Profiles of Geriatric Syndromes and Resources in Older Patients with Atrial Fibrillation
Abstract
1. Introduction
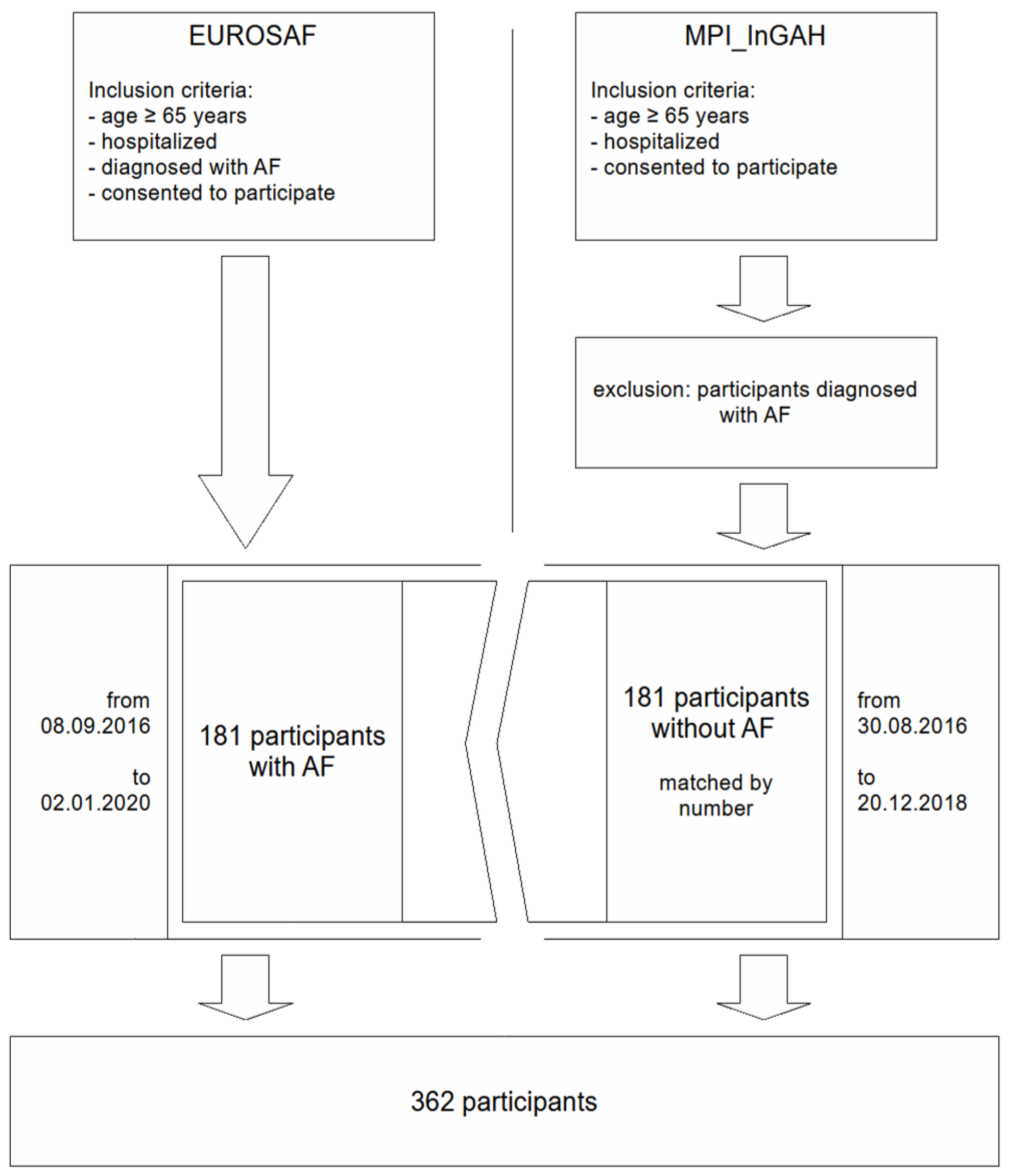
2. Methods
2.1. Study Population
2.2. Assessments
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Sample
3.2. GRs and GSs According to AF/NAF Group
3.3. GRs and GSs in the AF Group According to OACs Use
3.4. 1-Year Follow-Up Results
4. Discussion
Limitations of This Study
5. Practical Conclusions
- Assessment of GRs and GSs provides insight into profiles and the impact of frailty in patients with AF.
- Although not the number of GRs and GSs does not appear to be important, mnestic resources and sensorial impairment are significantly more common in patients with AF and should be assessed.
