Pharmaceutical Modulation of Intracranial Aneurysm Development and Rupture
Abstract
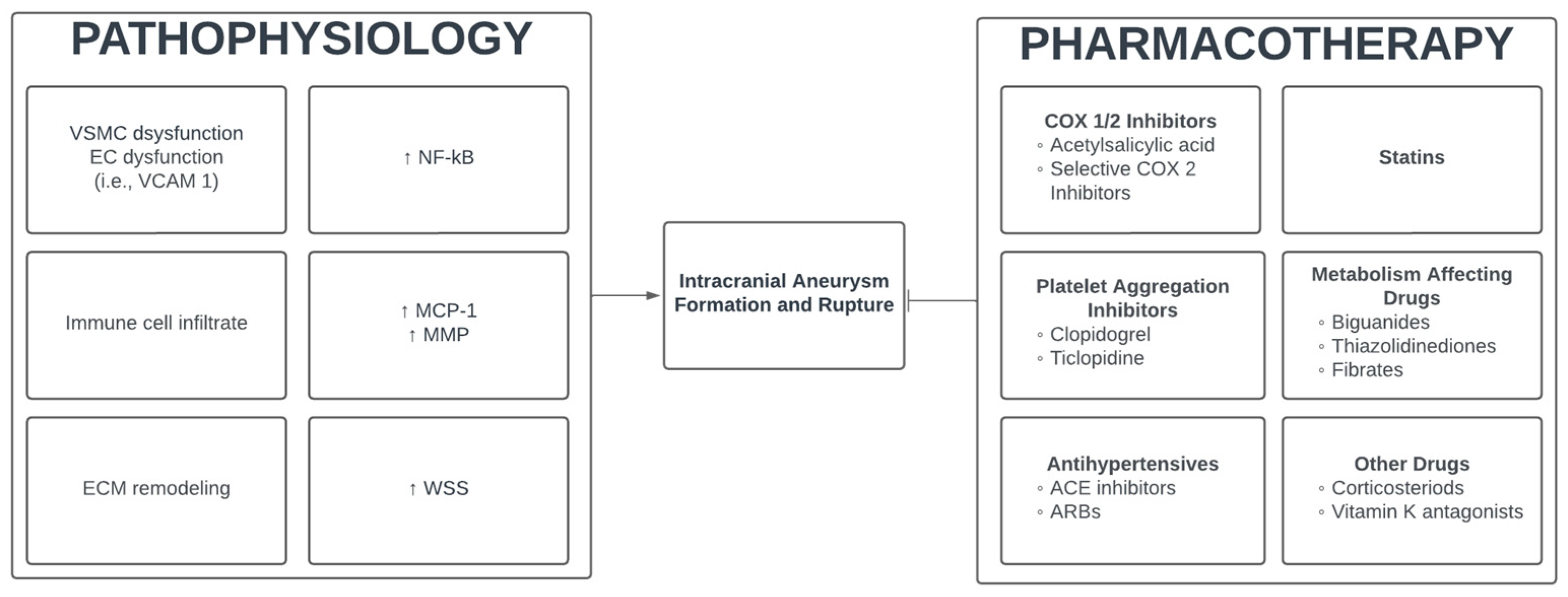
:1. Introduction
2. Pharmaceutical Modulation of Intracranial Aneurysms
2.1. Pathogenesis of Intracranial Aneurysms
2.2. Cyclooxygenase-1 and 2 Inhibitor (NSAIDS)
2.3. Selective Cyclooxygenase-2 Inhibitors
2.4. Platelet Aggregation Inhibitors
2.5. Antihypertensives
2.6. Hydroxymethylglutaryl-CoA Reductase Inhibitors (Statins)
2.7. Metabolism Affecting Drugs
2.8. Other Medications
| Study Author (Year) | No. of Pts | Medication | Association |
|---|---|---|---|
| Zanaty et al. (2020) [28] | N = 146 | ASA | Decreased IA growth (OR 0.19, CI 0.05–0.63, p = 0.007) |
| Weng et al. (2020) [29] | N = 272 | ASA | Decreased IA growth (HR 0.29, CI 0.11–0.77, p = 0.013) |
| Hasan et al. (2011) [30] | N = 271 | ASA | Decreased odds of hemorrhage (aOR 0.27, CI 0.11–0.67, p = 0.03) |
| Gross et al. (2014) [31] | N = 717 | ASA | Decreased rate of hemorrhage (40% vs. 28%, p = 0.016) |
| Hostettler et al. (2018) [32] | N = 2334 | ASA | Negative association with rupture (OR 0.28, CI 0.20–0.40, p < 0.001) |
| Can et al. (2018) [33] | N = 4619 | ASA | Negative association with rupture (OR 0.60, CI 0.45–0.80, p < 0.01) Dose response (OR 0.65, CI 0.53–0.81, p < 0.01) |
| Garcia-Rodriguez et al. (2013) [34] | N = 1340 | ASA > 3 years | Decreased risk of SAH (OR 0.63, CI 0.45–0.90) |
| Garbe et al. (2013) [38] | N = 2065 | ASA | Increased risk of SAH (OR 1.5, CI 1.2–2.0, p = 0.001) |
| Ewbank et al. (2023) [39] | N = 541 | ASA | No association with SAH (HR 1.15, CI 0.91–1.47, p = 0.24) |
| Pottegård et al. (2015) [37] | N = 5834 | ASA < 1 month | Increased risk of SAH (OR 1.75, CI 1.28–2.40) |
| ASA > 3 years | No association with SAH (OR 1.13, CI 0.86–1.49) | ||
| Raisanen et al. (2022) [47] | N = 1419 | COX2i | No association with IA formation (HR 0.63, CI 0.29–1.39, p = 0.249) |
| Risselada et al. (2011) [48] | N = 1004 | COX2i | Positive association with SAH (OR 2.35, CI 1.27–4.36) |
| Pottegård et al. (2015) [37] | N = 5834 | Clopidogrel < 1 month | Positive association with SAH (OR 2.33, CI 1.02–5.35), no significant relationship in long-term users. |
| Hudson et al. (2023) [54] | N = 921 | Clopidogrel | Lower likelihood of rupture (6.6% vs. 23.5%, p = 0.001) |
| Risselada et al. (2011) [48] | N = 1004 | Platelet Aggregation Inhibitors | Positive association with SAH (OR 1.32, CI 1.02–1.70) in case-control study, significance lost in case-crossover analysis. |
| Zhong et al. (2022) [61] | N = 3044 | ACEi | Negative association with rupture (OR 0.559, CI 0.442–0.709, p = 0.000) |
| ARBs | Negative association with rupture (OR 0.414, CI 0.315–0.542, p = 0.000) | ||
| Yoshimura et al. (2014) [67] | N = 421 | Statins | Negative association with rupture (aOR 0.30, CI 0.14–0.66) |
| Shimizu et al. (2021) [69] | N = 1197 | Statins | Negative association with rupture (OR 0.54, CI 0.38–0.77, p = 0.0008) |
| Terceno et al. (2021) [73] | N = 368 | Statins | No association with rupture (aOR 1.65, CI 0.83–3.31, p = 0.155) |
| Yoshida et al. (2021) [74] | N = 209 | Statins | No difference in IA growth, rupture, or “new bleb formation” (Log-rank p = 0.359) |
| Marbacher et al. (2012) [76] | N = 300 | Statins | No association with IA formation (OR 1.08, CI 0.69–1.69, p = 0.74) |
| Bekelis et al. (2015) [77] | N = 28,931 | Statins | No association with SAH (OR 1.03, CI 0.86–1.23, p = 0.730) |
