Hybrid Approach: Combining Surgical Thrombectomy and AngioJet™ Aspirational Thrombectomy in Limb Graft Occlusion Post-FEVAR with Fenestrated Anaconda™ and in ePTFE Bypass Graft Occlusion
Abstract
1. Background and Aim
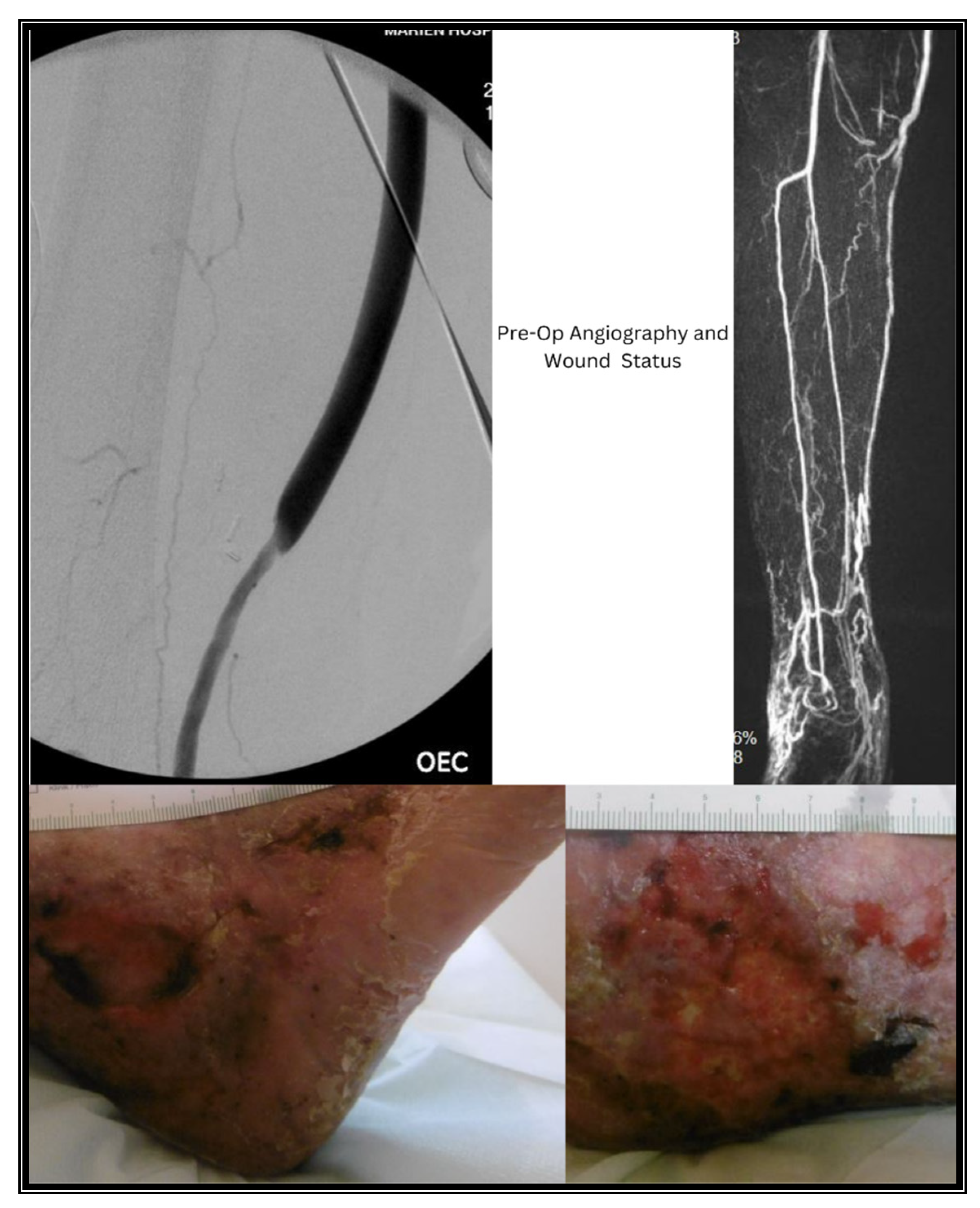
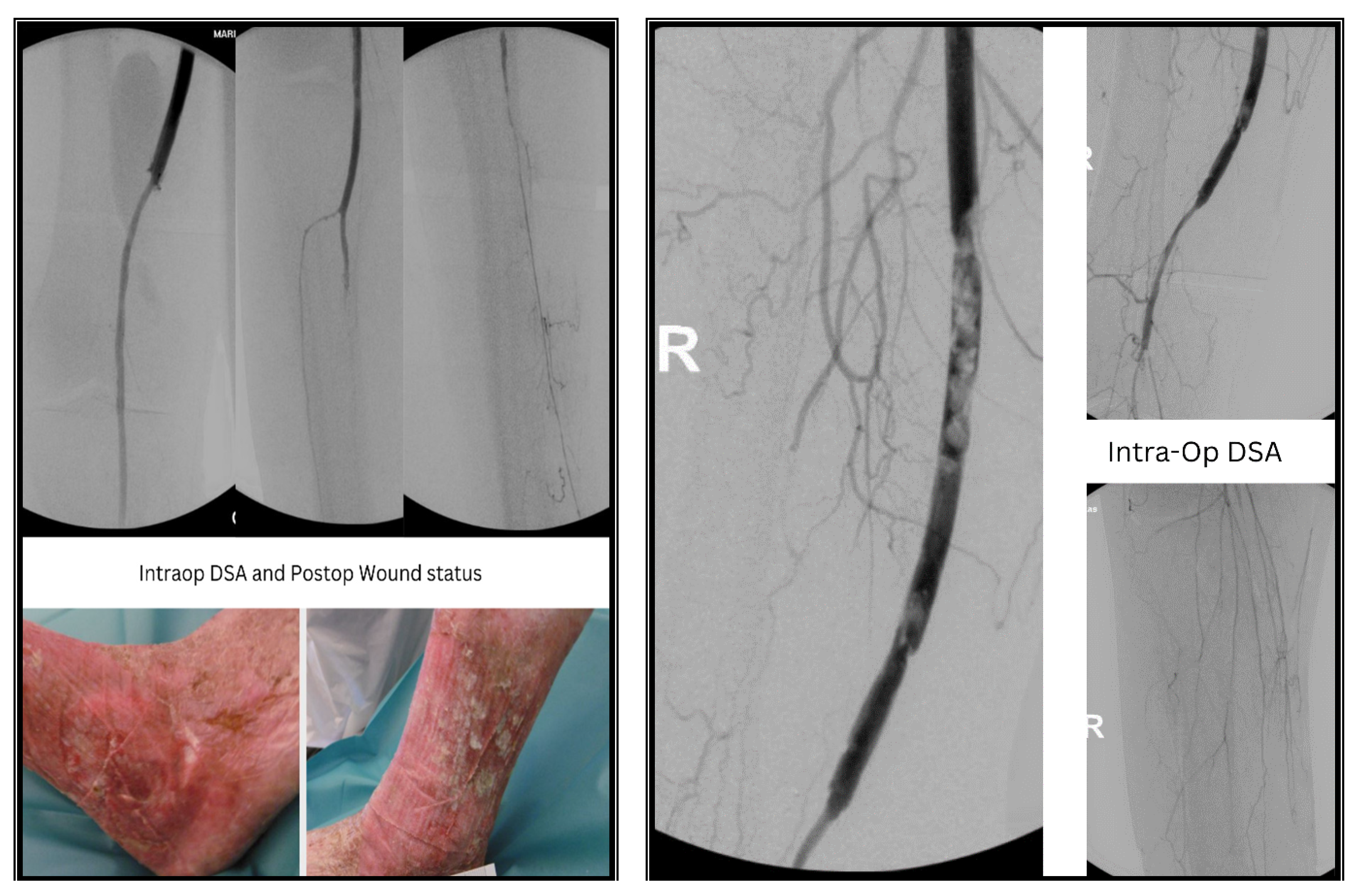
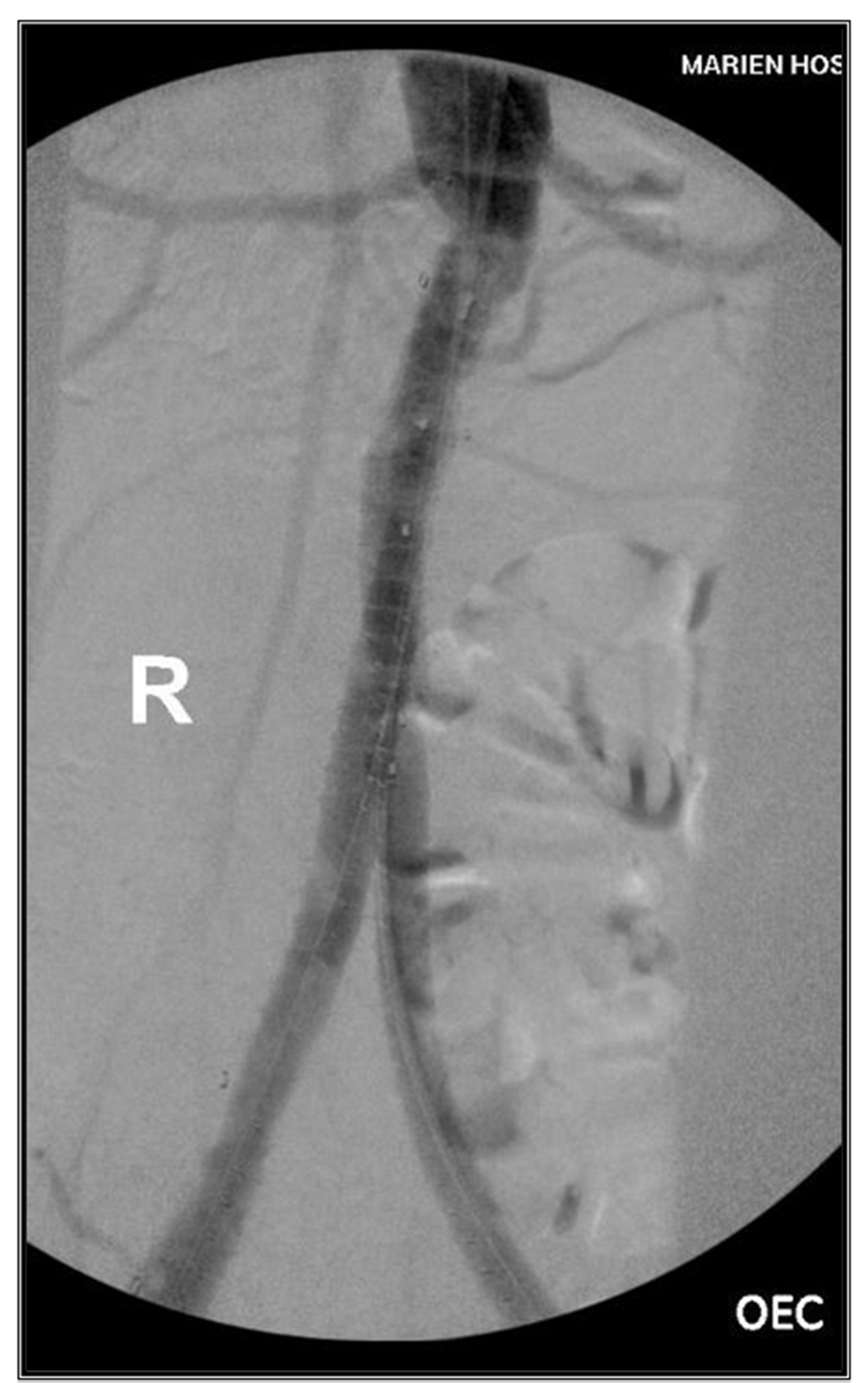
2. Case Presentations
2.1. Case I
2.2. Case II
3. Methods & Results
3.1. Case I
3.2. Case II
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogdanovic, M.; Stackelberg, O.; Lindström, D.; Ersryd, S.; Andersson, M.; Roos, H.; Siika, A.; Jonsson, M.; Roy, J. Limb Graft Occlusion Following Endovascular Aneurysm Repair for Infrarenal Abdominal Aortic Aneurysm with the Zenith Alpha, Excluder, and Endurant Devices: A Multicentre Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 532–539. [Google Scholar] [CrossRef]
- Ronsivalle, S.; Faresin, F.; Franz, F.; Pedon, L.; Rettore, C.; Zonta, L.; Olivieri, A. A new management for limb graft occlusion after endovascular aneurysm repair adding a vollmar ring stripper: The unclogging technique. Ann. Vasc. Surg. 2013, 27, 1216–1222. [Google Scholar] [CrossRef]