- Emotional resourced and chronic pain are more common in patients receiving OACs, while spiritual resources are less common, but the relationship needs further investigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 213, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort: The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Migdady, I.; Russman, A.; Buletko, A.B. Atrial Fibrillation and Ischemic Stroke: A Clinical Review. Semin. Neurol. 2021, 41, 348–364. [Google Scholar] [CrossRef]
- Eckardt, L.; Häusler, K.G.; Ravens, U.; Borggrefe, M.; Kirchhof, P. ESC-Leitlinien zum Vorhofflimmern 2016. Herz 2016, 41, 677–683. [Google Scholar] [CrossRef]
- Presta, R.; Brunetti, E.; Polidori, M.C.; Bo, M. Impact of frailty models on the prescription of oral anticoagulants and on the incidence of stroke, bleeding, and mortality in older patients with atrial fibrillation: A systematic review. Ageing Res. Rev. 2022, 82, 101761. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Veronese, N.; Polidori, M.C.; Strandberg, T.; Topinkova, E.; Cruz-Jentoft, A.J.; Custodero, C.; Maggi, S.; On Behalf of the EUROSAF Study Investigators. The role of prognostic stratification on prescription of anticoagulants in older patients with atrial fibrillation: A multicenter, observational, prospective European study (EUROSAF). Ann. Med. 2022, 54, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Banach, M.; Mah, D.; Ahmed, A.; Aronow, W.S. Role of Geriatric Syndromes in the Management of Atrial Fibrillation in Older Adults: A Narrative Review. J. Am. Med. Dir. Assoc. 2019, 20, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, D.L.; Foebel, A.D.; Marengoni, A.; Brandi, V.; Collamati, A.; Heckman, G.A.; Hirdes, J.; Bernabei, R.; Onder, G. Chronic diseases and geriatric syndromes: The different weight of comorbidity. Eur. J. Intern. Med. 2016, 27, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Fang, M.C.; Jeon, S.Y.; Gregorich, S.E.; Covinsky, K.E. Geriatric Syndromes and Atrial Fibrillation: Prevalence and Association with Anticoagulant Use in a National Cohort of Older Americans. J. Am. Geriatr. Soc. 2021, 69, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Becker, I.; Siri, G.; Brinkkötter, P.T.; Benzing, T.; Pilotto, A.; Polidori, M.C. The prognostic significance of geriatric syndromes and resources. Aging Clin. Exp. Res. 2020, 32, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Borghi, L.; Carugo, S.; Cavicchioli, M.; Faioni, E.M.; Negroni, M.S.; Vegni, E. Atrial fibrillation and psychological factors: A systematic review. PeerJ 2017, 5, e3537. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Ferrucci, L.; Franceschi, M.; D’Ambrosio, L.P.; Scarcelli, C.; Cascavilla, L.; Paris, F.; Placentino, G.; Seripa, D.; Dallapiccola, B.; et al. Development and Validation of a Multidimensional Prognostic Index for One-Year Mortality from Comprehensive Geriatric Assessment in Hospitalized Older Patients. Rejuvenation Res. 2008, 11, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Becker, I.; Siri, G.; Brinkkötter, P.T.; Benzing, T.; Pilotto, A.; Polidori, M.C. New associations of the Multidimensional Prognostic Index. Z. Für Gerontol. Geriatr. 2019, 52, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Pickert, L.; Meyer, A.M.; Becker, I.; Heeß, A.; Noetzel, N.; Brinkkötter, P.; Pilotto, A.; Benzing, T.; Polidori, M.C. Role of a multidimensional prognosis in-hospital monitoring for older patients with prolonged stay. Int. J. Clin. Pract. 2021, 75, e13989. [Google Scholar] [CrossRef] [PubMed]