| Jabbarli et al. (2023) [78] | N = 1960 | Statins | Positive association with IA formation (aOR 1.34, CI 1.02–1.78) |
| N = 2446 | Statins | Negative association with rupture (aOR 0.62, CI 0.47–0.81) | |
| Can et al. (2018) [68] | N = 4701 | Lipid-Lowering Medications | Negative association with rupture (OR 0.58, CI 0.47–0.71, p < 0.01) |
| Ruigrok et al. (2006) [92] | N = 1158 | Corticosteroids | Composite outcome of corticosteroids or a medical condition that may be treated with corticosteroids had a positive association with SAH (OR 1.67, CI 1.09–2.54, p = 0.016) |
| Pottegård et al. (2015) [37] | N = 5834 | Vitamin-K Antagonists | No association with SAH (OR 1.24, CI 0.86–1.77) in long-term users (>3 years) |
| Garbe et al. (2013) [38] | N = 2065 | Vitamin-K Antagonists | Positive association with SAH (OR 1.7, CI 1.3–2.3, p < 0.001) |
| Risselada et al. (2011) [48] | N = 1004 | Vitamin-K Antagonists | Positive association with SAH (OR 2.90, CI 1.27–6.65) in case-crossover, not significant in case-control |
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gasparotti, R.; Liserre, R. Intracranial aneurysms. Eur. Radiol. 2005, 15, 441–447. [Google Scholar] [CrossRef]
- Keedy, A. An overview of intracranial aneurysms. McGill J. Med. 2006, 9, 141–146. [Google Scholar] [CrossRef]
- Ajiboye, N.; Chalouhi, N.; Starke, R.M.; Zanaty, M.; Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. Sci. World J. 2015, 2015, 954954. [Google Scholar] [CrossRef]
- Ziu, E.; Khan Suheb, M.Z.; Mesfin, F.B. Subarachnoid Hemorrhage. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cras, T.Y.; Bos, D.; Ikram, M.A.; Vergouwen, M.D.I.; Dippel, D.W.J.; Voortman, T.; Adams, H.H.H.; Vernooij, M.W.; Roozenbeek, B. Determinants of the Presence and Size of Intracranial Aneurysms in the General Population: The Rotterdam Study. Stroke 2020, 51, 2103–2110. [Google Scholar] [CrossRef]
- Chandra, R.V.; Maingard, J.; Slater, L.-A.; Cheung, N.K.; Lai, L.T.; Gall, S.L.; Thrift, A.G.; Phan, T.G. A Meta-Analysis of Rupture Risk for Intracranial Aneurysms 10 mm or Less in Size Selected for Conservative Management without Repair. Front. Neurol. 2022, 12, 743023. [Google Scholar] [CrossRef]
- Roquer, J.; Cuadrado-Godia, E.; Guimaraens, L.; Conesa, G.; Rodriguez-Campello, A.; Capellades, J.; García-Arnillas, M.P.; Fernández-Candil, J.L.; Avellaneda-Gómez, C.; Giralt-Steinhauer, E.; et al. Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology 2020, 95, e1819–e1829. [Google Scholar] [CrossRef]
- van der Kamp, L.T.; Rinkel, G.J.E.; Verbaan, D.; van den Berg, R.; Vandertop, W.P.; Murayama, Y.; Ishibashi, T.; Lindgren, A.; Koivisto, T.; Teo, M.; et al. Risk of Rupture after Intracranial Aneurysm Growth. JAMA Neurol. 2021, 78, 1228–1235. [Google Scholar] [CrossRef]
- Liu, Z.; Ajimu, K.; Yalikun, N.; Zheng, Y.; Xu, F. Potential Therapeutic Strategies for Intracranial Aneurysms Targeting Aneurysm Pathogenesis. Front. Neurosci. 2019, 13, 1238. [Google Scholar] [CrossRef]
- Alnæs, M.S.; Isaksen, J.; Mardal, K.-A.; Romner, B.; Morgan, M.K.; Ingebrigtsen, T. Computation of Hemodynamics in the Circle of Willis. Stroke 2007, 38, 2500–2505. [Google Scholar] [CrossRef]
- Signorelli, F.; Sela, S.; Gesualdo, L.; Chevrel, S.; Tollet, F.; Pailler-Mattei, C.; Tacconi, L.; Turjman, F.; Vacca, A.; Schul, D.B. Hemodynamic Stress, Inflammation, and Intracranial Aneurysm Development and Rupture: A Systematic Review. World Neurosurg. 2018, 115, 234–244. [Google Scholar] [CrossRef]
