Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery
Abstract
1. Introduction
2. Methods
3. Properties of ICG and Description of Intraoperative Use
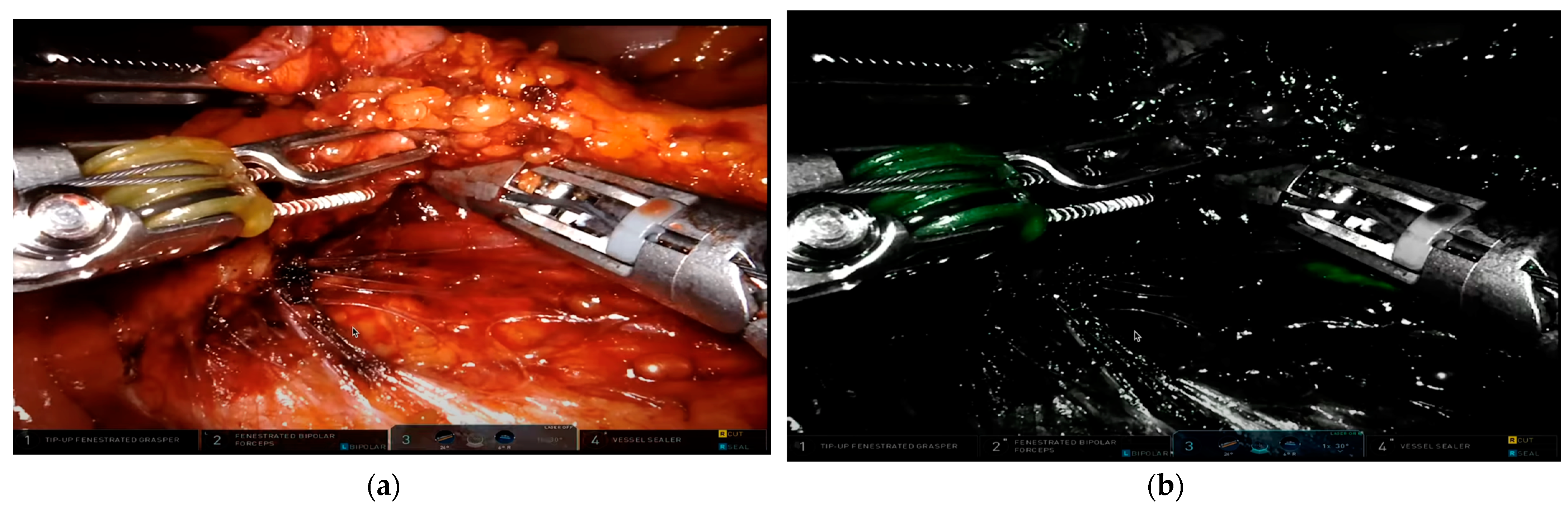
4. ICG in Evaluating Anastomotic Blood Supply
5. ICG for Lymphatic Mapping in Colorectal Surgery
6. Use of ICG for Intraoperative Localization of Ureters
7. Use of ICG for Preoperative Tumor Marking
8. ICG in Detecting Metastatic Disease in Colorectal Cancer
9. Discussion
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon. Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Patila, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Valisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of Indocyanine Green in Brain Tumor Surgery: Review of Clinical Evidence and Emerging Technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef] [PubMed]
- Burchell, H.B. Assessment of Clinical Value: Symposium on Diagnostic Applications of Indicator-Dilution Technics. Proc. Staff. Meet. Mayo Clin. 1957, 32, 551–553. [Google Scholar]
- Frangioni, J.V. In Vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Hochheimer, B.F. Angiography of the Retina with Indocyanine Green. Arch. Ophthalmol. 1971, 86, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, S.; Agrawal, A.; Kato, Y.; Sano, H. Intra Operative Indocyanine Green Video-Angiography in Cerebrovascular Surgery: An Overview with Review of Literature. Asian J. Neurosurg. 2011, 6, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Said, S.M.; Marey, G.; Hiremath, G. Intraoperative Fluorescence with Indocyanine Green in Congenital Cardiac Surgery: Potential Applications of a Novel Technology. JTCVS Tech. 2021, 8, 144–155. [Google Scholar] [CrossRef]
- Munabi, N.C.; Olorunnipa, O.B.; Goltsman, D.; Rohde, C.H.; Ascherman, J.A. The Ability of Intra-Operative Perfusion Mapping with Laser-Assisted Indocyanine Green Angiography to Predict Mastectomy Flap Necrosis in Breast Reconstruction: A Prospective Trial. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 449–455. [Google Scholar] [CrossRef]
- Jinno, H.; Ikeda, T.; Matsui, A.; Kitagawa, Y.; Kitajima, M.; Fujii, H.; Nakamura, K.; Kubo, A. Sentinel Lymph Node Biopsy in Breast Cancer Using Technetium-99m Tin Colloids of Different Sizes. Biomed. Pharmacother. 2002, 56 (Suppl. 1), 213s–216s. [Google Scholar] [CrossRef]
- Hirche, C.D.; Murawa, D.; Mohr, Z.; Kneif, S.; Hunerbein, M. Icg Fluorescence-Guided Sentinel Node Biopsy for Axillary Nodal Staging in Breast Cancer. Breast Cancer Res. Treat. 2010, 121, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Majlesara, A.; Golriz, M.; Hafezi, M.; Saffari, A.; Stenau, E.; Maier-Hein, L.; Muller-Stich, B.P.; Mehrabi, A. Indocyanine Green Fluorescence Imaging in Hepatobiliary Surgery. Photodiagn. Photodyn. Ther. 2017, 17, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S.; Cassinotti, E.; Fingerhut, A. Clinical Applications of Indocyanine Green (Icg) Enhanced Fluorescence in Laparoscopic Surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Safiejko, K.; Tarkowski, R.; Kozlowski, T.P.; Koselak, M.; Jachimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers 2022, 14, 1036. [Google Scholar] [CrossRef]