- Heeß, A.; Meyer, A.M.; Becker, I.; Noetzel, N.; Verleysdonk, J.; Rarek, M.; Benzing, T.; Polidori, M.C. The prognostic fingerprint of quality of life in older inpatients: Relationship to geriatric syndromes’ and resources’ profile. Z. Für Gerontol. Geriatr. 2022, 55, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Linn, B.S.; Linn, M.W.; Gurel, L. Cumulative illness rating scale. J. Am. Geriatr. Soc. 1968, 16, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in Development of the Index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients†. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sancarlo, D.; D’Onofrio, G.; Franceschi, M.; Scarcelli, C.; Niro, V.; Addante, F.; Copetti, M.; Ferrucci, L.; Fontana, L.; Pilotto, A. Validation of a modified-multidimensional prognostic index (m-MPI) including the mini nutritional assessment short-form (MNA-SF) for the prediction of one-year mortality in hospitalized elderly patients. J. Nutr. Health Aging. 2011, 15, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bliss, M.R.; McLaren, R.; Exton-Smith, A.N. Mattresses for preventing pressure sores in geriatric patients. Mon. Bull. Minist. Health Public Health Lab. Serv. 1966, 25, 238–268. [Google Scholar] [PubMed]
- Kozieł, M.; Teutsch, C.; Halperin, J.L.; Rothman, K.J.; Diener, H.-C.; Ma, C.-S.; Marler, S.; Lu, S.; Gurusamy, V.K.; Huisman, M.V.; et al. Atrial fibrillation comorbidities: Clinical characteristics antithrombotic treatment in GLORIA-AF. PLoS ONE 2021, 16, e0249524. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Linz, D.; Schotten, U. Dynamics of Atrial Fibrillation Mechanisms and Comorbidities. Annu. Rev. Physiol. 2021, 83, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Addante, F.; Franceschi, M.; Leandro, G.; Rengo, G.; D’Ambrosio, P.; Longo, M.G.; Rengo, F.; Pellegrini, F.; Dallapiccola, B.; et al. Multidimensional Prognostic Index Based on a Comprehensive Geriatric Assessment Predicts Short-Term Mortality in Older Patients with Heart Failure. Circ. Heart Fail. 2010, 3, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; EUROSAF Study Investigators; Argusti, A.; Canepa, E.; Polidori, M.C.; Maggi, S.; Strandberg, T.; Pilotto, A. Evaluating the effectiveness and risks of oral anticoagulant treatments in multimorbid frail older subjects with atrial fibrillation using the multidimensional prognostic index: The EURopean study of older subjects with atrial fibrillation—EUROSAF. Eur. Geriatr. Med. 2018, 9, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.R.; Tummolo, A.M.; Palmer, K.; Gravina, E.M.; Vetrano, D.L.; Bernabei, R.; Onder, G.; Acampora, N. Frailty and atrial fibrillation: A systematic review. Eur. J. Intern. Med. 2018, 56, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, B.; Palareti, G. Bleeding with anticoagulation therapy—Who is at risk, and how best to identify such patients. Thromb. Haemost. 