- Lai, X.L.; Deng, Z.F.; Zhu, X.G.; Chen, Z.H. Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-κB signaling pathway mediated inflammatory response. Biosci. Rep. 2019, 39, BSR20181909. [Google Scholar] [CrossRef]
- Cheng, W.T.; Wang, N. Correlation between MMP-2 and NF-κ B expression of intracranial aneurysm. Asian Pac. J. Trop. Med. 2013, 6, 570–573. [Google Scholar] [CrossRef]
- Fan, X.J.; Zhao, H.D.; Yu, G.; Zhong, X.L.; Yao, H.; Yang, Q.D. Role of inflammatory responses in the pathogenesis of human cerebral aneurysm. Genet. Mol. Res. 2015, 14, 9062–9070. [Google Scholar] [CrossRef]
- Jin, T.; An, Q.; Qin, X.; Qin, X.; Hu, Y.; Hu, J.; Zhou, B.; Leng, B. Resveratrol inhibits cerebral aneurysms in mice via downregulating the NF-κB pathway. Acta Biochim. Pol. 2022, 69, 613–618. [Google Scholar] [CrossRef]
- Li, S.; Tian, Y.; Huang, X.; Zhang, Y.; Wang, D.; Wei, H.; Dong, J.; Jiang, R.; Zhang, J. Intravenous transfusion of endothelial colony-forming cells attenuates vascular degeneration after cerebral aneurysm induction. Brain Res. 2014, 1593, 65–75. [Google Scholar] [CrossRef]
- Awtry, E.H.; Loscalzo, J. Aspirin. Circulation 2000, 101, 1206–1218. [Google Scholar] [CrossRef]
- Yin, M.-J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef]
- Chen, C.M.; Tung, Y.T.; Wei, C.H.; Lee, P.Y.; Chen, W. Anti-Inflammatory and Reactive Oxygen Species Suppression through Aspirin Pretreatment to Treat Hyperoxia-Induced Acute Lung Injury in NF-kappaB-Luciferase Inducible Transgenic Mice. Antioxidants 2020, 9, 429. [Google Scholar] [CrossRef]
- Jorda, A.; Aldasoro, M.; Aldasoro, C.; Guerra-Ojeda, S.; Iradi, A.; Vila, J.M.; Campos-Campos, J.; Valles, S.L. Action of low doses of Aspirin in Inflammation and Oxidative Stress induced by aβ(1-42) on Astrocytes in primary culture. Int. J. Med. Sci. 2020, 17, 834–843. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Bala, V.; Murry, D.J. Quantification of eicosanoids and their metabolites in biological matrices: A review. Bioanalysis 2018, 10, 2027–2046. [Google Scholar] [CrossRef]
- Dickinson, J.S.; Murphy, R.C. Mass spectrometric analysis of leukotriene A4 and other chemically reactive metabolites of arachidonic acid. J. Am. Soc. Mass Spectrom. 2002, 13, 1227–1234. [Google Scholar] [CrossRef]
- Gréen, K. Metabolism and pharmacokinetics of prostaglandin analogs in man. In Prostaglandins and Fertility Regulation; Toppozada, M.K., Bygdeman, M., Hafez, E.S.E., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 11–19. [Google Scholar]
- Kurban, S.; Mehmetoglu, I. Effects of acetylsalicylic acid on serum paraoxonase activity, Ox-LDL, coenzyme Q10 and other oxidative stress markers in healthy volunteers. Clin. Biochem. 2010, 43, 287–290. [Google Scholar] [CrossRef]
- Berg, K.; Langaas, M.; Ericsson, M.; Pleym, H.; Basu, S.; Nordrum, I.S.; Vitale, N.; Haaverstad, R. Acetylsalicylic acid treatment until surgery reduces oxidative stress and inflammation in patients undergoing coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2013, 43, 1154–1163. [Google Scholar] [CrossRef]