- Breuking, E.A.; van Varsseveld, O.C.; Harms, M.; Tytgat, S.; Hulscher, J.B.F.; Ruiterkamp, J. Safety and Feasibility of Indocyanine Green Fluorescence Angiography in Pediatric Gastrointestinal Surgery: A Systematic Review. J. Pediatr. Surg. 2023, 58, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, Z.; Qi, H.; Guo, Z.; Liu, Y.; Xiao, H.; Li, X. Safety and Efficacy of Indocyanine Green Near-Infrared Fluorescent Imaging-Guided Lymph Nodes Dissection During Radical Gastrectomy for Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 917541. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Beck, J.; Gerlach, R.; Zimmermann, M.; Seifert, V. Near-Infrared Indocyanine Green Video Angiography: A New Method for Intraoperative Assessment of Vascular Flow. Neurosurgery 2003, 52, 132–139; discussion 39. [Google Scholar] [PubMed]
- Meershoek, P.; KleinJan, G.H.; van Willigen, D.M.; Bauwens, K.P.; Spa, S.J.; van Beurden, F.; van Gennep, E.J.; Mottrie, A.M.; van der Poel, H.G.; Buckle, T.; et al. Multi-Wavelength Fluorescence Imaging with a Da Vinci Firefly-a Technical Look Behind the Scenes. J. Robot. Surg. 2021, 15, 751–760. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Gu, L. Development Review of Novel Laparoscope Technology. Zhongguo Yi Liao Qi Xie Za Zhi 2019, 43, 183–187. [Google Scholar]
- Zhu, B.; Sevick-Muraca, E.M. A Review of Performance of Near-Infrared Fluorescence Imaging Devices Used in Clinical Studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and Grading of Anastomotic Leakage Following Anterior Resection of the Rectum: A Proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Krukowski, Z.H.; Al-Khairy, G.; Russell, E.M.; Park, K.G. Systematic Review of the Definition and Measurement of Anastomotic Leak after Gastrointestinal Surgery. Br. J. Surg. 2001, 88, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Hirst, N.A.; Tiernan, J.P.; Millner, P.A.; Jayne, D.G. Systematic Review of Methods to Predict and Detect Anastomotic Leakage in Colorectal Surgery. Colorectal Dis. 2014, 16, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Gessler, B.; Eriksson, O.; Angenete, E. Treatment, and Consequences of Anastomotic Leakage in Colorectal Surgery. Int. J. Colorectal Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Kryzauskas, M.; Bausys, A.; Degutyte, A.E.; Abeciunas, V.; Poskus, E.; Bausys, R.; Dulskas, A.; Strupas, K.; Poskus, T. Risk Factors for Anastomotic Leakage and Its Impact on Long-Term Survival in Left-Sided Colorectal Cancer Surgery. World J. Surg. Oncol. 2020, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.K.; Chang, E.Y.; Jobe, B.A. Clinical Review: Healing in Gastrointestinal Anastomoses, Part I. Microsurgery 2006, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Hrabe, J. Anastomotic Technique-How to Optimize Success and Minimize Leak Rates. Clin. Colon. Rectal Surg. 2021, 34, 371–378. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion Assessment in Laparoscopic Left-Sided/Anterior Resection (Pillar Ii): A Multi-Institutional Study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef]
