Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue
Abstract
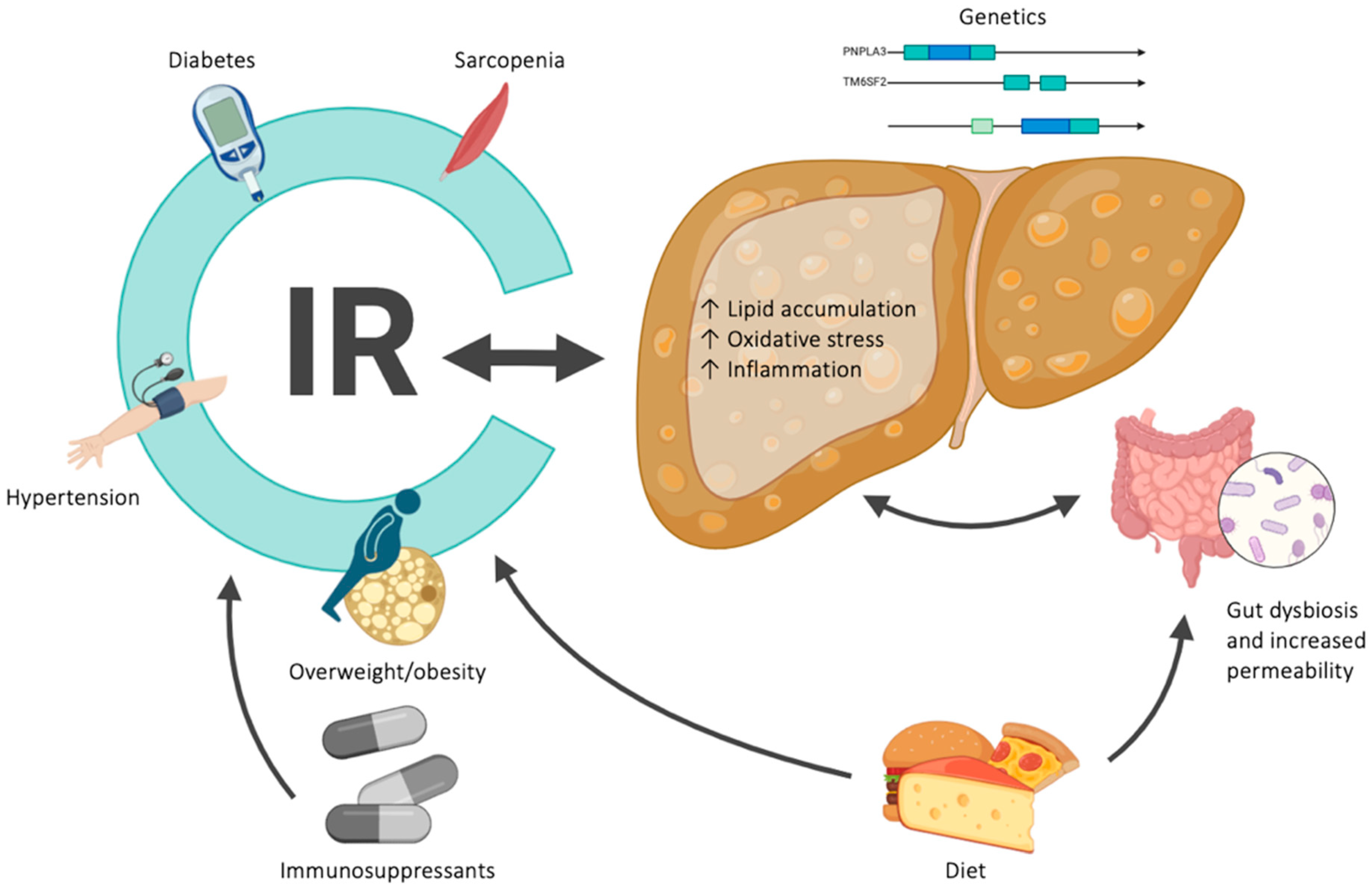
1. Introduction
2. Disease Burden: Epidemiology, Natural History, and Socio-Economic Impact
2.1. Epidemiology
2.2. Natural History
| Authors (Ref.), Publication Year | Study Design | Type of LT | F-Up * | Patient Nr. | Diagnostic Tools | Prevalence/Incidence | Risk Factors | Other Relevant Results | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-LT | Post-LT | ||||||||
| Adali [14], 2023 | Cross-sectional, prospective | LD and DD | NR | 122 | VCTE + CAP | 34%/NR | NR | DM2 | - |
| Mak [16], 2023 | Retrospective | LD and DD | NR | 549 | Biopsy and VCTE + CAP | 29%/NR | Cryptogenic cirrhosis | BMI, DM2, AH, dyslipidemia | Advanced fibrosis: 4% |
| Choudhary [18], 2024 | Prospective | LD | 62 | 117 | US scan | 33%/NR | BMI | Overweight, DM2, dyslipidemia | No effect of HS on OS |
| Vallin [21], 2014 | Prospective | LD and DD | 84 | 91 | Biopsy | NR/NR | NR | Recurrent NAFLD more aggressive than de novo NAFLD in terms of NASH and fibrosis development | At 5th year: NASH 17%, severe fibrosis 12% |
| Villeret [24], 2023 | Retrospective | LD and DD | 56 | 150 | Biopsy | NR/at 1st and 5th years: NAFLD 68% and 85% NASH 15% and 60% | BMI ≥ 31, ≥65 years | Low HDL cholesterol, CsA | At 5th year: F ≥ 2 and F3/4, 48% and 20% |
| Narayanan [25], 2019 | Retrospective | NR | 120 | 588 | Biopsy and US scan, CT, MR | NR/at 10th year: 48% (85% de novo vs. 15% recurrent) | Male sex, HCV, NASH | BMI | No effect of HS on survival; NASH is a risk factor for CV events |
| Miyaaki [26], 2019 | Retrospective | LD | 48 | 100 | Biopsy | NR/33% | Younger age | Donor steatosis, weight gain | NASH 27% |
| Balitzer [27], 2022 | Retrospective | NR | NR | 56 | Biopsy | NR/de novo and recurrent NASH: 13% vs. 6.5% | NR | NR | F3/4 in de novo vs. recurrent NASH: 10% vs. 42% but no difference in OS |
| Yalamanchili [29], 2010 | Retrospective | NR | NR | 257 | Biopsy | NR/At 1st, 2nd, 5th, and 10th years: 8%, 14%, 25%, and 33% | NR | NR | At 5th and 10th years: F4 5% and 10%, respectively |
| Hejlova [30], 2016 | Retrospective | NR | 65 | 548 | Biopsy | NR/At 1st and 10th years: 30% and 48%, respectively | ALD, female, BMI | BMI, triglycerides, alcohol consumption, DM2 | NASH: 10%; HS not associated with either higher fibrosis stage or survival |
| Dureja [32], 2011 | Retrospective | NR | 82 | 88 | Biopsy | NR/9% | BMI | BMI, triglycerides, prednisone dose at 6th months after LT | F3/4: 3.4%; no effect of HS on OS |
| Malik [33], 2009 | Case–control, retrospective | LD and DD | 36 | 98 | Biopsy | NR/70% | Younger age | Younger donor age and BMI, DM2, dyslipidemia, and MS | Recurrent NASH: 25%, F ≥ 2: 18%; no effect of HS on OS |
| Bhati [34], 2017 | Retrospective | NR | NR | 103 | Biopsy and VCTE + CAP | NR/88% | Female | Glucose and triglyceride levels | F 3/4: 27%; NASH: 41%; cirrhosis: 5% No OS difference among recurrent NAFLD vs. NASH |
| Galvin [35], 2019 | Retrospective | NR | 36 | 430 | Biopsy | NR/33% | HCV | DM2, BMI, weight gain, and SIR | F ≥ 2: 40%; no effect of HS on OS |
| Tejedor-Tejada [39], 2021 | Cross-sectional, retrospective | NR | 60 | 252 | NITs | 36%/NR | Male | Obesity, MS, DM2 | F ≥ 2: 58–86%;no effect of HS on OS |
| Gitto [40], 2018 | Retrospective | LD and DD | 120 | 194 | Biopsy and NITs | NR/20% | NR | MS, DM2, LD, Tac | Significantly lower long-term OS in patients with de novo NASH |
2.3. Socio-Economic Impact
3. Risk Factors
4. Diagnostic Assessment
5. Management: Preventing Strategies and Treatment Options
6. Discussion
7. Conclusions
8. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marchesini, G.; Roden, M.; Vettor, R. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Al Shabeeb, R.; Eberly, K.E.; Shah, D.; Nguyen, V.; Ong, J.; Henry, L.; Alqahtani, S.A. The changing epidemiology of adult liver transplantation in the United States in 2013–2022: The dominance of metabolic dysfunction–associated steatotic liver disease and alcohol-associated liver disease. Hepatol. Commun. 2023, 8, e0352. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)-50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Stepanova, M.; Kabbara, K.; Mohess, D.; Verma, M.; Roche-Green, A.; AlQahtani, S.; Ong, J.; Burra, P.; Younossi, Z.M. Nonalcoholic steatohepatitis is the most common indication for liver transplantation among the elderly: Data from the United States Scientific Registry of Transplant Recipients. Hepatol. Commun. 2022, 6, 1506–1515. [Google Scholar] [CrossRef]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.-N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, H.; Wang, C.; Chen, C.; Tang, J.; Zhou, M.; Hong, X.; Cheng, Y.; Wu, Q.; Zhang, X.; et al. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front. Endocrinol. 2022, 13, 934225. [Google Scholar] [CrossRef] [PubMed]
- Wongtrakul, W.; Charatcharoenwitthaya, N.; Charatcharoenwitthaya, P. Metabolic dysfunction-associated steatotic liver disease and the risk of mortality in individuals with type 2 diabetes: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2024, 36, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Paik, J.M.; Stepanova, M.; Ong, J.; Alqahtani, S.; Henry, L. Clinical profiles and mortality rates are similar for metabolic dysfunction-associated steatotic liver disease and non-alcoholic fatty liver disease. J. Hepatol. 2024, 80, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2024, 80, e76–e77. [Google Scholar] [CrossRef]