- Frösen, J.; Tulamo, R.; Heikura, T.; Sammalkorpi, S.; Niemelä, M.; Hernesniemi, J.; Levonen, A.-L.; Hörkkö, S.; Ylä-Herttuala, S. Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol. Commun. 2013, 1, 71. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; Rosenberger, G.; Walter, D.H.; Breuer, S.; Dimmeler, S.; Zeiher, A.M. Elevated C-Reactive Protein Levels and Impaired Endothelial Vasoreactivity in Patients With Coronary Artery Disease. Circulation 2000, 102, 1000–1006. [Google Scholar] [CrossRef]
- Zanaty, M.; Roa, J.A.; Nakagawa, D.; Chalouhi, N.; Allan, L.; Al Kasab, S.; Limaye, K.; Ishii, D.; Samaniego, E.A.; Jabbour, P.; et al. Aspirin associated with decreased rate of intracranial aneurysm growth. J. Neurosurg. JNS 2020, 133, 1478–1485. [Google Scholar] [CrossRef]
- Weng, J.-C.; Wang, J.; Li, H.; Jiao, Y.-M.; Fu, W.-L.; Huo, R.; Yan, Z.-H.; Xu, H.-Y.; Zhan, J.; Wang, S.; et al. Aspirin and Growth of Small Unruptured Intracranial Aneurysm. Stroke 2020, 51, 3045–3054. [Google Scholar] [CrossRef]
- Hasan, D.M.; Mahaney, K.B.; Brown, R.D., Jr.; Meissner, I.; Piepgras, D.G.; Huston, J.; Capuano, A.W.; Torner, J.C. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011, 42, 3156–3162. [Google Scholar] [CrossRef]
- Gross, B.A.; Rosalind Lai, P.M.; Frerichs, K.U.; Du, R. Aspirin and aneurysmal subarachnoid hemorrhage. World Neurosurg. 2014, 82, 1127–1130. [Google Scholar] [CrossRef]
- Hostettler, I.C.; Alg, V.S.; Shahi, N.; Jichi, F.; Bonner, S.; Walsh, D.; Bulters, D.; Kitchen, N.; Brown, M.M.; Houlden, H.; et al. Characteristics of Unruptured Compared to Ruptured Intracranial Aneurysms: A Multicenter Case–Control Study. Neurosurgery 2018, 83, 43–52. [Google Scholar] [CrossRef]
- Can, A.; Rudy, R.F.; Castro, V.M.; Yu, S.; Dligach, D.; Finan, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: A case-control study. Neurology 2018, 91, e1175–e1181. [Google Scholar] [CrossRef]
- García-Rodríguez, L.A.; Gaist, D.; Morton, J.; Cookson, C.; González-Pérez, A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology 2013, 81, 566–574. [Google Scholar] [CrossRef]
- Hasan, D.M.; Chalouhi, N.; Jabbour, P.; Magnotta, V.A.; Kung, D.K.; Young, W.L. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced MRI: Preliminary results. J. Neuroradiol. 2013, 40, 187–191. [Google Scholar] [CrossRef]
- Hasan, D.M.; Chalouhi, N.; Jabbour, P.; Dumont, A.S.; Kung, D.K.; Magnotta, V.A.; Young, W.L.; Hashimoto, T.; Winn, H.R.; Heistad, D. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: Preliminary results. J. Am. Heart Assoc. 2013, 2, e000019. [Google Scholar] [CrossRef]
- Pottegård, A.; García Rodríguez, L.A.; Poulsen, F.R.; Hallas, J.; Gaist, D. Antithrombotic drugs and subarachnoid haemorrhage risk. A nationwide case-control study in Denmark. Thromb. Haemost. 2015, 114, 1064–1075. [Google Scholar] [CrossRef]
- Garbe, E.; Kreisel, S.H.; Behr, S. Risk of subarachnoid hemorrhage and early case fatality associated with outpatient antithrombotic drug use. Stroke 2013, 44, 2422–2426. [Google Scholar] [CrossRef]
- Ewbank, F.; Birks, J.; Gaastra, B.; Hall, S.; Galea, I.; Bulters, D. Aspirin and Subarachnoid Haemorrhage in the UK Biobank. Transl. Stroke Res. 2023, 14, 490–498. [Google Scholar] [CrossRef]
- Vonkeman, H.E.; van de Laar, M.A. Nonsteroidal anti-inflammatory drugs: Adverse effects and their prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aoki, T.; Kataoka, H.; Shimamura, M.; Nakagami, H.; Wakayama, K.; Moriwaki, T.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. NF-κB Is a Key Mediator of Cerebral Aneurysm Formation. Circulation 2007, 116, 2830–2840. [Google Scholar] [CrossRef]