2009, 102, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Alves, M.; Bahat, G.; Boureau, A.S.; Ozkok, S.; Pfister, R.; Pilotto, A.; Veronese, N.; Bo, M.; On behalf of the Special Interest Group “Cardiovascular Diseases” of the EuGMS. Atrial fibrillation: A geriatric perspective on the 2020 ESC guidelines. Eur. Geriatr. Med. 2021, 13, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Veronese, N.; Polidori, M.C.; Strandberg, T.; Topinkova, E.; Cruz-Jentoft, A.; Custodero, C.; Maggi, S. Frailty and anticoagulants in older subjects with atrial fibrillation: The EUROSAF study. Age Ageing 2023, 52, afad216. [Google Scholar] [CrossRef]
- Zeng, S.; Zheng, Y.; Jiang, J.; Ma, J.; Zhu, W.; Cai, X. Effectiveness and Safety of DOACs vs. Warfarin in Patients with Atrial Fibrillation and Frailty: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 907197. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, M.; Ording, A.G.; Skjøth, F.; Larsen, T.B.; Nielsen, P.B. Effectiveness and safety of direct oral anticoagulation vs. warfarin in frail patients with atrial fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; Frobert, O.; Larsen, D.B.; Arendt-Nielsen, L.; Björkenheim, A. Patients with symptomatic permanent atrial fibrillation show quantitative signs of pain sensitisation. Open Heart. 2021, 8, e001699. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, H.; Xu, Y.; Wang, K.; Fu, Y.; Lu, Z. Hearing disorders, genetic predisposition, and risk of new-onset atrial fibrillation: A prospective cohort study in the UK biobank. Int. J. Cardiol. 2024, 401, 131829. [Google Scholar] [CrossRef] [PubMed]
- Rowe, F.J.; Hepworth, L.R.; Howard, C.; Cullen, C.; Sturgess, B.; Griffiths, N.; Lip, G.Y.H. Stroke-Related Visual Impairment; is There an Association with Atrial Fibrillation? J. Stroke Cerebrovasc. Dis. 2020, 29, 105186. [Google Scholar] [CrossRef] [PubMed]
- Kaewput, W.; Thongprayoon, C.; Rangsin, R.; Bathini, T.; Mao, M.A.; Cheungpasitporn, W. Associations of new-onset atrial fibrillation and severe visual impairment in type 2 diabetes: A multicenter nationwide study. World J. Cardiol. 2021, 13, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Liaw, W.; Bazemore, A.; Jetty, A.; Petterson, S.; Kushel, M. Adults with Housing Insecurity Have Worse Access to Primary and Preventive Care. J. Am. Board. Fam. Med. 2019, 32, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Parekh, T.; Xue, H.; Cheskin, L.J.; Cuellar, A.E. Food insecurity and housing instability as determinants of cardiovascular health outcomes: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1590–1608. [Google Scholar] [CrossRef] [PubMed]