- Ris, F.; Liot, E.; Buchs, N.C.; Kraus, R.; Ismael, G.; Belfontali, V.; Douissard, J.; Cunningham, C.; Lindsey, I.; Guy, R.; et al. Multicentre Phase Ii Trial of Near-Infrared Imaging in Elective Colorectal Surgery. Br. J. Surg. 2018, 105, 1359–1367. [Google Scholar] [CrossRef]
- Cassinotti, E.; Al-Taher, M.; Antoniou, S.A.; Arezzo, A.; Baldari, L.; Boni, L.; Bonino, M.A.; Bouvy, N.D.; Brodie, R.; Carus, T.; et al. European Association for Endoscopic Surgery (Eaes) Consensus on Indocyanine Green (Icg) Fluorescence-Guided Surgery. Surg. Endosc. 2023, 37, 1629–1648. [Google Scholar] [CrossRef]
- Tang, G.; Du, D.; Tao, J.; Wei, Z. Effect of Indocyanine Green Fluorescence Angiography on Anastomotic Leakage in Patients Undergoing Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials and Propensity-Score-Matched Studies. Front. Surg. 2022, 9, 815753. [Google Scholar] [CrossRef] [PubMed]
- NCCN, National Comprehensive Cancer Network. Colon Cancer Version 1.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 22 April 2024).
- Heald, R.J. The ‘Holy Plane’ of Rectal Surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized Surgery for Colonic Cancer: Complete Mesocolic Excision and Central Ligation-Technical Notes and Outcome. Colorectal Dis. 2009, 11, 354–364; discussion 64–65. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon. Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef] [PubMed]
- Hojo, K.; Koyama, Y.; Moriya, Y. Lymphatic Spread and Its Prognostic Value in Patients with Rectal Cancer. Am. J. Surg. 1982, 144, 350–354. [Google Scholar] [CrossRef]
- Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Hase, K. Prognostic Determinants of Patients with Lateral Nodal Involvement by Rectal Cancer. Ann. Surg. 2001, 234, 190–197. [Google Scholar] [CrossRef]
- Sugihara, K.; Kobayashi, H.; Kato, T.; Mori, T.; Mochizuki, H.; Kameoka, S.; Shirouzu, K.; Muto, T. Indication and Benefit of Pelvic Sidewall Dissection for Rectal Cancer. Dis. Colon. Rectum 2006, 49, 1663–1672. [Google Scholar] [CrossRef]
- Ohya, H.; Watanabe, J.; Suwa, H.; Suwa, Y.; Ozawa, M.; Ishibe, A.; Kunisaki, C.; Endo, I. Near-Infrared Imaging Using Indocyanine Green for Laparoscopic Lateral Pelvic Lymph Node Dissection for Clinical Stage Ii/Iii Middle-Lower Rectal Cancer: A Propensity Score-Matched Cohort Study. Dis. Colon. Rectum 2022, 65, 885–893. [Google Scholar] [CrossRef]
- Watanabe, J.; Ohya, H.; Sakai, J.; Suwa, Y.; Goto, K.; Nakagawa, K.; Ozawa, M.; Ishibe, A.; Suwa, H.; Kunisaki, C.; et al. Long-Term Outcomes of Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Lateral Pelvic Lymph Node Dissection for Clinical Stage Ii/Iii Middle-Lower Rectal Cancer: A Propensity Score-Matched Cohort Study. Tech. Coloproctol. 2023, 27, 759–767. [Google Scholar] [CrossRef]