- Hardy, T.; Wonders, K.; Younes, R.; Aithal, G.P.; Aller, R.; Allison, M.; Bedossa, P.; Betsou, F.; Boursier, J.; Brosnan, M.J.; et al. The European NAFLD Registry: A real-world longitudinal cohort study of nonalcoholic fatty liver disease. Contemp. Clin. Trials 2020, 98, 106175. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Nogueira, P.; Machado, M.V. Hepatic steatosis after liver transplantation: A systematic review and meta-analysis. Liver Transplant. 2023, 29, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Dhampalwar, S.; Saraf, N.; Soin, A.S. Metabolic dysfunction-associated steatotic liver disease: Where does non-alcoholic fatty liver disease in liver transplant recipients fit in this new definition? J. Hepatol. 2024, 80, e77–e79. [Google Scholar] [CrossRef] [PubMed]
- Adali, G.; Bilgic, N.M.; Kalaman, A.E.; Ozturk, O.; Ozdil, K. Prevalence and predictors of metabolic-associated fatty liver disease in liver transplant recipients: A cross-sectional prospective study. Hepatol. Forum 2023, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, S.; Cusi, G.; Lapenna, L.; Gazda, J.; Fonte, S.; Mattana, M.; Mennini, G.; Pasqualetti, P.; Merli, M. Diabetes and Metabolic Disorders: Their Impact on Cardiovascular Events in Liver Transplant Patients. Can. J. Gastroenterol. Hepatol. 2023, 2023, 2199193. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.Y.; Chan, A.C.; Wong, T.C.; Dai, W.C.; She, W.H.; Ma, K.W.; Sin, S.L.; Chu, K.W.; Seto, W.K.; Yuen, M.F.; et al. High prevalence of de novo metabolic dysfunction-associated fatty liver disease after liver transplantation and the role of controlled attenuation parameter. BMC Gastroenterol. 2023, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Glass, L.; Sharma, P.; Shannon, C.; Sonnenday, C.J.; Tincopa, M.A. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation 2019, 103, E345–E354. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Dhampalwar, S.; Saraf, N.; Rastogi, A.; Bhangui, P.; Soin, A.S. Post-transplant Non-alcoholic Fatty Liver Disease and Metabolic Syndrome After Living Donor Liver Transplantation in Indians. J. Clin. Exp. Hepatol. 2024, 14, 101281. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Losurdo, G.; Castellaneta, A.; Rendina, M.; Carparelli, S.; Leandro, G.; Di Leo, A. Systematic review with meta-analysis: De novo non-alcoholic fatty liver disease in liver-transplanted patients. Aliment. Pharmacol. Ther. 2018, 47, 704–714. [Google Scholar] [CrossRef]
- Vallin, M.; Guillaud, O.; Boillot, O.; Hervieu, V.; Scoazec, J.-Y.; Dumortier, J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: Natural history based on liver biopsy analysis. Liver Transplant. 2014, 20, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of non-alcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Deng, Y. Comparison of mortality outcomes in individuals with MASLD and/or MAFLD. J. Hepatol. 2024, 80, e62–e64. [Google Scholar] [CrossRef] [PubMed]
- Villeret, F.; Dharancy, S.; Erard, D.; Abergel, A.; Barbier, L.; Besch, C.; Boillot, O.; Boudjema, K.; Coilly, A.; Conti, F.; et al. Inevitability of disease recurrence after liver transplantation for NAFLD cirrhosis. JHEP Rep. 2023, 5, 100668. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Mara, K.; Izzy, M.; Dierkhising, R.; Heimbach, J.; Allen, A.M.; Watt, K.D. Recurrent or De Novo Allograft Steatosis and Long-term Outcomes After Liver Transplantation. Transplantation 2019, 103, e14–e21. [Google Scholar] [CrossRef] [PubMed]
- Miyaaki, H.; Miuma, S.; Taura, N.; Shibata, H.; Sasaki, R.; Soyama, A.; Hidaka, M.; Takatsuki, M.; Eguchi, S.; Nakao, K. Risk Factors and Clinical Course for Liver Steatosis or Nonalcoholic Steatohepatitis after Living Donor Liver Transplantation. Transplantation 2019, 103, 109–112. [Google Scholar] [CrossRef]
- Balitzer, D.; Tsai, J.-H.; Gill, R.M. Clinicopathologic features of de novo non-alcoholic steatohepatitis in the post-transplant setting. Diagn. Pathol. 2022, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Germani, G.; Laryea, M.; Rubbia-Brandt, L.; Egawa, H.; Burra, P.; O’Grady, J.; Watt, K.D. Management of Recurrent and De Novo NAFLD/NASH After Liver Transplantation. Transplantation 2019, 103, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Yalamanchili, K.; Saadeh, S.; Klintmalm, G.B.; Jennings, L.W.; Davis, G.L. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or non-alcoholic fatty liver disease. Liver Transpl. 2010, 16, 431–439. [Google Scholar] [CrossRef]
- Hejlova, I.; Honsova, E.; Sticova, E.; Lanska, V.; Hucl, T.; Spicak, J.; Jirsa, M.; Trunecka, P. Prevalence and risk factors of steatosis after liver transplantation and patient outcomes. Liver Transplant. 2016, 22, 644–655. [Google Scholar] [CrossRef]