- Miralles, M.; Wester, W.; Sicard, G.A.; Thompson, R.; Reilly, J.M. Indomethacin inhibits expansion of experimental aortic aneurysms via inhibition of the cox2 isoform of cyclooxygenase. J. Vasc. Surg. 1999, 29, 884–892; discussion 892–893. [Google Scholar] [CrossRef]
- Ghoshal, S.; Loftin, C.D. Cyclooxygenase-2 inhibition attenuates abdominal aortic aneurysm progression in hyperlipidemic mice. PLoS ONE 2012, 7, e44369. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M.; Matsuoka, T.; Yamamoto, K.; Furuyashiki, T.; Kataoka, H.; Narumiya, S. PGE(2) -EP(2) signalling in endothelium is activated by haemodynamic stress and induces cerebral aneurysm through an amplifying loop via NF-κB. Br. J. Pharmacol. 2011, 163, 1237–1249. [Google Scholar] [CrossRef]
- Hasan, D.; Hashimoto, T.; Kung, D.; Macdonald, R.L.; Winn, H.R.; Heistad, D. Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: Preliminary results. Stroke 2012, 43, 1964–1967. [Google Scholar] [CrossRef]
- Räisänen, S.; Huttunen, J.; Huuskonen, T.J.; von Und Zu Fraunberg, M.; Koivisto, T.; Jääskeläinen, J.E.; Lindgren, A.; Frösen, J. Risk factor management matters more than pharmaceutical cyclooxygenase-2 inhibition in the prevention of de novo intracranial aneurysms. Eur. J. Neurol. 2022, 29, 2734–2743. [Google Scholar] [CrossRef]
- Risselada, R.; Straatman, H.; Van Kooten, F.; Dippel, D.W.J.; Van Der Lugt, A.; Niessen, W.J.; Firouzian, A.; Herings, R.M.C.; Sturkenboom, M.C.J.M. Platelet aggregation inhibitors, vitamin K antagonists and risk of subarachnoid hemorrhage. J. Thromb. Haemost. 2011, 9, 517–523. [Google Scholar] [CrossRef]
- Shimizu, K.; Aoki, T.; Etminan, N.; Hackenberg, K.A.M.; Tani, S.; Imamura, H.; Kataoka, H.; Sakai, N. Associations Between Drug Treatments and the Risk of Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-analysis. Transl. Stroke Res. 2022, 14, 833–841. [Google Scholar] [CrossRef]
- Smyth, E.M.; Grosser, T.; Wang, M.; Yu, Y.; FitzGerald, G.A. Prostanoids in health and disease. J. Lipid. Res. 2009, 50, S423–S428. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Lopez, R.A.; Hai, O. Antiplatelet Medications. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tu, X.; Chen, X.; Xie, Y.; Shi, S.; Wang, J.; Chen, Y.; Li, J. Anti-inflammatory renoprotective effect of clopidogrel and irbesartan in chronic renal injury. J. Am. Soc. Nephrol. 2008, 19, 77–83. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Egashira, K.; Hashimoto, N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009, 40, 942–951. [Google Scholar] [CrossRef]
- Hudson, J.S.; Nowicki, K.W.; Lucke-Wold, B.; Gersey, Z.C.; Dodd, W.S.; Alattar, A.; McCarthy, D.J.; Agarwal, P.; Mehdi, Z.; Lang, M.J.; et al. Clopidogrel Is Associated with Reduced Likelihood of Aneurysmal Subarachnoid Hemorrhage: A Multi-Center Matched Retrospective Analysis. Transl. Stroke Res. 2023. [Google Scholar] [CrossRef]
- Tada, Y.; Wada, K.; Shimada, K.; Makino, H.; Liang, E.I.; Murakami, S.; Kudo, M.; Kitazato, K.T.; Nagahiro, S.; Hashimoto, T.; et al. Roles of hypertension in the rupture of intracranial aneurysms. Stroke 2014, 45, 579–586. [Google Scholar] [CrossRef]
- Schievink, W.I. Intracranial Aneurysms. N. Engl. J. Med. 1997, 336, 28–40. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Inci, S.; Spetzler, R.F. Intracranial aneurysms and arterial hypertension: A review and hypothesis. Surg. Neurol. 2000, 53, 530–540; discussion 540–542. [Google Scholar] [CrossRef]