- Sims, M.; Kershaw, K.N.; Breathett, K.; Jackson, E.A.; Lewis, L.M.; Mujahid, M.S.; Suglia, S.F.; On Behalf of the American Heart Association Council on Epidemiology and Prevention and Council on Quality of Care and Outcomes Research. Importance of Housing and Cardiovascular Health and Well-Being: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e000089. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Tran, A.M.; Ahsan, M.J.; Niu, F.; Walters, R.W.; Kim, M.H. Relationship of health-related social needs and hospital readmissions in patients following a hospitalization for atrial fibrillation. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 36, 100340. [Google Scholar] [CrossRef] [PubMed]
- Black, G.; Davis, B.A.; Mitchell, B.N.; Sanderson, C. The Relationship Between Spirituality and Compliance in Patients with Heart Failure. Prog. Cardiovasc. Nurs. 2006, 21, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Badanta, B.; Rivilla-García, E.; Lucchetti, G.; De Diego-Cordero, R. The influence of spirituality and religion on critical care nursing: An integrative review. Nurs. Crit. Care 2022, 27, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Fenger-Grøn, M.; Vestergaard, C.H.; Frost, L.; Davydow, D.S.; Parner, E.T.; Christensen, B.; Ribe, A.R. Depression and Uptake of Oral Anticoagulation Therapy in Patients with Atrial Fibrillation: A Danish Nationwide Cohort Study. Med. Care 2020, 58, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Michal, M.; Prochaska, J.H.; Keller, K.; Göbel, S.; Coldewey, M.; Ullmann, A.; Schulz, A.; Lamparter, H.; Münzel, T.; Reiner, I.; et al. Symptoms of depression and anxiety predict mortality in patients undergoing oral anticoagulation: Results from the thrombEVAL study program. Int. J. Cardiol. 2015, 187, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Königsbrügge, O.; Ay, C. Atrial fibrillation in patients with end-stage renal disease on hemodialysis: Magnitude of the problem and new approach to oral anticoagulation. Res. Pract. Thromb. Haemost. 2019, 3, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Kyriakoulis, I.; Adamou, A.; Stamatiou, I.; Chlorogiannis, D.D.; Kardoutsos, I.; Koukousaki, D.; Ntaios, G. Efficacy and safety of direct oral anticoagulants vs vitamin K antagonists in patients with atrial fibrillation and end-stage renal disease on hemodialysis: A systematic review and meta-analysis. Eur. J. Intern. Med. 2024, 119, 45–52. [Google Scholar] [CrossRef] [PubMed]
| Total N = 362 n (%) | AF N = 181 n (%) | NAF N = 181 n (%) | Univariate p Value * | p Value (Logistic Regression) | |
|---|---|---|---|---|---|
| Age, mean (SD) ** | 77.65 (5.83) | 77.78 (5.8) | 77.53 (5.9) | 0.686 | 0.551 |
| Female sex | 140 (38.7) | 68 (37.6) | 72 (39.8) | 0.666 | 0.337 |
| Hemodialysis after discharge | 117 (32.3) | 66 (36.5) | 51 (28.2) | 0.092 | |
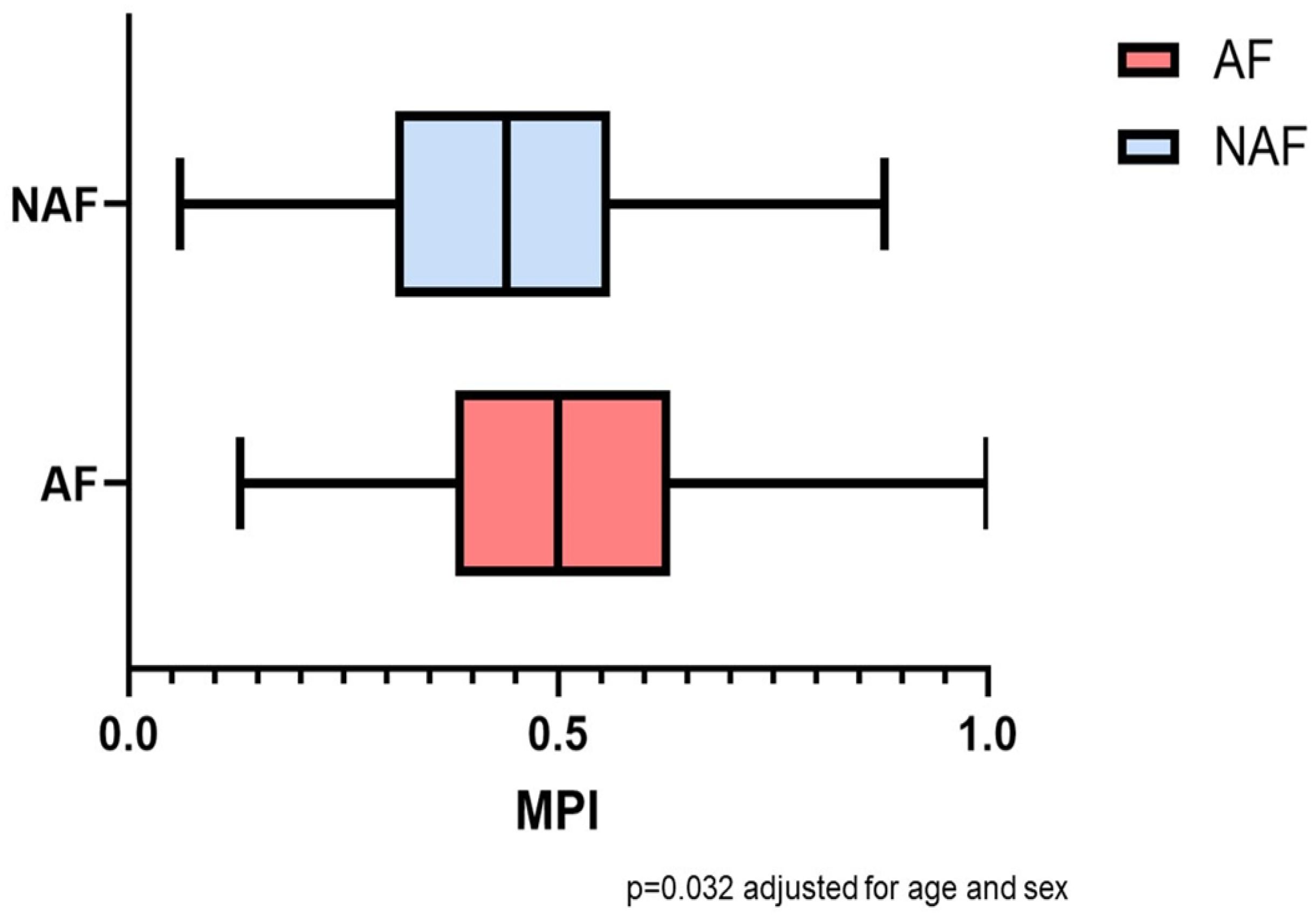
| MPI admission, mean (SD) ** | 0.49 (0.17) | 0.51 (0.16) | 0.46 (0.18) | 0.032 | 0.021 |
| MPI discharge, mean (SD) ** | 0.47 (0.16) | 0.49 (0.15) | 0.45 (0.17) | 0.263 | 0.040 |
| MPI subcategories (discharge), median (IQR) *** | |||||
| CIRS comorbidity index (discharge), mean (SD) | 5.38 (1.87) | 5.98 (1.68) | 4.77 (1.86) | <0.001, r = 0.317 | <0.001 |
| Activities of Daily Living | 5 (3) | 5 (3) | 5 (3) | 0.237 | |
| Instrumental Activities of Daily Living | 5 (4) | 5 (4) | 5 (4) | 0.438 | |
