Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methodology
2.1. Data Sources and Search Strategy
2.2. Study Assessment
2.3. Eligibility Criteria
2.4. Data Extraction and Quality Assessment
2.5. Endpoint Definition
2.6. Data Synthesis
3. Results
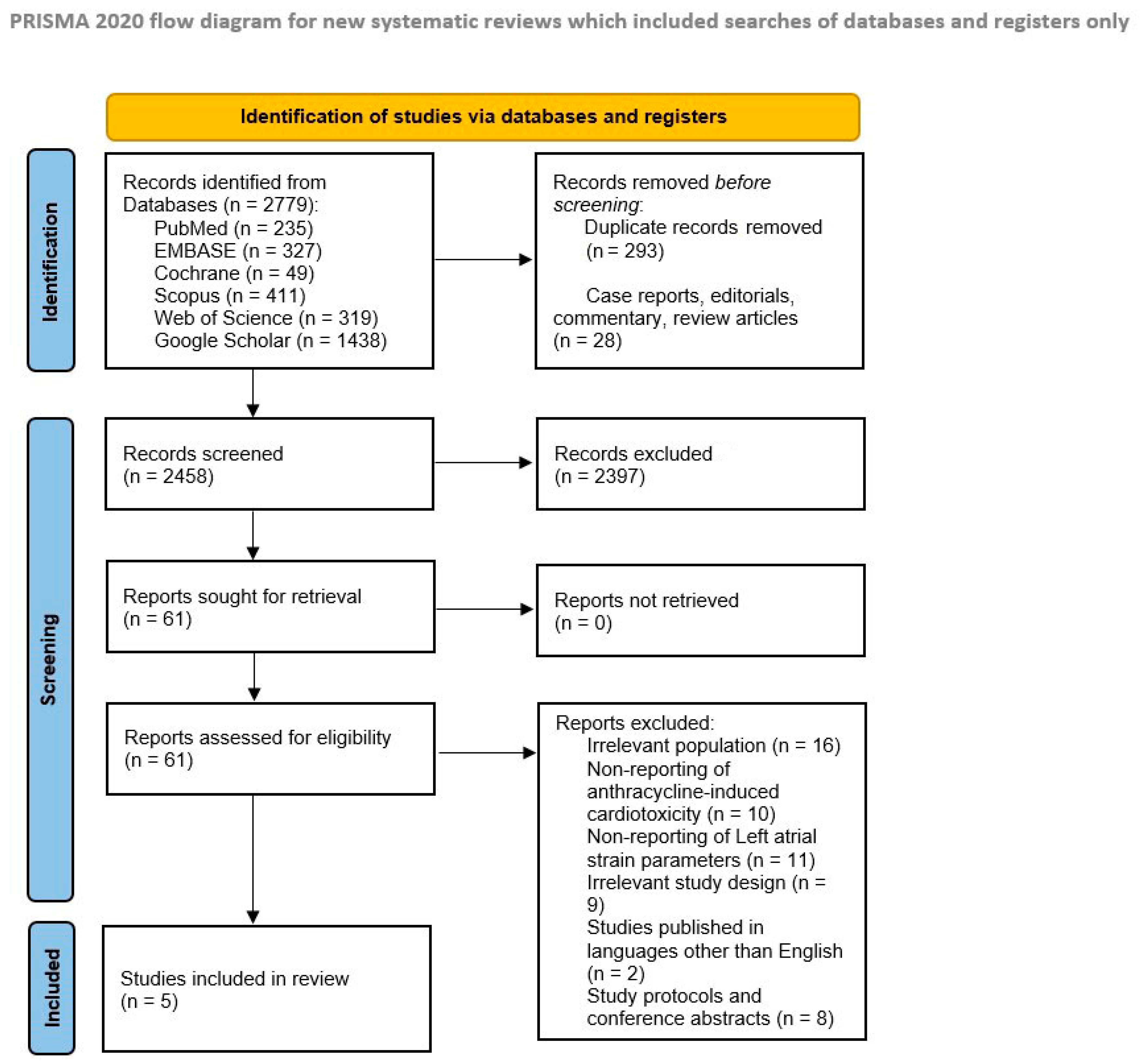
3.1. Literature Search Results
3.2. Study Characteristics and Risk of Bias Assessment
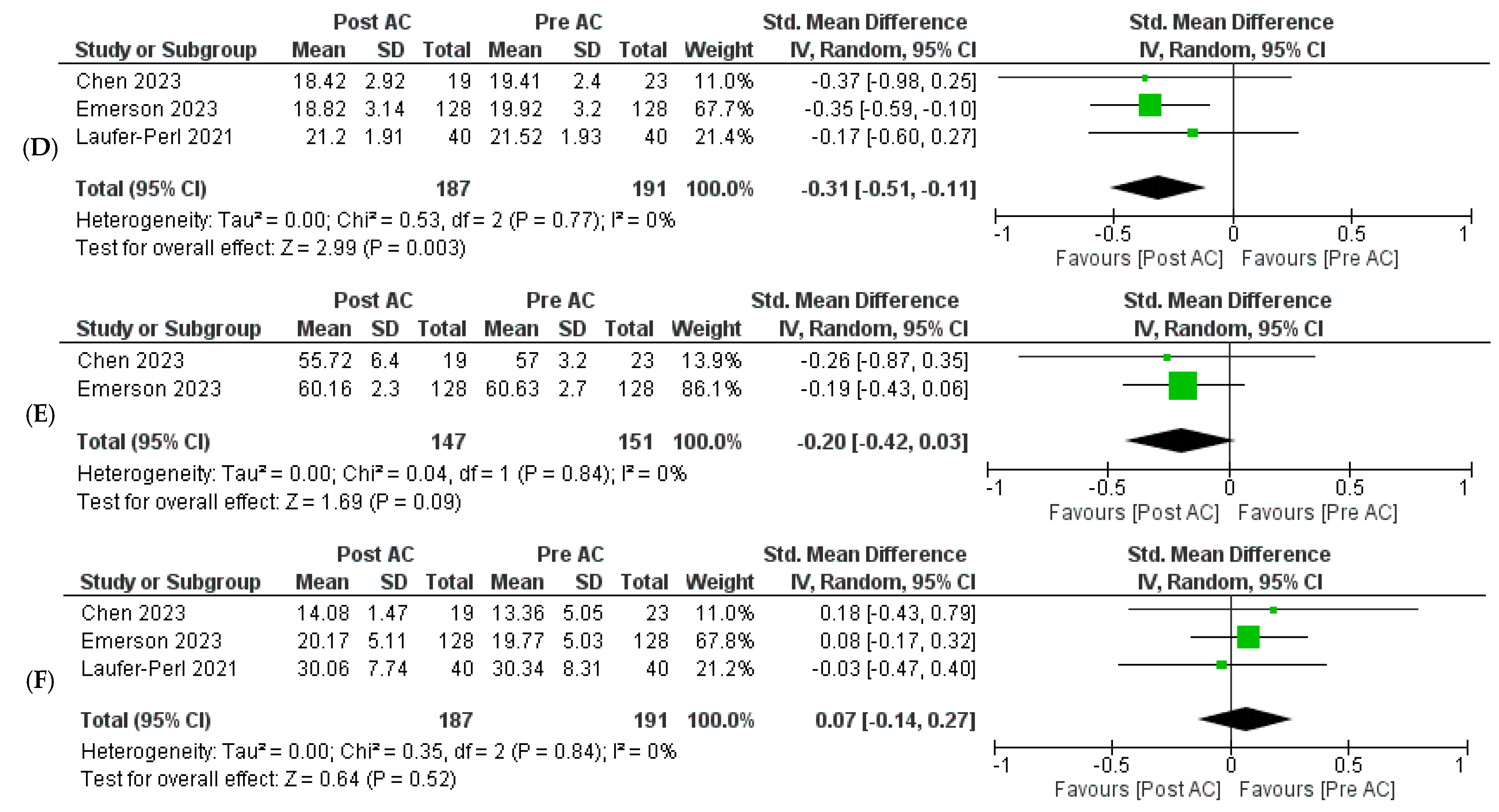
3.3. Outcomes
3.3.1. Primary Outcomes
3.3.2. Secondary Outcomes
3.4. Publication Bias
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Anthracycline | AC |
| Anthracycline-induced cardiac dysfunction | ACD |
| American Society of Echocardiography | ASE |
| Chemotherapy-related cardiac dysfunction | CTRCD |
| European Association of Cardiovascular Imaging | EACVI |
| Left atrial strain | LAS |
| Left atrial reservoir strain | LASr |
| Left atrial conduit strain | LAScd |
| Left atrial contractile strain | LASct |
| Left atrial volume index | LAVI |
| Left ventricular ejection fraction | LVEF |
| Left ventricular global longitudinal strain | LV GLS |
| Standardized mean difference | SMD |
| Speckle-tracking echocardiography | STE |
References
- Bansal, N.; Adams, M.J.; Ganatra, S.; Colan, S.D.; Aggarwal, S.; Steiner, R.; Amdani, S.; Lipshultz, E.R.; Lipshultz, S.E. Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valcovici, M.; Andrica, F.; Serban, C.; Dragan, S. Cardiotoxicity of anthracycline therapy: Current perspectives. Arch. Med. Sci. 2016, 12, 428–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamphuis, J.A.M.; Linschoten, M.; Cramer, M.J.; Doevendans, P.A.; Asselbergs, F.W.; Teske, A.J. Early- and late anthracycline-induced cardiac dysfunction: Echocardiographic characterization and response to heart failure therapy. Cardiooncology 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawicki, K.T.; Sala, V.; Prever, L.; Hirsch, E.; Ardehali, H.; Ghigo, A. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, K.C.; Zhang, Y.; Sturgeon, K.; Sinoway, L.I.; Trifiletti, D.M.; Chinchilli, V.M.; Zaorsky, N.G. Fatal heart disease among cancer patients. Nat. Commun. 2020, 11, 2011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801, Erratum in Eur. Heart J. 2018, 39, 839. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.-Y.; Lee, C.-H.; Su, P.-L.; Li, S.-S.; Chen, M.-Y.; Chen, Y.-P.; Hsu, Y.-T.; Tsai, W.-C.; Liu, P.-Y.; Chen, T.