- Fatmawati, H.S.A.; Santoso, A.G. Hypertension as a Determining Factor in the Rupture of Intracranial Aneurysms, Diagnosed by 64-MDCT Angiography. Makara J. Health Res. 2017, 21, 49–53. [Google Scholar] [CrossRef]
- Zhong, P.; Lu, Z.; Li, T.; Lan, Q.; Liu, J.; Wang, Z.; Chen, S.; Huang, Q. Association Between Regular Blood Pressure Monitoring and the Risk of Intracranial Aneurysm Rupture: A Multicenter Retrospective Study with Propensity Score Matching. Transl. Stroke Res. 2022, 13, 983–994. [Google Scholar] [CrossRef]
- Zhong, P.; Lu, Z.; Li, Z.; Li, T.; Lan, Q.; Liu, J.; Chen, S.; Huang, Q. Effect of Renin-Angiotensin-Aldosterone System Inhibitors on the Rupture Risk Among Hypertensive Patients With Intracranial Aneurysms. Hypertension 2022, 79, 1475–1486. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Landmesser, U.; Bahlmann, F.; Mueller, M.; Spiekermann, S.; Kirchhoff, N.; Schulz, S.; Manes, C.; Fischer, D.; de Groot, K.; Fliser, D.; et al. Simvastatin versus ezetimibe: Pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation 2005, 111, 2356–2363. [Google Scholar] [CrossRef]
- Yoshida, M.; Sawada, T.; Ishii, H.; Gerszten, R.E.; Rosenzweig, A.; Gimbrone, M.A., Jr.; Yasukochi, Y.; Numano, F. Hmg-CoA reductase inhibitor modulates monocyte-endothelial cell interaction under physiological flow conditions in vitro: Involvement of Rho GTPase-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, A.; Shibui, T.; Kohashi, K.; Kosugi, M.; Kusama, Y.; Atarashi, H.; Shimizu, W. Differential Effects of Atorvastatin and Pitavastatin on Inflammation, Insulin Resistance, and the Carotid Intima-Media Thickness in Patients with Dyslipidemia. J. Atheroscler. Thromb. 2015, 22, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Murakami, Y.; Saitoh, M.; Yokoi, T.; Aoki, T.; Miura, K.; Ueshima, H.; Nozaki, K. Statin use and risk of cerebral aneurysm rupture: A hospital-based case-control study in Japan. J. Stroke Cerebrovasc. Dis. 2014, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; Cai, T.; et al. Lipid-Lowering Agents and High HDL (High-Density Lipoprotein) Are Inversely Associated With Intracranial Aneurysm Rupture. Stroke 2018, 49, 1148–1154. [Google Scholar] [CrossRef]
- Shimizu, K.; Imamura, H.; Tani, S.; Adachi, H.; Sakai, C.; Ishii, A.; Kataoka, H.; Miyamoto, S.; Aoki, T.; Sakai, N. Candidate drugs for preventive treatment of unruptured intracranial aneurysms: A cross-sectional study. PLoS ONE 2021, 16, e0246865. [Google Scholar] [CrossRef] [PubMed]
- Matouk, C.C.; Mandell, D.M.; Günel, M.; Bulsara, K.R.; Malhotra, A.; Hebert, R.; Johnson, M.H.; Mikulis, D.J.; Minja, F.J. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery 2013, 72, 492–496; discussion 496. [Google Scholar] [CrossRef]
- Xia, J.; Peng, F.; Chen, X.; Yang, F.; Feng, X.; Niu, H.; Xu, B.; Liu, X.; Guo, J.; Zhong, Y.; et al. Statins may Decrease Aneurysm wall Enhancement of Unruptured Fusiform Intracranial Aneurysms: A high-resolution 3T MRI Study. Transl. Stroke Res. 2023. [Google Scholar] [CrossRef]
- Kang, H.; Tian, D.-C.; Yang, X.; Zhang, Y.; Li, W.; Sui, B.; Duan, Y.; Zhang, Y.; Liu, J.; Wang, K.; et al. A Randomized Controlled Trial of Statins to Reduce Inflammation in Unruptured Cerebral Aneurysms. JACC Cardiovasc. Imaging 2022, 15, 1668–1670. [Google Scholar] [CrossRef]
