Left Bundle Branch Area Pacing to Overcome Coronary Sinus Anatomy-Related Technical Problems Encountered during Implantation of Biventricular CRT—A Case Report
Abstract
:1. Introduction
2. Materials and Methods
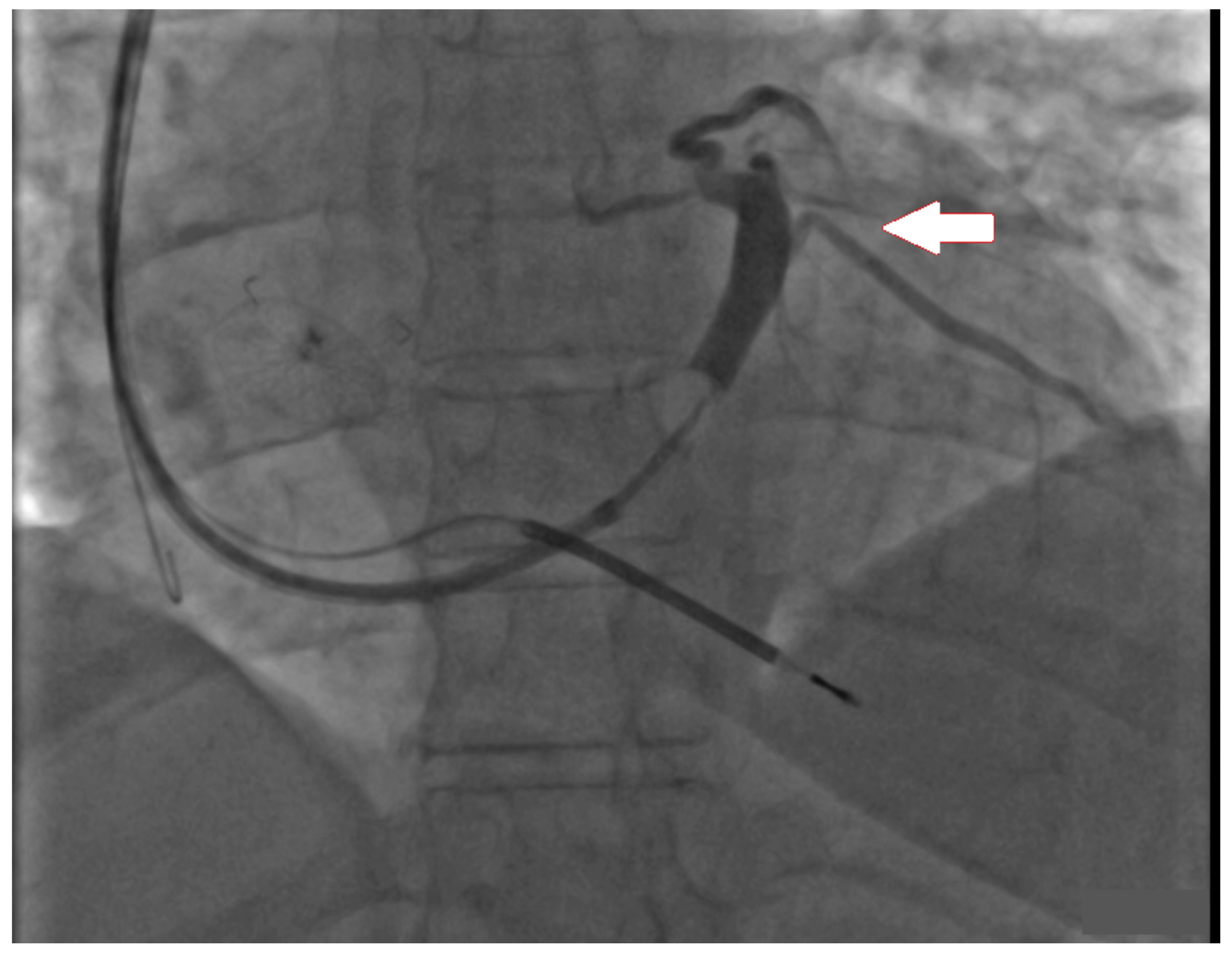
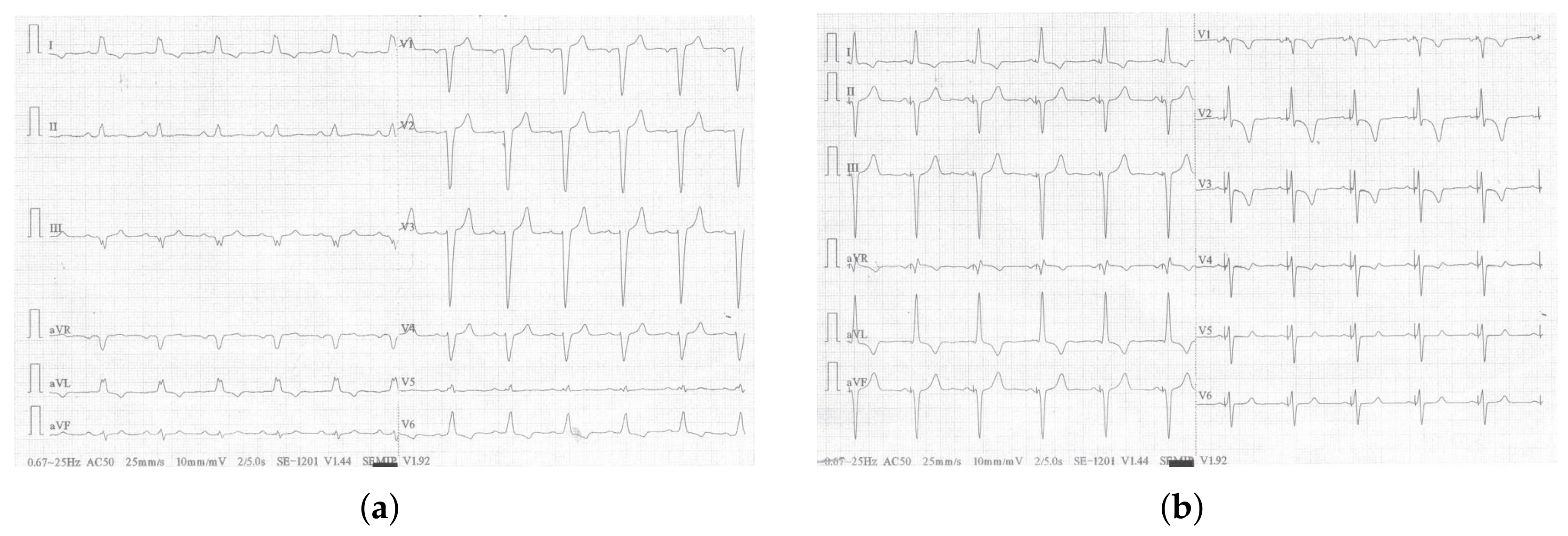
3. Case Report
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somma, V.; Ha, F.J.; Palmer, S.; Mohamed, U.; Agarwal, S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023, 20, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Norm, C.; Kaye, D.M.; Povsic, T.J.; Dickstein, K. Beyond pharmacological treatment: An insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 2019, 393, 1045–1055. [Google Scholar]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Auricchio, A.; Stellbrink, C.; Sack, S.; Block, M.; Vogt, J.; Bakker, P.; Huth, C.; Schöndube, F.; Wolfhard, U.; Böcker, D.; et al. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J. Am. Coll. Cardiol. 2002, 39, 2026–2033. [Google Scholar] [CrossRef]
- Johansen, J.B.; Nielsen, J.C.; Kristensen, J.S.; Gaard, N.C. Troubleshooting the difficult left ventricular lead placement in cardiac resynchronization therapy: Current status and future perspectives. Expert Rev. Med. Devices 2022, 19, 341–352. [Google Scholar] [CrossRef]
- Deshmukh, P.; Casavant, D.A.; Romanyshyn, M.; Anderson, K. Permanent, direct His-bundle pacing: A novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000, 101, 869–877. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A novel pacing strategy with low and stable output: Pacing the left bundle branch immediately beyond the conduction block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized trial of left bundle branch vs biventricular pacing for cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Zalavadia, D.; Haseeb, A.; Dye, C.; Madan, N.; Skeete, J.R.; Vipparthy, S.C.; Young, W.; Ravi, V.; Rajakumar, C.; et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef]
- Gui, Y.; Ye, L.; Wu, L.; Mai, H.; Yan, Q.; Wang, L. Clinical outcomes associated with His-purkinje system pacing vs. biventricular pacing, in cardiac resynchronization therapy: A meta-analysis. Front. Cardiovasc. Med. 2022, 9, 707148. [Google Scholar] [CrossRef]
- Tung, R.; Upadhyay, G.A. Defining Left Bundle Branch Block Patterns in Cardiac Resynchronisation Therapy: A Return to His Bundle Recordings. Arrhythm. Electrophysiol. Rev. 2020, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Duque, M.; Aristizabal, J.; Marin, J.; Niño, C.; Bastidas, O.; Ruiz, L.M.; Matos, C.D.; Hoyos, C.; Hincapie, D.; et al. The Emerging Role of Left Bundle Branch Area Pacing for Cardiac Resynchronisation Therapy. Arrhythm. Electrophysiol. Rev. 2023, 12, e29. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S. Biventricular versus right ventricular pacing in heart failure patients with atrioventricular block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Martinelli Filho, M.; de Siqueira, S.F.; Costa, R.; Greco, O.T.; Moreira, L.F.; D’avila, A.; Heist, E.K. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: The COMBAT study. J. Card. Fail. 2010, 16, 293–300. [Google Scholar] [CrossRef]