- Han, X.; Liu, G.; Li, T.; Guo, X. Application of the AngioJet Ultra Thrombectomy Device for the Percutaneous Mechanical Treatment (PMT) of Iliac Limb Occlusion after Endovascular Aneurysm Repair (EVAR). Ann. Vasc. Surg. 2022, 78, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Abisi, S.; Badawy, A. Percutaneous mechanical thrombectomy for limb graft occlusion after endovascular aneurysm repair: Results of a case series. Vascular 2024, 32, 546–549. [Google Scholar] [CrossRef]
- Meng, X.H.; Xie, X.P.; Liu, Y.C.; Huang, C.P.; Wang, L.J.; Liu, H.Y.; Fang, X.; Zhang, G.H. Observation of the effect of angiojet to treat acute lower extremity arterial embolization. World J. Clin. Cases 2023, 11, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Wissgott, C.; Kamusella, P.; Richter, A.; Klein-Wiegel, P.; Steinkamp, H.J. Mechanical rotational thrombectomy for treatment thrombolysis in acute and subacute occlusion of femoropopliteal arteries: Retrospective analysis of the results from 1999 to 2005. Rofo 2008, 180, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Wissgott, C.; Kamusella, P.; Richter, A.; Klein-Weigel, P.; Schink, T.; Steinkamp, H.J. Treatment of acute femoropopliteal bypass graft occlusion: Comparison of mechanical rotational thrombectomy with ultrasound-enhanced lysis. Rofo 2008, 180, 547–552. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puvvala, G.K.; Loukas, K.; Donas, K.P.; Hinkelmann, J.; Faiz, B.-F.; Gerado, L.V.; Psyllas, A. Hybrid Approach: Combining Surgical Thrombectomy and AngioJet™ Aspirational Thrombectomy in Limb Graft Occlusion Post-FEVAR with Fenestrated Anaconda™ and in ePTFE Bypass Graft Occlusion. J. Clin. Med. 2024, 13, 4002. https://doi.org/10.3390/jcm13144002
Puvvala GK, Loukas K, Donas KP, Hinkelmann J, Faiz B-F, Gerado LV, Psyllas A. Hybrid Approach: Combining Surgical Thrombectomy and AngioJet™ Aspirational Thrombectomy in Limb Graft Occlusion Post-FEVAR with Fenestrated Anaconda™ and in ePTFE Bypass Graft Occlusion. Journal of Clinical Medicine. 2024; 13(14):4002. https://doi.org/10.3390/jcm13144002
Chicago/Turabian StylePuvvala, Gowri Kiran, Karamperidis Loukas, Konstantinos P. Donas, Juergen Hinkelmann, Ba-Fadhl Faiz, Luna Vidriales Gerado, and Anastasios Psyllas. 2024. "Hybrid Approach: Combining Surgical Thrombectomy and AngioJet™ Aspirational Thrombectomy in Limb Graft Occlusion Post-FEVAR with Fenestrated Anaconda™ and in ePTFE Bypass Graft Occlusion" Journal of Clinical Medicine 13, no. 14: 4002. https://doi.org/10.3390/jcm13144002
APA StylePuvvala, G. K., Loukas, K., Donas, K. P., Hinkelmann, J., Faiz, B.-F., Gerado, L. V., & Psyllas, A. (2024). Hybrid Approach: Combining Surgical Thrombectomy and AngioJet™ Aspirational Thrombectomy in Limb Graft Occlusion Post-FEVAR with Fenestrated Anaconda™ and in ePTFE Bypass Graft Occlusion. Journal of Clinical Medicine, 13(14), 4002. https://doi.org/10.3390/jcm13144002