- Choudhary, N.S.; Saigal, S. Preventive Strategies for Nonalcoholic Fatty Liver Disease after Liver Transplantation. J. Clin. Exp. Hepatol. 2019, 9, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Dureja, P.; Mellinger, J.; Agni, R.; Chang, F.; Avey, G.; Lucey, M.; Said, A. NAFLD Recurrence in Liver Transplant Recipients. Transplantation 2011, 91, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.M.; Devera, M.E.; Fontes, P.; Shaikh, O.; Sasatomi, E.; Ahmad, J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transplant. 2009, 15, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Bhati, C.; Idowu, M.O.; Sanyal, A.J.; Rivera, M.; Driscoll, C.; Stravitz, R.T.; Kohli, D.R.; Matherly, S.; Puri, P.; Gilles, H.; et al. Long-term Outcomes in Patients Undergoing Liver Transplantation for Nonalcoholic Steatohepatitis-Related Cirrhosis. Transplantation 2017, 101, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Galvin, Z.; Rajakumar, R.; Chen, E.; Adeyi, O.; Selzner, M.; Grant, D.; Sapisochin, G.; Greig, P.; Cattral, M.; McGilvray, I.; et al. Predictors of De Novo Nonalcoholic Fatty Liver Disease After Liver Transplantation and Associated Fibrosis. Liver Transplant. 2019, 25, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, J.M.; de La Melena, V.; Orloff, S.L.; Corless, C.L.; Rosen, H.R.; Olyaei, A.J. Late mortality after orthotopic liver transplantation. Am. J. Surg. 2001, 181, 475–479. [Google Scholar] [CrossRef]
- Serrano, M.T.; Sabroso, S.; Esteban, L.M.; Berenguer, M.; Fondevila, C.; Lorente, S.; Cortés, L.; Sanchez-Antolin, G.; Nuño, J.; De la Rosa, G.; et al. Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl. Int. 2022, 35, 10263. [Google Scholar] [CrossRef]
- Tejedor-Tejada, J.; Valenzuela, E.F.; Muñoz, R.N.; Gómez, L.H.; García-Pajares, F.; Álvarez, C.A.; Sánchez-Martín, F.; Alonso-Martín, C.; Sánchez-Antolín, G. De-novo nonalcoholic fatty liver disease at 5 years after liver transplantation: Prevalence and predictive factors. Eur. J. Gastroenterol. Hepatol. 2021, 33, 399–406. [Google Scholar] [CrossRef]
- Gitto, S.; Benedetto, F.; Maria, N.; Tarantino, G.; Serra, V.; Maroni, L.; Cescon, M.; Pinna, A.; Andreone, P.; Villa, E. De novo non-alcoholic steatohepatitis is associated with a five-fold long-term increased mortality in liver transplant recipients. J. Hepatol. 2017, 66, S41. [Google Scholar] [CrossRef]
- Lim, W.H.; Tan, C.; Xiao, J.; Tan, D.J.H.; Ng, C.H.; Yong, J.N.; Fu, C.; Chan, K.E.; Zeng, R.W.; Ren, Y.P.; et al. De novo metabolic syndrome after liver transplantation: A meta-analysis on cumulative incidence, risk factors, and outcomes. Liver Transplant. 2023, 29, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, J.; Verbeek, J.; van Malenstein, H.; Laleman, W.; Cassiman, D.; Verslype, C.; van der Merwe, S.; Jochmans, I.; Sainz-Barriga, M.; Monbaliu, D.; et al. Liver-Related and Cardiovascular Outcome of Patients Transplanted for Nonalcoholic Fatty Liver Disease: A European Single-Center Study. Transplant. Proc. 2021, 53, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Fussner, L.A.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Leise, M.D.; Watt, K.D. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transplant. 2015, 21, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Hastie, D.J.; Lee, K.J.; Addonizio, E.; Fan, G.H.; Chou, H.; Jung, D.; Lee, K.; Lominadze, Z. The trends in cost associated with liver transplantation in the US: Analysis of weighted hospital data. Liver Transplant. 2023, 29, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hilst, C.S.; IJtsma, A.J.; Slooff, M.J.; TenVergert, E.M. Cost of Liver Transplantation: A Systematic Review and Meta-Analysis Comparing the United States with Other OECD Countries. Med. Care Res. Rev. 2009, 66, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Balp, M.-M.; Krieger, N.; Przybysz, R.; Way, N.; Cai, J.; Zappe, D.; McKenna, S.J.; Wall, G.; Janssens, N.; Tapper, E. The burden of non-alcoholic steatohepatitis (NASH) among patients from Europe: A real-world patient-reported outcomes study. JHEP Rep. 2019, 1, 154–161. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.; Finnegan, A.; Dhillon, H.; Ruiz-Casas, L.; Pedra, G.; Franks, B.; Morgan, G.; Hebditch, V.; Jönsson, B.; Mabhala, M.; et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: The GAIN study. JHEP Rep. 2020, 2, 100142. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Lazarus, J.V.; Newsome, P.N.; Serfaty, L.; Aghemo, A.; Augustin, S.; Tsochatzis, E.; de Ledinghen, V.; Bugianesi, E.; Romero-Gomez, M.; et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: A cost-of-illness analysis. Liver Int. 2021, 41, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Tampi, R.P.; Racila, A.; Qiu, Y.; Burns, L.; Younossi, I.; Nader, F. Economic and Clinical Burden of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes in the US. Diabetes Care 2020, 43, 283–289. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Allen, A.M.; Arab, J.P.; Carrieri, P.; Noureddin, M.; Alazawi, W.; Alkhouri, N.; Alqahtani, S.A.; Arrese, M.; et al. A global research priority agenda to advance public health responses to fatty liver disease. J. Hepatol. 2023, 79, 618–634. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Spongberg, C.; Martinino, A.; Giovinazzo, F. Exploring the Multifaceted Landscape of MASLD: A Comprehensive Synthesis of Recent Studies, from Pathophysiology to Organoids and Beyond. Biomedicines 2024, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Zhao, W.; Ma, J.; Toshiyoshi, M.M. Patatin-like phospholipase domain-containing 3 gene (PNPLA3) polymorphic (rs738409) single nucleotide polymorphisms and susceptibility to nonalcoholic fatty liver disease: A meta-analysis of twenty studies. Medicine 2023, 102, e33110. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Auer, C.; Glodny, B.; Posch, U.; Steitzer, H.; Lanzer, G.; Pratschke, J.; Biebl, M.; Steurer, M.; Graziadei, I.; et al. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin. Gastroenterol. Hepatol. 2013, 11, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.W.; Lee, K.; Seo, S.; Park, M.Y.; Ahn, S.W.; Hong, S.K.; Yoon, K.C.; Kim, H.S.; Choi, Y.; et al. Effect of PNPLA3 I148M polymorphism on histologically proven non-alcoholic fatty liver disease in liver transplant recipients. Hepatol. Res. 2018, 48, e162–e171. [Google Scholar] [CrossRef] [PubMed]