| Mini Nutritional Assessment Short Form | 9 (6) | 8 (6) | 9 (7) | 0.052 | |
| Short Portable Mental Status Questionnaire | 1 (2) | 1 (3) | 1 (2) | 0.002, r = 0.162 | |
| Exton Smith Scale | 16 (6) | 16 (4) | 16 (7) | 0.949 | |
| Number of drugs, mean (SD) | 9.73 (3.979) | 10.31 (3.72) | 9.16 (4.15) | 0.004, r = 0.149 | |
| Living conditions | 1 (2) | 1 (2) | 1 (2) | 0.537 | |
| CIRS subcategories (admission), n (%) | |||||
| CIRS subcategory: psychiatric disease | 6 (1.7) | 4 (2.2) | 2 (1.1) | 0.410 | |
| CIRS subcategory: metabolic disease | 120 (33.1) | 56 (30.9) | 64 (35.4) | 0.372 | |
| CIRS subcategory: neurological disease | 34 (9.4) | 12 (6.6) | 22 (12.2) | 0.072 | |
| CIRS subcategory: musculoskeletal disease | 50 (13.8) | 24 (13.3) | 26 (14.4) | 0.761 | |
| CIRS subcategory: genitourinary disease | 46 (12.7) | 24 (13.3) | 22 (12.2) | 0.752 | |
| CIRS subcategory: kidney disease | 197 (54.4) | 103 (56.9) | 94 (51.9) | 0.342 | |
| CIRS subcategory: liver disease | 10 (2.8) | 4 (2.2) | 6 (3.3) | 0.521 | |
| CIRS subcategory: disease of lower gastrointestinal tract | 20 (5.5) | 11 (6.1) | 9 (5.0) | 0.645 | |
| CIRS subcategory: disease of upper gastrointestinal tract | 21 (5.8) | 6 (3.3) | 15 (8.3) | 0.043 | |
| CIRS subcategory: disease of eyes, ears, nose, larynx, pharynx | 13 (3.6) | 6 (3.3) | 7 (3.9) | 0.778 | |
| CIRS subcategory: respiratory disease | 77 (21.3) | 35 (19.3) | 42 (23.2) | 0.369 | |
| CIRS subcategory: vascular, hematological, lymphatic disease | 78 (21.5) | 37 (20.4) | 41 (22.7) | 0.609 | |
| CIRS subcategory: hypertension | 27 (7.5) | 8 (4.4) | 19 (10.5) | 0.028 | |
| CIRS subcategory: heart disease | 77 (21.3) | 37 (20.4) | 40 (22.1) | 0.700 | |
| GRs and GSs | |||||
| Number of GRs, mean (SD) ** | 5.8 (2) | 5.97 (2.1) | 6.64 (1.9) | 0.117 | |
| Physical resources | 153 (42.3) | 68 (37.6) | 85 (47) | 0.070 | 0.308 |
| Age-appropriate living conditions | 234 (64.6) | 102 (56.4) | 132 (72.9) | <0.001 | 0.051 |
| Social resources | 313 (86.5) | 152 (84) | 161 (89) | 0.167 | |
| Financial resources | 213 (58.8) | 106 (58.6) | 107 (59.1) | 0.915 | |
| Spiritual resources | 152 (42) | 82 (45.3) | 70 (38.7) | 0.201 | |
| Motivational resources | 224 (61.9) | 124 (68.5) | 100 (55.2) | 0.009 | 0.014 |
| Emotional resources | 255 (70.4) | 132 (72.9) | 123 (68) | 0.300 | |
| Mnestic resources | 175 (48.3) | 115 (63.5) | 60 (33.1) | <0.001 | <0.001 |
| Competence-related resource | 171 (47.2) | 89 (49.2) | 82 (45.3) | 0.461 | |
| Intellectual resources | 195 (53.9) | 109 (60.2) | 86 (47.5) | 0.015 | 0.316 |
| Number of GSs, mean (SD) ** | 5.66 (2.5) | 6.24 (2.4) | 5.07 (2.6) | 0.746 | 0.046 |
| Incontinence | 146 (40.3) | 70 (38.7) | 76 (42) | 0.520 | |
| Instability | 242 (66.9) | 139 (76.8) | 103 (56.9) | <0.001 | 0.220 |
| Immobility | 142 (39.2) | 77 (42.5) | 65 (35.9) | 0.196 | |
| Cognitive Impairment | 35 (9.7) | 17 (9.4) | 18 (9.9) | 0.859 | |
| Inanition | 136 (37.6) | 74 (40.9) | 62 (34.3) | 0.193 | |
| Chronic Pain | 161 (44.5) | 87 (48.1) | 74 (40.9) | 0.169 | |
| Polypharmacy | 297 (82) | 150 (82.9) | 147 (81.2) | 0.681 | |
| Irritability/Depression | 62 (17.1) | 32 (17.7) | 30 (16.6) | 0.780 | |
