 (16.7) | |
| Minimal, n (%) | 17 (34.0) | 11 (36.7) | |
| Mild, n (%) | 14 (28.0) | 11 (36.7) | |
| Moderate, n (%) | 11 (22.0) | 3 (10.0) | |
| Severe, n (%) | 0 | 0 | |
| Tricuspid regurgitation grade | |||
| None, n (%) | 8 (16.0) | 3 (10.0) | |
| Minimal, n (%) | 20 (40.0) | 14 (46.7) | |
| Mild, n (%) | 14 (28.0) | 11 (36.7) | |
| Moderate, n (%) | 5 (10.0) | 0 | |
| Severe, n (%) | 3 (6.0) | 2 (6.7) | |
| Beta-blocker, n (%) | 47 (94.0) | 27 (90.0) | 0.667 |
| ACEi/ARB, n (%) | 40 (80.0) | 24 (80.0) | 1.000 |
| MRA, n (%) | 38 (76.0) | 19 (63.3) | 0.226 |
| Digoxin, n (%) | 13 (26.0) | 11 (36.7) | 0.313 |
| Antiarrhythmic drugs, n (%) | 50 (100) | 30 (100) | |
| Flecainide, n (%) | 9 (18.0) | 9 (30.0) | |
| Propafenone, n (%) | 1 (2.0) | 2 (6.7) | |
| Amiodarone, n (%) | 38 (76.0) | 15 (50.0) | |
| Dronedarone | 1 (2.0) | 0 | |
| Sotalol | 1 (2.0) | 4 (13.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kwon, C.H. Real-World Outcomes of a Rhythm Control Strategy for Atrial Fibrillation Patients with Reduced Left Ventricular Ejection Fraction (<50%). J. Clin. Med. 2024, 13, 3285. https://doi.org/10.3390/jcm13113285
Choi J-H, Kwon CH. Real-World Outcomes of a Rhythm Control Strategy for Atrial Fibrillation Patients with Reduced Left Ventricular Ejection Fraction (<50%). Journal of Clinical Medicine. 2024; 13(11):3285. https://doi.org/10.3390/jcm13113285
Chicago/Turabian StyleChoi, Ji-Hoon, and Chang Hee Kwon. 2024. "Real-World Outcomes of a Rhythm Control Strategy for Atrial Fibrillation Patients with Reduced Left Ventricular Ejection Fraction (<50%)" Journal of Clinical Medicine 13, no. 11: 3285. https://doi.org/10.3390/jcm13113285
APA StyleChoi, J.-H., & Kwon, C. H. (2024). Real-World Outcomes of a Rhythm Control Strategy for Atrial Fibrillation Patients with Reduced Left Ventricular Ejection Fraction (<50%). Journal of Clinical Medicine, 13(11), 3285. https://doi.org/10.3390/jcm13113285