-Y.; et al. Subtle cardiac dysfunction in lymphoma patients receiving low to moderate dose chemotherapy. Sci. Rep. 2021, 11, 7100. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.H.; Kim, H.Y.; Cho, J.Y.; Yoon, H.J.; Hong, Y.J.; Park, H.W.; Kim, J.H.; Ahn, Y.; Jeong, M.H.; et al. Left atrial longitudinal strain as a predictor of Cancer therapeutics-related cardiac dysfunction in patients with breast Cancer. Cardiovasc. Ultrasound 2020, 18, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mondillo, S.; Cameli, M.; Caputo, M.L.; Lisi, M.; Palmerini, E.; Padeletti, M.; Ballo, P. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J. Am. Soc. Echocardiogr. 2011, 24, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.W.V.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; Cochrane: London, UK, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Volume 2, pp. 1–2. [Google Scholar]
- Chen, J.; Cheng, C.; Fan, L.; Xu, X.; Chen, J.; Feng, Y.; Tang, Y.; Yang, C. Assessment of left heart dysfunction to predict doxorubicin cardiotoxicity in children with lymphoma. Front. Pediatr. 2023, 11, 1163664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emerson, P.; Stefani, L.; Boyd, A.; Richards, D.; Hui, R.; Altman, M.; Thomas, L. Alterations in Left Atrial Strain in Breast Cancer Patients Immediately Post Anthracycline Exposure. Heart Lung Circ. 2023, 33, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Perl, M.; Arias, O.; Dorfman, S.S.; Baruch, G.; Rothschild, E.; Beer, G.; Hasson, S.P.; Arbel, Y.; Rozenbaum, Z.; Topilsky, Y.; et al. Left Atrial Strain changes in patients with breast cancer during anthracycline therapy. Int. J. Cardiol. 2021, 330, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Nolan, M.; Amir, E.; Brezden-Masley, C.; Yan, A.; Thampinathan, B.; Woo, A.; Bernd, W.; Thavendiranathan, P. Temporal changes in left atrial function in women with HER2+ breast cancer receiving sequential anthracyclines and trastuzumab therapy. J. Am. Coll. Cardiol. 2018, 71, A1524. [Google Scholar] [CrossRef]
- Patel, N.R.; Chyu, C.K.; Satou, G.M.; Halnon, N.J.; Nguyen, K.L. Left atrial function in children and young adult cancer survivors treated with anthracyclines. Echocardiography 2018, 35, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef]