| Sensorial impairment | 237 (65.5) | 142 (78.5) | 95 (52.5) | <0.001 | 0.003 |
| Insomnia | 165 (45.6) | 90 (49.7) | 75 (41.4) | 0.113 | |
| Irritable colon | 142 (39.2) | 71 (39.2) | 71 (39.2) | 1.000 | |
| Iatrogenic disease | 38 (10.5) | 23 (12.7) | 15 (8.3) | 0.170 | |
| Incoherence/Delirium | 14 (3.9) | 9 (5) | 5 (2.8) | 0.276 | |
| Impoverishment | 22 (6.1) | 13 (7.2) | 9 (5) | 0.379 | |
| Isolation | 23 (6.4) | 11 (6.1) | 12 (6.6) | 0.829 | |
| Fluid/Electrolyte problems | 114 (31.5) | 69 (38.1) | 45 (24.9) | 0.007 | 0.218 |
| Swallowing disorder | 59 (16.3) | 36 (19.9) | 23 (12.7) | 0.064 | 0.531 |
| GRs (%) > GSs (%) | 294 (81.2) | 148 (81.8) | 146 (80.7) | 0.788 |
| OACs Total N = 89 N (%) | |
|---|---|
| VKAs prescription (N = 181) | 58 (32) |
| DOACs prescription (N = 181) | 31 (17.1) |
| Apixaban | 15 (8.3) |
| Dabigatran | 1 (0.6.) |
| Edoxaban | 3 (1.7) |
| Rivaroxaban | 6 (3.3) |
| Component not specified | 6 (3.3) |
| Antiplatelet therapy | 66 (36.4) |
| Total N = 181 n (%) | OACs N = 91 n (%) | No OACs N = 90 n (%) | Univariate p Value * | p Value (Logistic Regression) | |
|---|---|---|---|---|---|
| Age, mean (SD) ** | 77.78 (5.8) | 77.19 (5.6) | 78.38 (5.9) | 0.167 | |
| Female | 68 (37.6) | 35 (38.5) | 33 (36.7) | 0.803 | |
| CIRS comorbidity index (discharge), mean (SD) | 5.98 (1.68) | 5.82 (1.91) | 6.14 (1.40) | 0.201 | |
| Hemodialysis after discharge | 66 (36.5) | 24 (26.4) | 42 (46.7) | 0.005 | 0.003 |
| MPI admission, mean (SD) ** | 0.52 (0.16) | 0.50 (0.16) | 0.53 (0.16) | 0.179 | |
| MPI discharge, mean (SD) ** | 0.49 (0.15) | 0.48 (0.15) | 0.51 (0.15) | 0.164 | |
| CHA2DS2-VASc, mean (SD) ** | 4.67 (1.5) | 4.74 (1.4) | 4.60 (1.6) | 0.546 | |
| HAS-BLED, mean (SD) ** | 2.73 (0.9) | 2.66 (0.9) | 2.81 (0.9) | 0.250 | |
| Number of GRs, mean (SD) ** | 5.97 (2.1) | 5.92 (1.96) | 6.01 (2.2) | 0.777 | |
| Physical resources | 68 (37.6) | 32 (35.2) | 36 (40) | 0.502 | |
| Age-appropriate living conditions | 102 (56.4) | 44 (48.4) | 58 (64.4) | 0.029 | 0.021 |
| Social resources | 152 (84) | 74 (81.3) | 78 (86.7) | 0.327 | |
| Financial resources | 106 (58.6) | 57 (62.6) | 49 (54.4) | 0.263 | |
| Spiritual resources | 82 (45.3) | 33 (36.3) | 49 (54.4) | 0.014 | 0.002 |
| Motivational resources | 124 (68.5) | 67 (73.6) | 57 (63.3) | 0.136 | |
| Emotional resources | 132 (72.9) | 73 (80.2) | 59 (65.6) | 0.026 | 0.010 |
| Mnestic resources | 115 (63.5) | 62 (68.1) | 53 (58.9) | 0.196 | |
| Competence-related resource | 89 (49.2) | 46 (50.5) | 43 (47.8) | 0.709 | |
| Intellectual resources | 109 (60.2) | 53 (58.2) | 56 (62.2) | 0.584 | |
| Number of GSs, mean (SD) ** | 6.24 (2.4) | 6.18 (2.46) | 6.31 (2.25) | 0.700 | |
| Incontinence | 70 (38.7) | 32 (35.2) | 38 (42.2) | 0.330 | |
| Instability | 139 (76.8) | 73 (80.2) | 66 (73.3) | 0.272 | |
| Immobility | 77 (42.5) | 32 (35.2) | 45 (50) | 0.044 | 0.246 |
| Cognitive Impairment | 17 (9.4) | 7 (7.7) | 10 (11.1) | 0.431 | |
| Inanition | 74 (40.9) | 37 (40.7) | 37 (41.1) | 0.951 | |
| Chronic Pain | 87 (48.1) | 51 (56) | 36 (40) | 0.031 | 0.040 |
| Polypharmacy | 150 (82.9) | 74 (81.3) | 76 (84.4) | 0.577 | |