- Trunečka, P.; Míková, I.; Dlouhá, D.; Hubáček, J.A.; Honsová, E.; Kolesár, L.; Lánská, V.; Fraňková, S.; Šperl, J.; Jirsa, M.; et al. Donor PNPLA3 rs738409 genotype is a risk factor for graft steatosis. A post-transplant biopsy-based study. Dig. Liver Dis. 2018, 50, 490–495. [Google Scholar] [CrossRef]
- Liu, Z.T.; Chen, T.C.; Lu, X.X.; Cheng, J.; Xie, H.Y.; Zhou, L.; Zheng, S.S. PNPLA3 I148M variant affects non-alcoholic fatty liver disease in liver transplant recipients. World J. Gastroenterol. 2015, 21, 10054–10056. [Google Scholar] [CrossRef] [PubMed]
- Míková, I.; Neřoldová, M.; Hubáček, J.A.; Dlouhá, D.; Jirsa, M.; Honsová, E.; Sticová, E.; Lánská, V.; Špičák, J.; Trunečka, P. Donor PNPLA3 and TM6SF2 Variant Alleles Confer Additive Risks for Graft Steatosis After Liver Transplantation. Transplantation 2020, 104, 526–534. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Tran, Q.T.; Kovalic, A.J.; Bontha, S.V.; Jiang, Y.; Kedia, S.; Karri, S.; Mupparaju, V.; Podila, P.S.B.; Verma, R.; et al. Clinical and Genetic Risk Factors of Recurrent Nonalcoholic Fatty Liver Disease After Liver Transplantation. Clin. Transl. Gastroenterol. 2021, 12, e00302. [Google Scholar] [CrossRef]
- Chiu, K.-W.; Goto, S.; Nakano, T.; Hu, T.-H.; Chen, D.-W.; Huang, K.-T.; Hsu, L.-W.; Chen, C.-L. Genetic polymorphisms of the hepatic pathways of fatty liver disease after living donor liver transplantation. Liver Int. 2018, 38, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Beckebaum, S.; Iacob, R.; Gheorghe, C.; Cicinnati, V.; Popescu, I.; Gheorghe, L. Genetic and Life Style Risk Factors for Recurrent Non-alcoholic Fatty Liver Disease Following Liver Transplantation. Front. Nutr. 2022, 8, 787430. [Google Scholar] [CrossRef] [PubMed]
- Kelava, T.; Turcic, P.; Markotic, A.; Ostojic, A.; Sisl, D.; Mrzljak, A. Importance of genetic polymorphisms in liver transplantation outcomes. World J. Gastroenterol. 2020, 26, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Lee, K.W.; Yi, N.J.; Lee, H.W.; Hong, G.; Choi, Y.; You, T.; Suh, S.W.; Jang, J.J.; et al. Histologically proven non-alcoholic fatty liver disease and clinically related factors in recipients after liver transplantation. Clin. Transplant. 2014, 28, 521–529. [Google Scholar] [CrossRef]
- Vanlerberghe, B.T.; van Malenstein, H.; Sainz-Bariga, M.; Jochmans, I.; Cassiman, D.; Monbaliu, D.; van der Merwe, S.; Pirenne, J.; Nevens, F.; Verbeek, J. Utility and prognostic value of diagnosing MAFLD in patients undergoing liver transplantation for alcohol-related liver disease. Clin. Transplant. 2023, 37, e14965. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Sterling, R.K. Posttransplant Metabolic Syndrome. Int. J. Hepatol. 2012, 2012, 891516. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, M.F.; Weinmann, A.; Lohse, N.; Tönissen, H.; Koch, S.; Schattenberg, J.; Hoppe-Lotichius, M.; Zimmermann, T.; Galle, P.R.; Hansen, T.; et al. Metabolic syndrome and its association with fatty liver disease after orthotopic liver transplantation. Transpl. Int. 2013, 26, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Spiritos, Z.; Abdelmalek, M.F. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 13. [Google Scholar] [CrossRef]
- Dumortier, J.; Giostra, E.; Belbouab, S.; Morard, I.; Guillaud, O.; Spahr, L.; Boillot, O.; Rubbia-Brandt, L.; Scoazec, J.-Y.; Hadengue, A. Non-Alcoholic Fatty Liver Disease in Liver Transplant Recipients: Another Story of “Seed and Soil”. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 613–620. [Google Scholar] [CrossRef]
- Charlton, M.; Levitsky, J.; Aqel, B.; O’Grady, J.; Hemibach, J.; Rinella, M.; Fung, J.; Ghabril, M.; Thomason, R.; Burra, P.; et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2018, 102, 727–743. [Google Scholar] [CrossRef] [PubMed]
- De Simone, P.; Battistella, S.; Lai, Q.; Ducci, J.; D’Arcangelo, F.; Marchetti, P.; Russo, F.P.; Burra, P. Immunosuppression for older liver transplant recipients. Transplant. Rev. 2024, 38, 100817. [Google Scholar] [CrossRef] [PubMed]
- Alten, T.A.; Negm, A.A.; Voigtländer, T.; Jaeckel, E.; Lehner, F.; Brauner, C.; Wedemeyer, H.; Manns, M.P.; Lankisch, T.O. Safety and performance of liver biopsies in liver transplant recipients. Clin. Transplant. 2014, 28, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Midia, M.; Devang, O.; Shuster, A.; Midia, R.; Muir, J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: A scoping review. Diagn. Interv. Radiol. 2019, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Tian, G.; Kong, D.; Jiang, T.; Li, L. Complications After Percutaneous Ultrasound-Guided Liver Biopsy. J. Ultrasound Med. 2020, 39, 1355–1365. [Google Scholar] [CrossRef]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Colca, J.R.; Iwashita, J.; Koch, G.G.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef]
- Bohte, A.E.; van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2011, 21, 87–97. [Google Scholar] [CrossRef]