- Georgiou, P.; Tan, E.; Gouvas, N.; Antoniou, A.; Brown, G.; Nicholls, R.J.; Tekkis, P. Extended Lymphadenectomy Versus Conventional Surgery for Rectal Cancer: A Meta-Analysis. Lancet Oncol. 2009, 10, 1053–1062. [Google Scholar] [CrossRef]
- Su, H.; Xu, Z.; Bao, M.; Luo, S.; Liang, J.; Pei, W.; Guan, X.; Liu, Z.; Jiang, Z.; Zhang, M.; et al. Lateral Pelvic Sentinel Lymph Node Biopsy Using Indocyanine Green Fluorescence Navigation: Can It Be a Powerful Supplement Tool for Predicting the Status of Lateral Pelvic Lymph Nodes in Advanced Lower Rectal Cancer. Surg. Endosc. 2023, 37, 4088–4096. [Google Scholar] [CrossRef]
- You, Y.N.; Hardiman, K.M.; Bafford, A.; Poylin, V.; Francone, T.D.; Davis, K.; Paquette, I.M.; Steele, S.R.; Feingold, D.L.; Colon On Behalf of the Clinical Practice Guidelines Committee of the American Society of, and Surgeons Rectal. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis. Colon. Rectum 2020, 63, 1191–1222. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Real-Time Indocyanine Green Fluorescence Imaging-Guided Complete Mesocolic Excision in Laparoscopic Flexural Colon Cancer Surgery. Dis. Colon. Rectum 2016, 59, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Kawada, K.; Itatani, Y.; Okamura, R.; Oshima, N.; Okada, T.; Hida, K.; Obama, K. Timing of Real-Time Indocyanine Green Fluorescence Visualization for Lymph Node Dissection During Laparoscopic Colon Cancer Surgery. Langenbecks Arch. Surg. 2023, 408, 38. [Google Scholar] [CrossRef] [PubMed]
- Ribero, D.; Mento, F.; Sega, V.; Conte, D.L.; Mellano, A.; Spinoglio, G. Icg-Guided Lymphadenectomy During Surgery for Colon and Rectal Cancer-Interim Analysis of the Greenlight Trial. Biomedicines 2022, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Marcelissen, T.A.; Den Hollander, P.P.; Tuytten, T.R.; Sosef, M.N. Incidence of Iatrogenic Ureteral Injury During Open and Laparoscopic Colorectal Surgery: A Single Center Experience and Review of the Literature. Surg. Laparosc. Endosc. Percutan Tech. 2016, 26, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.M.; Putt, K.S.; Srinivasarao, M.; Low, P.S. Design of a near Infrared Fluorescent Ureter Imaging Agent for Prevention of Ureter Damage During Abdominal Surgeries. Molecules 2021, 26, 3739. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.G.; Hompes, R.; Birks, J.; Mortensen, N.J.; Jones, O.; Lindsey, I.; Guy, R.; George, B.; Cunningham, C.; Yeung, T.M. Methylene Blue Fluorescence of the Ureter During Colorectal Surgery. Surg. Endosc. 2018, 32, 4036–4043. [Google Scholar] [CrossRef] [PubMed]
- Grosek, J.; Tomazic, A. Key Clinical Applications for Indocyanine Green Fluorescence Imaging in Minimally Invasive Colorectal Surgery. J. Minim. Access Surg. 2020, 16, 308–314. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Wexner, S.D. Ureter Identification Utilizing Indocyanine Green (Icg) Imaging in Colorectal Surgery: A Systematic Review of the Literature. Mini-Invasive Surg. 2022, 6, 51. [Google Scholar] [CrossRef]