- Terceño, M.; Remollo, S.; Silva, Y.; Bashir, S.; Werner, M.; Vera-Monge, V.A.; Serena, J.; Castaño, C. Effect of combined acetylsalicylic acid and statins treatment on intracranial aneurysm rupture. PLoS ONE 2021, 16, e0247153. [Google Scholar] [CrossRef]
- Yoshida, K.; Uwano, I.; Sasaki, M.; Takahashi, O.; Sakai, N.; Tsuruta, W.; Nakase, H.; Ogasawara, K.; Osato, T.; Takahashi, J.C.; et al. Small Unruptured Aneurysm Verification-prevention Effect against Growth of Cerebral Aneurysm Study Using Statin. Neurol. Med. Chir. 2021, 61, 442–451. [Google Scholar] [CrossRef]
- Sandvei, M.S.; Romundstad, P.R.; Müller, T.B.; Vatten, L.; Vik, A. Risk Factors for Aneurysmal Subarachnoid Hemorrhage in a Prospective Population Study. Stroke 2009, 40, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Schläppi, J.A.; Fung, C.; Hüsler, J.; Beck, J.; Raabe, A. Do statins reduce the risk of aneurysm development? A case-control study. J. Neurosurg. 2012, 116, 638–642. [Google Scholar] [CrossRef]
- Bekelis, K.; Smith, J.; Zhou, W.; MacKenzie, T.A.; Roberts, D.W.; Skinner, J.; Morden, N.E. Statins and subarachnoid hemorrhage in Medicare patients with unruptured cerebral aneurysms. Int. J. Stroke 2015, 10, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Jabbarli, R.; Darkwah Oppong, M.; Chihi, M.; Dinger, T.F.; Said, M.; Rodemerk, J.; Dammann, P.; Schmidt, B.; Deuschl, C.; Guberina, N.; et al. Regular medication as a risk factor for intracranial aneurysms: A comparative case-control study. Eur. Stroke J. 2023, 8, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Damanhouri, Z.A.; Alkreathy, H.M.; Alharbi, F.A.; Abualhamail, H.; Ahmad, M.S. A Review of the Impact of Pharmacogenetics and Metabolomics on the Efficacy of Metformin in Type 2 Diabetes. Int. J. Med. Sci. 2023, 20, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Di Magno, L.; Di Pastena, F.; Bordone, R.; Coni, S.; Canettieri, G. The Mechanism of Action of Biguanides: New Answers to a Complex Question. Cancers 2022, 14, 3220. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, Y.; Liu, P.; Song, Y.; Liu, Y.; Ying, L.; Quan, K.; Yu, G.; Fan, Z.; Zhu, W. Metformin inhibits intracranial aneurysm formation and progression by regulating vascular smooth muscle cell phenotype switching via the AMPK/ACC pathway. J. Neuroinflamm. 2020, 17, 191. [Google Scholar] [CrossRef]
- Hsueh, W.A.; Law, R.E. PPARγ and Atherosclerosis. Arterioscler.Thromb. Vasc. Biol. 2001, 21, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.M.; Starke, R.M.; Gu, H.; Wilson, K.; Chu, Y.; Chalouhi, N.; Heistad, D.D.; Faraci, F.M.; Sigmund, C.D. Smooth Muscle Peroxisome Proliferator–Activated Receptor γ Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo. Hypertension 2015, 66, 211–220. [Google Scholar] [CrossRef]
- Shimada, K.; Furukawa, H.; Wada, K.; Korai, M.; Wei, Y.; Tada, Y.; Kuwabara, A.; Shikata, F.; Kitazato, K.T.; Nagahiro, S.; et al. Protective Role of Peroxisome Proliferator-Activated Receptor-γ in the Development of Intracranial Aneurysm Rupture. Stroke 2015, 46, 1664–1672. [Google Scholar] [CrossRef]