- Dasarathy, S.; Dasarathy, J.; Khiyami, A.; Joseph, R.; Lopez, R.; McCullough, A.J. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J. Hepatol. 2009, 51, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Webb, M.; Yeshua, H.; Zelber-Sagi, S.; Santo, E.; Brazowski, E.; Halpern, Z.; Oren, R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. Am. J. Roentgenol. 2009, 192, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.-M.; Gilja, O.H.; Klauser, A.S.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 2: Clinical Applications. Ultraschall Med.-Eur. J. Ultrasound 2013, 34, 238–253. [Google Scholar] [CrossRef]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis. Ultrasound Q. 2016, 32, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Zong, F.; Jiang, T. Alcoholic/Nonalcoholic Fatty Liver Disease Detection with TransientElastography: A Detailed Review and Meta-analysis. Curr. Med. Imaging 2023, 20, e230223213941. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Petroff, D.; Blank, V.; Newsome, P.N.; Voican, C.S.; Thiele, M.; de Lédinghen, V.; Baumeler, S.; Chan, W.K.; Perlemuter, G.; Cardoso, A.C.; et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: An individual patient data meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Chayanupatkul, M.; Dasani, D.B.; Sogaard, K.; Schiano, T.D. The Utility of Assessing Liver Allograft Fibrosis and Steatosis Post-Liver Transplantation Using Transient Elastography With Controlled Attenuation Parameter. Transplant. Proc. 2021, 53, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Kollmeier, J.; Böhm, S.; Müller, J.; Kovacs, P.; Tröltzsch, M.; Weimann, A.; Bartels, M.; Rosendahl, J.; Mössner, J.; et al. Noninvasive characterization of graft steatosis after liver transplantation. Scand. J. Gastroenterol. 2015, 50, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Idowu, M.O.; Stromberg, K.; Sima, A.; Lee, E.; Patel, S.; Ghaus, S.; Driscoll, C.; Sterling, R.K.; John, B.; et al. Diagnostic Performance of Vibration-Controlled Transient Elastography in Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2021, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- Petta, S.; Sebastiani, G.; Viganò, M.; Ampuero, J.; Wong, V.W.S.; Boursier, J.; Berzigotti, A.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 806. [Google Scholar] [CrossRef]
- Dumont, C.; Lanthier, N.; Dahlqvist, G. Fibrosis and steatosis of the liver graft: Are non-invasive tests useful? A short review. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102194. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Tazari, M.; Sebastiani, G. Performance of transient elastography and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: A meta-analysis. PLoS ONE 2017, 12, e0185192. [Google Scholar] [CrossRef]
- Andersson, A.; Kelly, M.; Imajo, K.; Nakajima, A.; Fallowfield, J.A.; Hirschfield, G.; Pavlides, M.; Sanyal, A.J.; Noureddin, M.; Banerjee, R.; et al. Clinical Utility of Magnetic Resonance Imaging Biomarkers for Identifying Nonalcoholic Steatohepatitis Patients at High Risk of Progression: A Multicenter Pooled Data and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2451–2461. [Google Scholar] [CrossRef]
- Gu, J.; Liu, S.; Du, S.; Zhang, Q.; Xiao, J.; Dong, Q.; Xin, Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: A meta-analysis. Eur. Radiol. 2019, 29, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Li, M.; Hamilton, G.; Zhang, Y.N.; Song, B. Diagnostic accuracy of hepatic proton density fat fraction measured by magnetic resonance imaging for the evaluation of liver steatosis with histology as reference standard: A meta-analysis. Eur. Radiol. 2019, 29, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Munaganuru, N.; Barnard, A.; Wang, J.L.; Kaulback, K.; Argo, C.K.; Singh, S.; Fowler, K.J.; Sirlin, C.B.; Loomba, R. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 2274–2283.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Xia, C.; Wang, Y.; Tang, Q.; Li, J.; Zhou, X.H. Toward standardized premarket evaluation of computer aided diagnosis/detection products: In-sights from FDA-approved products. Expert Rev. Med. Devices 2020, 17, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Keskin, O.; Celik, A.; Savas, B.; Elhan, A.H.; Idilman, R.; Karcaaltincaba, M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016, 57, 271–278. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Hajek, M.; Sedivy, P.; Mikova, I.; Trunecka, P.; Dezortova, M. Lipid Profile and Hepatic Fat Content Measured by 1H MR Spectroscopy in Patients before and after Liver Transplantation. Metabolites 2021, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Hájek, M.; Dezortová, M.; Wagnerová, D.; Škoch, A.; Voska, L.; Hejlová, I.; Trunečka, P. MR spectroscopy as a tool for in vivo determination of steatosis in liver transplant recipients. Magn. Reson. Mater. Phys. Biol. Med. 2011, 24, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Venkatesh, S.K.; Loomba, R.; Wang, Z.; Sirlin, C.; Chen, J.; Yin, M.; Miller, F.H.; Low, R.N.; Hassanein, T.; et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: A diagnostic accuracy systematic review and individual participant data pooled analysis. Eur. Radiol. 2016, 26, 1431–1440. [Google Scholar] [CrossRef]
- Jehangir, M.; Nazir, R.; Jang, A.; Rana, A.; Rafique, S.; Dar, F.S. Macrovesicular steatosis in living related liver donors: Correlation of biopsy findings with CT liver attenuation index and body mass index. Clin. Transplant. 2016, 30, 1016–1020. [Google Scholar] [CrossRef]