- Peltrini, R.; Podda, M.; Castiglioni, S.; Di Nuzzo, M.M.; D’Ambra, M.; Lionetti, R.; Sodo, M.; Luglio, G.; Mucilli, F.; Di Saverio, S.; et al. Intraoperative Use of Indocyanine Green Fluorescence Imaging in Rectal Cancer Surgery: The State of the Art. World J. Gastroenterol. 2021, 27, 6374–6386. [Google Scholar] [CrossRef] [PubMed]
- Zocola, E.; Meyer, J.; Christou, N.; Liot, E.; Toso, C.; Buchs, N.C.; Ris, F. Role of Near-Infrared Fluorescence in Colorectal Surgery. World J. Gastroenterol. 2021, 27, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Garoufalia, Z.; Wexner, S.D. Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. J. Clin. Med. 2023, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Ohue, M.; Noura, S.; Yano, M.; Sasaki, Y.; Kishi, K.; Yamada, T.; Miyashiro, I.; Ohigashi, H.; Iishi, H.; et al. Surgical Usefulness of Indocyanine Green as an Alternative to India Ink for Endoscopic Marking. Surg. Endosc. 2009, 23, 347–351. [Google Scholar] [CrossRef]
- Sparks, R.; Power, S.; Kearns, E.; Clarke, A.; Mohan, H.M.; Brannigan, A.; Mulsow, J.; Shields, C.; Cahill, R.A. Fallibility of Tattooing Colonic Neoplasia Ahead of Laparoscopic Resection: A Retrospective Cohort Study. Ann. R. Coll. Surg. Engl. 2023, 105, 126–131. [Google Scholar] [CrossRef]
- Hammond, D.C.; Lane, F.R.; Mackeigan, J.M.; Passinault, W.J. Endoscopic Tattooing of the Colon: Clinical Experience. Am. Surg. 1993, 59, 205–210. [Google Scholar]
- Son, G.M.; Ahn, H.M.; Lee, I.Y.; Ha, G.W. Multifunctional Indocyanine Green Applications for Fluorescence-Guided Laparoscopic Colorectal Surgery. Ann. Coloproctol. 2021, 37, 133–140. [Google Scholar] [CrossRef]
- Satoyoshi, T.; Okita, K.; Ishii, M.; Hamabe, A.; Usui, A.; Akizuki, E.; Okuya, K.; Nishidate, T.; Yamano, H.; Nakase, H.; et al. Timing of Indocyanine Green Injection Prior to Laparoscopic Colorectal Surgery for Tumor Localization: A Prospective Case Series. Surg. Endosc. 2021, 35, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.N.; Pease, P.; Ibbotson, J.P. Clustering of Crohn’s Disease in a Cotswold Village. Q. J. Med. 1986, 59, 473–478. [Google Scholar]
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal Cancer Liver Metastases-a Population-Based Study on Incidence, Management and Survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Fong, Y.; Cohen, A.M.; Fortner, J.G.; Enker, W.E.; Turnbull, A.D.; Coit, D.G.; Marrero, A.M.; Prasad, M.; Blumgart, L.H.; Brennan, M.F. Liver Resection for Colorectal Metastases. J. Clin. Oncol. 1997, 15, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence after Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 Consecutive Cases. Ann Surg 1999, 230, 309–318; discussion 18–21. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Zaidi, N.; Berber, E. An Initial Report on the Intraoperative Use of Indocyanine Green Fluorescence Imaging in the Surgical Management of Liver Tumorss. J. Surg. Oncol. 2016, 114, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.D.; Wilton, J.C. Can Intra-Operative Fluorescence Play a Significant Role in Hepatobiliary Surgery? Eur. J. Surg. Oncol. 