- Jones, A.; Deb, R.; Torsney, E.; Howe, F.; Dunkley, M.; Gnaneswaran, Y.; Gaze, D.; Nasr, H.; Loftus, I.M.; Thompson, M.M.; et al. Rosiglitazone Reduces the Development and Rupture of Experimental Aortic Aneurysms. Circulation 2009, 119, 3125–3132. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.-C. Mechanism of Action of Fibrates on Lipid and Lipoprotein Metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Cullen, B.; Rush, C.; Moran, C.S.; Secomb, E.; Wood, F.; Daugherty, A.; Campbell, J.H.; Norman, P.E. Peroxisome proliferator-activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis 2010, 210, 51–56. [Google Scholar] [CrossRef]
- Krishna, S.M.; Seto, S.W.; Moxon, J.V.; Rush, C.; Walker, P.J.; Norman, P.E.; Golledge, J. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am. J. Pathol. 2012, 181, 706–718. [Google Scholar] [CrossRef]
- Moxon, J.V.; Rowbotham, S.E.; Pinchbeck, J.L.; Lazzaroni, S.M.; Morton, S.K.; Moran, C.S.; Quigley, F.; Jenkins, J.S.; Reid, C.M.; Cavaye, D.; et al. A Randomised Controlled Trial Assessing the Effects of Peri-operative Fenofibrate Administration on Abdominal Aortic Aneurysm Pathology: Outcomes From the FAME Trial. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Pinchbeck, J.L.; Moxon, J.V.; Rowbotham, S.E.; Bourke, M.; Lazzaroni, S.; Morton, S.K.; Matthews, E.O.; Hendy, K.; Jones, R.E.; Bourke, B.; et al. Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME -2 Trial. J. Am. Heart Assoc. 2018, 7, e009866. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- Ruigrok, Y.M.; Dekkers, P.J.; Bromberg, J.E.; Algra, A.; Rinkel, G.J. Corticosteroid use and risk of aneurysmal subarachnoid haemorrhage. J. Neurol. 2006, 253, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Matur, A.V.; Yamani, A.S.; Robinson, M.W.; Smith, M.S.; Shirani, P.; Grossman, A.W.; Prestigiacomo, C.J. Association between underlying autoimmune disease and small aneurysm size at rupture. J. Neurosurg. 2023, 138, 701–708. [Google Scholar] [CrossRef]
- Patel, S.; Singh, R.; Preuss, C.V.; Patel, N. Warfarin. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shima, Y.; Sasagawa, S.; Ota, N.; Oyama, R.; Tanaka, M.; Kubota-Sakashita, M.; Kawakami, H.; Kobayashi, M.; Takubo, N.; Ozeki, A.N.; et al. Increased PDGFRB and NF-κB signaling caused by highly prevalent somatic mutations in intracranial aneurysms. Sci. Transl. Med. 2023, 15, eabq7721. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Zhang, Z.; Zhao, R.; Wang, C.; Hou, W.; Huang, Q.; Liu, J. Pharmacological inhibition of epidermal growth factor receptor attenuates intracranial aneurysm formation by modulating the phenotype of vascular smooth muscle cells. CNS Neurosci. Ther. 2022, 28, 64–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crane, A.; Shanahan, R.M.; Hudson, J.S.; Nowicki, K.W.; Gersey, Z.C.; Agarwal, P.; Jacobs, R.C.; Lang, M.J.; Gross, B. Pharmaceutical Modulation of Intracranial Aneurysm Development and Rupture. J. Clin. Med. 2024, 13, 3324. https://doi.org/10.3390/jcm13113324
Crane A, Shanahan RM, Hudson JS, Nowicki KW, Gersey ZC, Agarwal P, Jacobs RC, Lang MJ, Gross B. Pharmaceutical Modulation of Intracranial Aneurysm Development and Rupture. Journal of Clinical Medicine. 2024; 13(11):3324. https://doi.org/10.3390/jcm13113324
Chicago/Turabian StyleCrane, Alex, Regan M. Shanahan, Joseph S. Hudson, Kamil W. Nowicki, Zachary C. Gersey, Prateek Agarwal, Rachel C. Jacobs, Michael J. Lang, and Bradley Gross. 2024. "Pharmaceutical Modulation of Intracranial Aneurysm Development and Rupture" Journal of Clinical Medicine 13, no. 11: 3324. https://doi.org/10.3390/jcm13113324