- Zheng, D.; Tian, W.; Zheng, Z.; Gu, J.; Guo, Z.; He, X. Accuracy of computed tomography for detecting hepatic steatosis in donors for liver transplantation: A meta-analysis. Clin. Transplant. 2017, 31, e13013. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Kahn, H.S.; Bellentani, S.; Tiribelli, C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Otgonsuren, M.; Estep, M.J.; Hossain, N.; Younossi, E.; Frost, S.; Henry, L.; Hunt, S.; Fang, Y.; Goodman, Z.; Younossi, Z.M. A single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J. Gastroenterol. Hepatol. 2014, 29, 2006–2013. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Lassailly, G.; Diaz, E.; Clement, K.; Caïazzo, R.; Tordjman, J.; Munteanu, M.; Perazzo, H.; Demol, B.; Callafe, R.; et al. Performance of Biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in Patients with Severe Obesity: Meta Analysis of Individual Patient Data. PLoS ONE 2012, 7, e30325. [Google Scholar] [CrossRef]
- Fedchuk, L.; Nascimbeni, F.; Pais, R.; Charlotte, F.; Housset, C.; Ratziu, V.; the LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2014, 40, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Lawitz, E.J.; Alkhouri, N.; Wong, V.W.-S.; Romero-Gomez, M.; Okanoue, T.; Trauner, M.; Kersey, K.; Li, G.; Han, L.; et al. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology 2019, 70, 1521–1530. [Google Scholar] [CrossRef]
- Viganò, M.; Pugliese, N.; Cerini, F.; Turati, F.; Cimino, V.; Ridolfo, S.; Rocchetto, S.; Foglio, F.; Terrin, M.; La Vecchia, C.; et al. Accuracy of FIB-4 to detect elevated liver stiffness measurements in patients with non-alcoholic fatty liver disease: A cross-sectional study in referral centers. Int. J. Mol. Sci. 2022, 23, 12489. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Neves Souza, L.; Isted, A.; Fitzpatrick, E.; Vimalesvaran, S.; Cotoi, C.; Amin, S.; Heaton, N.; Quaglia, A.; Dhawan, A. AST-to-platelet ratio index in non-invasive assessment of long-term graft fibrosis following pediatric liver transplantation. Pediatr. Transplant. 2016, 20, 222–226. [Google Scholar] [CrossRef] [PubMed]
- El Serafy, M.A.; Kassem, A.M.; Omar, H.; Mahfouz, M.S.; Raziky, M.E.S.E. APRI test and hyaluronic acid as non-invasive diagnostic tools for post HCV liver fibrosis: Systematic review and meta-analysis. Arab. J. Gastroenterol. 2017, 18, 51–57. [Google Scholar] [CrossRef]
- El-Meteini, M.; Sakr, M.; Eldorry, A.; Mohran, Z.; Abdelkader, N.A.; Dabbous, H.; Montasser, I.; Refaie, R.; Salah, M.; Aly, M. Non-Invasive Assessment of Graft Fibrosis After Living Donor Liver Transplantation: Is There Still a Role for Liver Biopsy? Transplant. Proc. 2019, 51, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Kamphues, C.; Lotz, K.; Röcken, C.; Berg, T.; Eurich, D.; Pratschke, J.; Neuhaus, P.; Neumann, U.P. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clin. Transplant. 2010, 24, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Pissaia, A.; Borderie, D.; Bernard, D.; Scatton, O.; Calmus, Y.; Conti, F. APRI and FIB-4 Scores Are Useful After Liver Transplantation Independently of Etiology. Transplant. Proc. 2009, 41, 679–681. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Petta, S.; Carraro, A.; Byrne, C.D.; Targher, G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver trans-plantation. Nat. Rev. Endocrinol. 2022, 18, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Mara, K.; Dierkhising, R.; Watt, K.D. Gender, Race and Disease Etiology Predict De Novo Malignancy Risk After Liver Transplantation: Insights for Future Individualized Cancer Screening Guidance. Transplantation 2019, 103, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Fan, J.G.; Francque, S.M. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United Eur. Gastroenterol. J. 2024, 12, 177–186. [Google Scholar] [CrossRef]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef]
- Totti, V.; Tamè, M.; Burra, P.; Mosconi, G.; Roi, G.S.; Sella, G.; Ermolao, A.; Ferrarese, A.; Sgarzi, S.; Savino, G.; et al. Physical Condition, Glycemia, Liver Function, and Quality of Life in Liver Transplant Recipients After a 12-Month Supervised Exercise Program. Transplant. Proc. 2019, 51, 2952–2957. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Koshiaris, C.; Henry, J.A.; Noreik, M.; Morris, E.; Manoharan, I.; Tudor, K.; Bodenham, E.; Dunnigan, A.; Jebb, S.A.; et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021, 115, 154455. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Cassiman, D.; Roelants, M.; Vandenplas, G.; Van der Merwe, S.W.; Mertens, A.; Libbrecht, L.; Verslype, C.; Fevery, J.; Aerts, R.; Pirenne, J.; et al. Orlistat treatment is safe in overweight and obese liver transplant recipients: A prospective, open label trial. Transpl. Int. 2006, 19, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Diwan, T.S.; Rice, T.C.; Heimbach, J.K.; Schauer, D.P. Liver Transplantation and Bariatric Surgery: Timing and Outcomes. Liver Transplant. 2018, 24, 1280–1287. [Google Scholar] [CrossRef]
- Dumortier, J.; Erard, D.; Villeret, F.; Faitot, F.; Duvoux, C.; Faure, S.; Francoz, C.; Gugenheim, J.; Hardwigsen, J.; Hiriart, J.-B.; et al. Bariatric surgery and liver transplantation, here we are now: A French nationwide retrospective study. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102164. [Google Scholar] [CrossRef]
- Lee, Y.; Tian, C.; Lovrics, O.; Soon, M.S.; Doumouras, A.G.; Anvari, M.; Hong, D. Bariatric surgery before, during, and after liver transplantation: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2020, 16, 1336–1347. [Google Scholar] [CrossRef]