- Bloom, M.W.; Hamo, C.E.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, G.M.; Lyon, A.R.; et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ. Heart Fail. 2016, 9, e002661. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Force, T.; Ewer, M.S.; De Keulenaer, G.W.; Suter, T.M.; Anker, S.D.; Avkiran, M.; De Azambuja, E.; Balligand, J.L.; Brutsaert, D.L.; et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2011, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Kasliwal, R.R. How do I do it? Speckle-tracking echocardiography. Indian Heart J. 2013, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Machino-Ohtsuka, T.; Nakazawa, Y.; Iida, N.; Sasamura, R.; Bando, H.; Chiba, S.; Tasaka, N.; Ishizu, T.; Murakoshi, N.; et al. Early Detection and Prediction of Anthracycline-Induced Cardiotoxicity―A Prospective Cohort Study. Circ. J. 2024, 88, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Giang, M.N.; Nguyen, H.H.; Vo, D.T.; Ho Huynh Quang, T.; Phan, D.T.H.; Chau, N.H. Superiority of left heart deformation in early anthracycline-related cardiac dysfunction detection. Open Heart 2023, 10, e002493. [Google Scholar] [CrossRef] [PubMed]
- Anqi, Y.; Yu, Z.; Mingjun, X.; Xiaoli, K.; Mengmeng, L.; Fangfang, L.; Mei, Z. Use of echocardiography to monitor myocardial damage during anthracycline chemotherapy. Echocardiography 2019, 36, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Pripon, S.; Parv, A.; Duncea, C.; Ciuleanu, T.E. Altered left ventricular diastolic performance in oncologic patients treated with epirubicin. Congest. Heart Fail. 2007, 13, 215–220. [Google Scholar] [CrossRef]
- Tassanmangina, S.; Codorean, D.; Metivier, M.; Costa, B.; Himberlin, C.; Jouannaud, C.; Blaise, A.M.; Elaerts, J.; Nazeyrollas, P. Tissue Doppler imaging and conventional echocardiography after anthracycline treatment in adults: Early and late alterations of left ventricular function during a prospective study. Eur. J. Echocardiogr. 2006, 7, 141–146. [Google Scholar] [CrossRef]

| Study ID | Country | Study Design | Type of Cancer n (%) | Type of Anthracycline n (%) | |||
|---|---|---|---|---|---|---|---|
| Doxorubicin | Daunorubicin | Epirubicin | Other | ||||
| Chen et al., 2023 [16] | China | Prospective cohort study | Lymphoma = 23 (100) | 23 (100) | 0 | 0 | 0 |
| Emerson et al., 2023 [17] | Australia | Prospective cohort study | HER2-negative breast cancer = 128 (100) | NR | 0 | NR | 0 |