- Cano, O.; Navarrete-Navarro, J.; Jover, P.; Osca, J.; Izquierdo, M.; Navarro, J.; Ayala, H.D.; Martínez-Dolz, L. Conduction System Pacing for Cardiac Resynchronization Therapy. J. Cardiovasc. Dev. Dis. 2023, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Szotek, M.; Drużbicki, Ł.; Sabatowski, K.; Amoroso, G.R.; De Schouwer, K.; Matusik, P.T. Transcatheter Aortic Valve Implantation and Cardiac Conduction Abnormalities: Prevalence, Risk Factors and Management. J. Clin. Med. 2023, 12, 6056. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, G.; Le Bloa, M.; Teres Castillo, C.; Graf, D.; Carroz, P.; Ascione, C.; Porretta, A.P.; Pascale, P.; Pruvot, E. Conduction System Pacing versus Conventional Biventricular Pacing for Cardiac Resynchronization Therapy: Where Are We Heading? J. Clin. Med. 2023, 12, 6288. [Google Scholar] [CrossRef]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef]
- Wu, S.; Su, L.; Vijayaraman, P.; Zheng, R.; Cai, M.; Xu, L.; Shi, R.; Huang, Z.; Whinnett, Z.I.; Huang, W. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: Nonrandomized On-Treatment Comparison With His Bundle Pacing and Biventricular Pacing. Can. J. Cardiol. 2021, 37, 319–328. [Google Scholar] [CrossRef]
- Michalik, J.; Dabrowska-Kugacka, A.; Kosmalska, K.; Moroz, R.; Kot, A.; Lewicka, E.; Szolkiewicz, M. Hemodynamic Effects of Permanent His Bundle Pacing Compared to Right Ventricular Pacing Assessed by Two-Dimensional Speckle-Tracking Echocardiography. Int. J. Environ. Res. Public Health 2021, 18, 11721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, Y.; Wang, Z.; Jin, Q.; Qiu, Z.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: A prospective, multi-centre, observational study. Europace 2022, 24, 807–816. [Google Scholar] [CrossRef] [PubMed]

| Baseline | 0D | 1D | 6M | |
|---|---|---|---|---|
| Threshold [V/ms] | - | 0.5/0.4 | 0.5/0.4 | 0.25/0.4 |
| R-wave [mV] | - | 14.5 | 15.6 | 15.7 |