- Mosko, J.D.; Nguyen, G.C. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Safwan, M.; Collins, K.M.; Abouljoud, M.S.; Salgia, R. Outcome of liver transplantation in patients with prior bariatric surgery. Liver Transplant. 2017, 23, 1415–1421. [Google Scholar] [CrossRef]
- Zamora-Valdes, D.; Watt, K.D.; Kellogg, T.A.; Poterucha, J.J.; Di Cecco, S.R.; Francisco-Ziller, N.M.; Taner, T.; Rosen, C.B.; Heimbach, J.K. Long-term outcomes of patients undergoing simultaneous liver transplantation and sleeve gastrectomy. Hepatology 2018, 68, 485–495. [Google Scholar] [CrossRef]
- Salomone, F.; Sharaiha, R.Z.; Boškoski, I. Endoscopic bariatric and metabolic therapies for non-alcoholic fatty liver disease: Evidence and perspectives. Liver Int. 2020, 40, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Guckelberger, O. Long-term medical comorbidities and their management: Hypertension/cardiovascular disease. Liver Transplant. 2009, 15, S75–S78. [Google Scholar] [CrossRef] [PubMed]
- Galioto, A.; Semplicini, A.; Zanus, G.; Fasolato, S.; Sticca, A.; Boccagni, P.; Frigo, A.C.; Cillo, U.; Gatta, A.; Angeli, P. Nifedipine versus carvedilol in the treatment of de novo arterial hypertension after liver transplantation: Results of a controlled clinical trial. Liver Transplant. 2008, 14, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Najeed, S.A.; Saghir, S.; Hein, B.; Neff, G.; Shaheen, M.; Ijaz, H.; Khan, I.A. Management of hypertension in liver transplant patients. Int. J. Cardiol. 2011, 152, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.C.; Taler, S.J.; Canzanello, V.J.; Schwartz, L.; Augustine, J.E. Posttransplantation hypertension related to calcineurin inhibitors. Liver Transplant. 2000, 6, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.; Cohen, D.E.; Chalasani, N.; Harrison, S.A. An assessment by the Statin Liver Safety Task Force: 2014 update. J. Clin. Lipidol. 2014, 8, S47–S57. [Google Scholar] [CrossRef] [PubMed]
- Fatourou, E.M.; Tsochatzis, E.A. Management of metabolic syndrome and cardiovascular risk after liver transplantation. Lancet Gastroenterol. Hepatol. 2019, 4, 731–741. [Google Scholar] [CrossRef]
- Lucey, M.R.; Terrault, N.; Ojo, L.; Hay, J.E.; Neuberger, J.; Blumberg, E.; Teperman, L.W. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transplant. 2013, 19, 3–26. [Google Scholar] [CrossRef]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transplant. 2014, 14, 1992–2000. [Google Scholar] [CrossRef]
- Grancini, V.; Resi, V.; Palmieri, E.; Pugliese, G.; Orsi, E. Management of diabetes mellitus in patients undergoing liver transplantation. Pharmacol. Res. 2019, 141, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Luther, P.; Baldwin, D. Pioglitazone in the management of diabetes mellitus after transplantation. Am. J. Transplant. 2004, 4, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, G.; Baldwin, D. Rosiglitazone therapy of posttransplant diabetes mellitus. Transplantation 2005, 80, 1402–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Providencia, R.; Jiang, W.; Liu, M.; Yu, L.; Gu, C.; Chang, A.C.Y.; Ma, H. Association of Metformin with the Mortality and Incidence of Cardiovascular Events in Patients with Pre-existing Cardiovascular Diseases. Drugs 2022, 82, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020, 63, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.-P. Intestinal glucagon-like peptide-1 effects on food intake: Physiological relevance and emerging mechanisms. Peptides 2020, 131, 170342. [Google Scholar] [CrossRef]
- Carbone, L.J.; Angus, P.W.; Yeomans, N.D. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 23–31. [Google Scholar] [CrossRef]
- Dong, Y.; Lv, Q.; Li, S.; Wu, Y.; Li, L.; Li, J.; Zhang, F.; Sun, X.; Tong, N. Efficacy and safety of glucagon-like peptide-1 receptor agonists in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 284–295. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Targher, G. Glucagon-Like Peptide-1 Receptor Agonists for Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: An Updated Meta-Analysis of Randomized Controlled Trials. Metabolites 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Byrne, C.D.; Targher, G. Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: A systematic review. Lancet Gastroenterol. Hepatol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024; online ahead of print. [Google Scholar]
- Thangavelu, T.; Lyden, E.; Shivaswamy, V. A Retrospective Study of Glucagon-Like Peptide 1 Receptor Agonists for the Management of Diabetes After Transplantation. Diabetes Ther. 2020, 11, 987–994. [Google Scholar] [CrossRef]
- Noble, J.; Terrec, F.; Malvezzi, P.; Rostaing, L. Adverse effects of immunosuppression after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.D.; Charlton, M.R. Metabolic syndrome and liver transplantation: A review and guide to management. J. Hepatol. 2010, 53, 199–206. [Google Scholar] [CrossRef]
- Fairfield, C.; Penninga, L.; Powell, J.; Harrison, E.M.; Wigmore, S.J. Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients. Cochrane Database Syst. Rev. 2018, 2018, CD007606. [Google Scholar] [CrossRef]
- Krasnoff, J.B.; Vintro, A.Q.; Ascher, N.L.; Bass, N.M.; Paul, S.M.; Dodd, M.J.; Painter, P.L. A randomized trial of exercise and dietary counseling after liver transplantation. Am. J. Transplant. 2006, 6, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Rinella, M.; Patel, D.; McCague, K.; Heimbach, J.; Watt, K. Everolimus Is Associated with Less Weight Gain than Tacrolimus 2 Years after Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation 2017, 101, 2873. [Google Scholar] [CrossRef]