2017, 43, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Morimoto, M.; Ogura, T.; Sugimori, K.; Takebayashi, S.; Okada, M.; Tanaka, K. Ablation Therapy Guided by Contrast-Enhanced Sonography with Sonazoid for Hepatocellular Carcinoma Lesions Not Detected by Conventional Sonography. J. Ultrasound Med. 2008, 27, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, F.B.; Mulder, B.G.S.; Meijer, R.P.J.; Bonsing, B.A.; Hartgrink, H.H.; Mieog, J.S.D.; Zlitni, A.; Park, S.M.; Sarasqueta, A.F.; Vahrmeijer, A.L.; et al. Real-Time Surgical Margin Assessment Using Icg-Fluorescence During Laparoscopic and Robot-Assisted Resections of Colorectal Liver Metastases. Ann. Transl. Med. 2020, 8, 1448. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.; Lemmens, V.E.; Nienhuijs, S.W.; Luyer, M.D.; de Hingh, I.H. Peritoneal Carcinomatosis of Colorectal Origin: Incidence, Prognosis and Treatment Options. World J. Gastroenterol. 2012, 18, 5489–5494. [Google Scholar] [CrossRef]
- Gelli, M.; Huguenin, J.F.; Cerebelli, C.; Benhaim, L.; Honore, C.; Elias, D.; Goere, D. Strategies to Prevent Peritoneal Carcinomatosis Arising from Colorectal Cancer. Future Oncol. 2017, 13, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Gheza, F.; Molfino, S.; Arru, L.; Vaira, M.; Giacopuzzi, S. Indocyanine Green Fluorescence-Guided Intraoperative Detection of Peritoneal Carcinomatosis: Systematic Review. BMC Surg. 2020, 20, 158. [Google Scholar] [CrossRef]
- Simion, L.; Ionescu, S.; Chitoran, E.; Rotaru, V.; Cirimbei, C.; Madge, O.L.; Nicolescu, A.C.; Tanase, B.; Dicu-Andreescu, I.G.; Dinu, D.M.; et al. Indocyanine Green (Icg) and Colorectal Surgery: A Literature Review on Qualitative and Quantitative Methods of Usage. Medicina 2023, 59, 1530. [Google Scholar] [CrossRef]
- Schneider, C.; Johnson, S.P.; Gurusamy, K.; Cook, R.J.; Desjardins, A.E.; Hawkes, D.J.; Davidson, B.R.; Walker-Samuel, S. Identification of Liver Metastases with Probe-Based Confocal Laser Endomicroscopy at Two Excitation Wavelengths. Lasers Surg. Med. 2017, 49, 280–292. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalafi, S.; Botero Fonnegra, C.; Reyes, A.; Hui, V.W. Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. J. Clin. Med. 2024, 13, 4003. https://doi.org/10.3390/jcm13144003
Khalafi S, Botero Fonnegra C, Reyes A, Hui VW. Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. Journal of Clinical Medicine. 2024; 13(14):4003. https://doi.org/10.3390/jcm13144003
Chicago/Turabian StyleKhalafi, Shayan, Cristina Botero Fonnegra, Ana Reyes, and Vanessa W. Hui. 2024. "Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery" Journal of Clinical Medicine 13, no. 14: 4003. https://doi.org/10.3390/jcm13144003
APA StyleKhalafi, S., Botero Fonnegra, C., Reyes, A., & Hui, V. W. (2024). Developments in the Use of Indocyanine Green (ICG) Fluorescence in Colorectal Surgery. Journal of Clinical Medicine, 13(14), 4003. https://doi.org/10.3390/jcm13144003