| QRSd [ms] | 165 | 105 | 105 | 105 |
| V6 RWPT [ms] | - | 65 | - | - |
| V6-V1 int.p [ms] | - | 35 | - | - |
| 6MWD [m] | 310 | - | 375 | 379 |
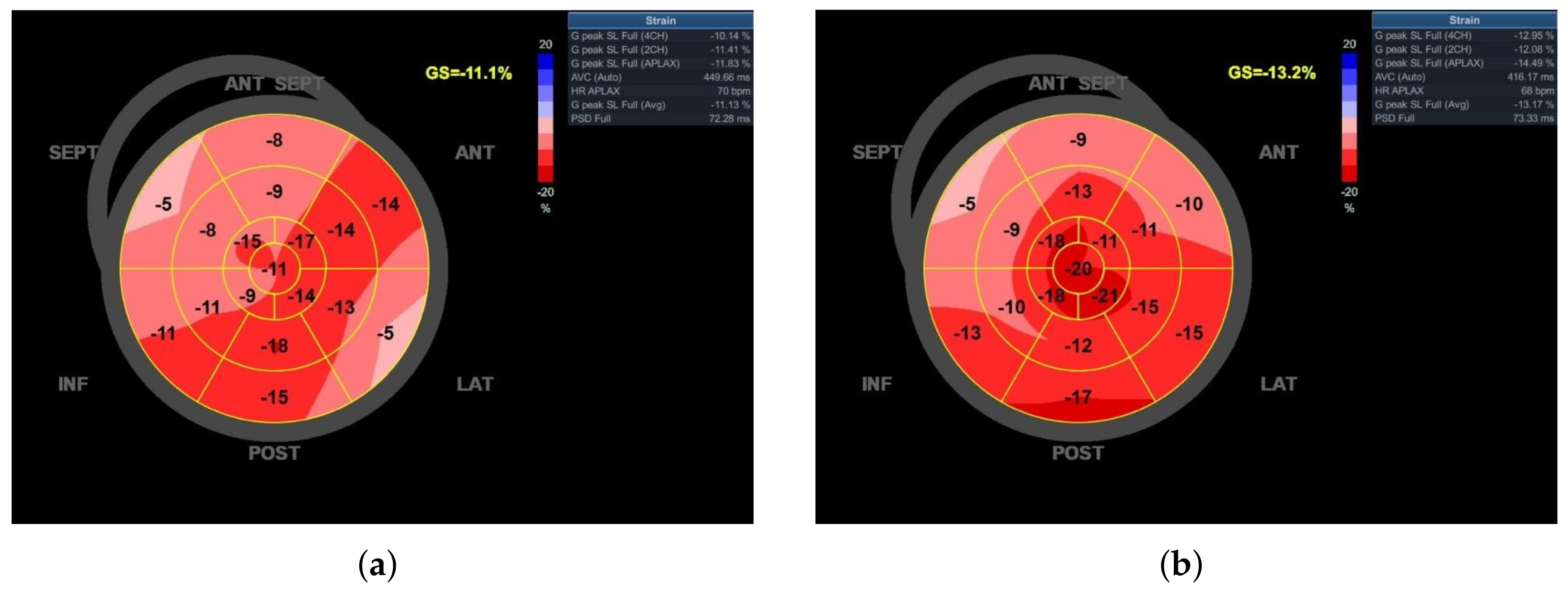
| LV-GLS [%] | −11.1 | - | −13.2 | −13.4 |
| LVEF [%] | 30 | - | 30 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalik, J.; Moroz, R.; Szołkiewicz, M.; Dąbrowska-Kugacka, A.; Daniłowicz-Szymanowicz, L. Left Bundle Branch Area Pacing to Overcome Coronary Sinus Anatomy-Related Technical Problems Encountered during Implantation of Biventricular CRT—A Case Report. J. Clin. Med. 2024, 13, 3307. https://doi.org/10.3390/jcm13113307
Michalik J, Moroz R, Szołkiewicz M, Dąbrowska-Kugacka A, Daniłowicz-Szymanowicz L. Left Bundle Branch Area Pacing to Overcome Coronary Sinus Anatomy-Related Technical Problems Encountered during Implantation of Biventricular CRT—A Case Report. Journal of Clinical Medicine. 2024; 13(11):3307. https://doi.org/10.3390/jcm13113307
Chicago/Turabian StyleMichalik, Jędrzej, Roman Moroz, Marek Szołkiewicz, Alicja Dąbrowska-Kugacka, and Ludmiła Daniłowicz-Szymanowicz. 2024. "Left Bundle Branch Area Pacing to Overcome Coronary Sinus Anatomy-Related Technical Problems Encountered during Implantation of Biventricular CRT—A Case Report" Journal of Clinical Medicine 13, no. 11: 3307. https://doi.org/10.3390/jcm13113307
APA StyleMichalik, J., Moroz, R., Szołkiewicz, M., Dąbrowska-Kugacka, A., & Daniłowicz-Szymanowicz, L. (2024). Left Bundle Branch Area Pacing to Overcome Coronary Sinus Anatomy-Related Technical Problems Encountered during Implantation of Biventricular CRT—A Case Report. Journal of Clinical Medicine, 13(11), 3307. https://doi.org/10.3390/jcm13113307