- Haddad, E.; McAlister, V.; Renouf, E.; Malthaner, R.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst. Rev. 2006, 2010, CD005161. [Google Scholar] [CrossRef]
- Neal, D.A.; Gimson, A.E.; Gibbs, P.; Alexander, G.J. Beneficial effects of converting liver transplant recipients from cyclosporine to tacrolimus on blood pressure, serum lipids, and weight. Liver Transplant. 2001, 7, 533–539. [Google Scholar] [CrossRef]
- Pacana, T.; Sanyal, A.J. Vitamin E and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Parmar, D.; Shaikh, F.; Forsgren, M.; Patel, S.; Bui, A.T.; Boyett, S.; Patel, V.; Sanyal, A.J. Saroglitazar improves nonalcoholic fatty liver disease and metabolic health in liver transplant recipients. Liver Transplant. 2023, 29, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Kokkorakis, M.; Muzurović, E.; Volčanšek, Š.; Chakhtoura, M.; Hill, M.A.; Mikhailidis, D.P.; Mantzoros, C.S. Steatotic Liver Disease: Pathophysiology and Emerging Pharmacotherapies. Pharmacol. Rev. 2024, 76, 454–499. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Bugianesi, E. Dietary and pharmacological treatment in patients with metabolic-dysfunction associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.N.; Watson, C.; Barlev, A.; Stevenson, M.; Dharnidharka, V.R. Mean lifetime survival estimates following solid organ transplantation in the US and UK. J. Med. Econ. 2022, 25, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.N.; Lim, W.H.; Ng, C.H.; Tan, D.J.H.; Xiao, J.; Tay, P.W.L.; Lin, S.Y.; Syn, N.; Chew, N.; Nah, B.; et al. Outcomes of Nonalcoholic Steatohepatitis After Liver Transplantation: An Updated Meta-Analysis and Systematic Review. Clin. Gastroenterol. Hepatol. 2023, 21, 45–54.e6. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Reyes, J.; Kashyap, R.; Dodson, S.F.; Demetris, A.J.; Ruppert, K.; Abu-Elmagd, K.; Marsh, W.; Madariaga, J.; Mazariegos, G.; et al. Long-Term Survival After Liver Transplantation in 4,000 Consecutive Patients at a Single Center. Ann. Surg. 2000, 232, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Ong, E.Y.H.; Chung, C.H.; Ong, C.E.Y.; Koh, B.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Xiao, J.; Wong, Z.Y.; et al. Longitudinal Outcomes Associated With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-analysis of 129 Studies. Clin. Gastroenterol. Hepatol. 2024, 22, 488–498. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Bhave, M.; Te, H.S.; Feinglass, J.; Alvarez, L.; Rinella, M.E. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology 2012, 56, 1741–1750. [Google Scholar] [CrossRef]
- Boga, S.; Munoz-Abraham, A.S.; I Rodriguez-Davalos, M.; Emre, S.H.; Jain, D.; Schilsky, M.L. Host factors are dominant in the development of post-liver transplant non-alcoholic steatohepatitis. World J. Hepatol. 2016, 8, 659–664. [Google Scholar] [CrossRef][Green Version]
- Jiang, M.-J.; Wu, M.-C.; Duan, Z.-H.; Wu, J.; Xu, X.-T.; Li, J.; Meng, Q.-H. Prevalence and clinical impact of sarcopenia in liver transplant recipients: A meta-analysis. World J. Gastroenterol. 2024, 30, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Vali, Y.; Alzoubi, O.; Staufer, K.; Trauner, M.; Paternostro, R.; Stauber, R.E.; Holleboom, A.G.; Van Dijk, A.M.; et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: An individual participant data meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gabarre, P.; Loens, C.; Tamzali, Y.; Barrou, B.; Jaisser, F.; Tourret, J. Immunosuppressive therapy after solid organ transplantation and the gut microbiota: Bidirectional interactions with clinical consequences. Am. J. Transplant. 2022, 22, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef]
| Corticosteroid | CNIs (Tac, CyA) | mTORis (EVR, SIR) | Antiproliferative Agents (MPA, AZA) | Monoclonal Antibodies (Basiliximab, Thymoglobulin) | |
|---|---|---|---|---|---|
| Main physiopathological mechanism | ↑ IR ↓ Insulin secretion ↑ Lipogenesis ↑ Gluconeogenesis ↑ Appetite | ↓ Insulin secretion Altered lipid metabolism ↑ Vasoconstriction | ↑ IR Altered lipid metabolism | Minimal direct metabolism effect | - |
| Weight gain | + | + | - | - | - |
| Hyperglycemia | + | + (>Tac) | + | - | - |
| Dyslipidemia | + | + (>CyA) | + | - | - |
| Arterial hypertension | + | + | + | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, A.; Loglio, A.; Neri, F.; Camagni, S.; Pasulo, L.; Lucà, M.G.; Trevisan, R.; Fagiuoli, S.; Viganò, M. Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue. J. Clin. Med. 2024, 13, 3871. https://doi.org/10.3390/jcm13133871
Savino A, Loglio A, Neri F, Camagni S, Pasulo L, Lucà MG, Trevisan R, Fagiuoli S, Viganò M. Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue. Journal of Clinical Medicine. 2024; 13(13):3871. https://doi.org/10.3390/jcm13133871
Chicago/Turabian StyleSavino, Alberto, Alessandro Loglio, Flavia Neri, Stefania Camagni, Luisa Pasulo, Maria Grazia Lucà, Roberto Trevisan, Stefano Fagiuoli, and Mauro Viganò. 2024. "Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue" Journal of Clinical Medicine 13, no. 13: 3871. https://doi.org/10.3390/jcm13133871
APA StyleSavino, A., Loglio, A., Neri, F., Camagni, S., Pasulo, L., Lucà, M. G., Trevisan, R., Fagiuoli, S., & Viganò, M. (2024). Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue. Journal of Clinical Medicine, 13(13), 3871. https://doi.org/10.3390/jcm13133871