| Irritability/Depression | 32 (17.7) | 14 (15.4) | 18 (20) | 0.416 | |
| Sensorial impairment | 142 (78.5) | 69 (75.8) | 73 (81.1) | 0.387 | |
| Insomnia | 90 (49.7) | 48 (52.7) | 42 (46.7) | 0.413 | |
| Irritable colon | 71 (39.2) | 35 (38.5) | 36 (40) | 0.832 | |
| Iatrogenic disease | 23 (12.7) | 12 (13.2) | 11 (12.2) | 0.846 | |
| Incoherence/Delirium | 9 (5) | 5 (5.5) | 4 (4.4) | 0.745 | |
| Impoverishment | 13 (7.2) | 6 (6.6) | 7 (7.8) | 0.758 | |
| Isolation | 11 (6.1) | 6 (6.6) | 5 (5.6) | 0.770 | |
| Fluid/Electrolyte problems | 69 (38.1) | 36 (39.6) | 33 (36.7) | 0.689 | |
| Swallowing disorder | 36 (19.9) | 19 (20.9) | 17 (18.9) | 0.737 | |
| GRs (%) > GSs (%) | 148 (81.8) | 77 (84.6) | 71 (78.9) | 0.318 |
| Total N = 154 n (%) | Survived N = 82 n (%) | Not Survived N = 72 n (%) | Univariate p Value * | p Value (Logistic Regression) | Odds Ratio (Lower/Upper Value of 95% Confidence Interval) | |
|---|---|---|---|---|---|---|
| Age, mean (SD) ** | 77.45 (5.9) | 76.93 (5.8) | 78.04 (6.0) | 0.244 | 0.446 | 0.977 (0.922/1.036) |
| Female | 60 (39) | 37 (45.1) | 23 (31.9) | 0.094 | 0.084 | 1.848 (0.920/3.711) |
| CIRS at discharge, mean (SD) ** | 6.06 (1.67) | 5.84 (1.81) | 6.32 (1.47) | 0.077 | ||
| Hemodialysis after discharge | 60 (39) | 23 (28) | 37 (51.4) | 0.003 | 0.022 | 0.440 (0.218/0.886) |
| MPI admission, mean (SD) ** | 0.51 (0.16) | 0.48 (0.15) | 0.55 (0.16) | 0.005 | 0.814 | 0.579 (0.006/54.578) |
| MPI discharge, mean (SD) ** | 0.49 (0.15) | 0.45 (0.12) | 0.53 (0.17) | 0.001 | 0.009 | 0.040 (0.004/0.448) |
| OAC therapy | 80 (51.9)) | 49 (59.8) | 31 (43.1) | 0.038 | 0.157 | 1.649 (0.824/3.300) |
| Number of GRs, mean (SD) ** | 5.06 (2.1) | 6.32 (2.1) | 5.78 (2.0) | 0.104 | ||
| Number of GSs, mean (SD) ** | 6.27 (2.4) | 6.17 (2.33) | 6.39 (2.50) | 0.578 | ||
| GRs (%) > GSs (%) | 127 (82.5) | 70 (85.4) | 57 (79.2) | 0.313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verleysdonk, J.; Noetzel, N.; Becker, I.; Pickert, L.; Benzing, T.; Pfister, R.; Polidori, M.C.; Affeldt, A.M. Profiles of Geriatric Syndromes and Resources in Older Patients with Atrial Fibrillation. J. Clin. Med. 2024, 13, 4009. https://doi.org/10.3390/jcm13144009
Verleysdonk J, Noetzel N, Becker I, Pickert L, Benzing T, Pfister R, Polidori MC, Affeldt AM. Profiles of Geriatric Syndromes and Resources in Older Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2024; 13(14):4009. https://doi.org/10.3390/jcm13144009
Chicago/Turabian StyleVerleysdonk, Joshua, Nicolas Noetzel, Ingrid Becker, Lena Pickert, Thomas Benzing, Roman Pfister, Maria Cristina Polidori, and Anna Maria Affeldt. 2024. "Profiles of Geriatric Syndromes and Resources in Older Patients with Atrial Fibrillation" Journal of Clinical Medicine 13, no. 14: 4009. https://doi.org/10.3390/jcm13144009
APA StyleVerleysdonk, J., Noetzel, N., Becker, I., Pickert, L., Benzing, T., Pfister, R., Polidori, M. C., & Affeldt, A. M. (2024). Profiles of Geriatric Syndromes and Resources in Older Patients with Atrial Fibrillation. Journal of Clinical Medicine, 13(14), 4009. https://doi.org/10.3390/jcm13144009