| Laufer-Perl et al., 2021 [18] | Israel | Prospective cohort study | Hormone receptor-positive breast cancer = 21 (52.5) HER2-positive breast cancer = 11 (27.5) Triple-negative breast cancer = 8 (20) | 40 (100) | 0 | 0 | 0 |
| Meloche et al., 2018 [19] | Canada | Prospective cohort study | HER2-positive breast cancer = 51 (100) | 0 | 0 | 51 (100) | 0 |
| Patel et al., 2018 [20] | United States | Retrospective cohort study | Osteosarcoma = 19 (36) Ewing sarcoma = 11 (21) Leukemia = 8 (15) Other = 15 (28) | 40 (78) | 7 (14) | NR | 4 (8) |
| Study ID | Total No. of Participants | Age in Years (Mean SD) | Female n (%) | Weight (kg) | BMI (Mean, SD) | Hypertension n (%) | Diabetes Mellitus n (%) | Hyperlipidemia n (%) | Smoking n (%) | Cumulative Anthracycline Dosage (mg/m2) (Mean ± SD) | Time Elapsed between Baseline and Post-AC Therapy Echocardiograms (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2023 [16] | 23 | 9.34 ± 3.59 | 3 (13) | 29.28 ± 14.22 | NR | NR | NR | NR | NR | 50 | NR |
| Emerson et al., 2023 [17] | 128 | 54.7 ± 9.6 | NR | 74.23 ± 14.92 | 29.05 ± 6.52 | 39 (30) | 9 (7) | 27 (21) | 38 (30) | 195.6 ± 73.5 | 3 |
| Laufer-Perl et al., 2021 [18] | 40 | 51 ± 12 | 40 (100) | NR | 27 ± 6 | 12 (30) | 3 (7.5) | 8 (20) | 5 (12.5) | 237 ± 13.24 | 3.18 ± 0.62 |
| Meloche et al., 2018 [19] | 51 | 50.5 ± 9.8 | 51 (100) | NR | NR | NR | NR | NR | NR | 300 | NR |
| Patel et al., 2018 [20] | 55 | 13 ± 5 | 12 (22) | NR | NR | NR | NR | NR | NR | 308.6 ± 152 | 10.3 ± 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, A.; Abbasi, H.Q.; Yakkali, S.; Khan, A.M.; Tariq, M.D.; Sohail, A.H.; Khan, R. Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3904. https://doi.org/10.3390/jcm13133904
Goyal A, Abbasi HQ, Yakkali S, Khan AM, Tariq MD, Sohail AH, Khan R. Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(13):3904. https://doi.org/10.3390/jcm13133904
Chicago/Turabian StyleGoyal, Aman, Haleema Qayyum Abbasi, Shreyas Yakkali, Abdul Moiz Khan, Muhammad Daoud Tariq, Amir Humza Sohail, and Rozi Khan. 2024. "Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 13: 3904. https://doi.org/10.3390/jcm13133904
APA StyleGoyal, A., Abbasi, H. Q., Yakkali, S., Khan, A. M., Tariq, M. D., Sohail, A. H., & Khan, R. (2024). Left Atrial Strain as a Predictor of Early Anthracycline-Induced Chemotherapy-Related Cardiac Dysfunction: A Pilot Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(13), 3904. https://doi.org/10.3390/jcm13133904





