Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring
Abstract
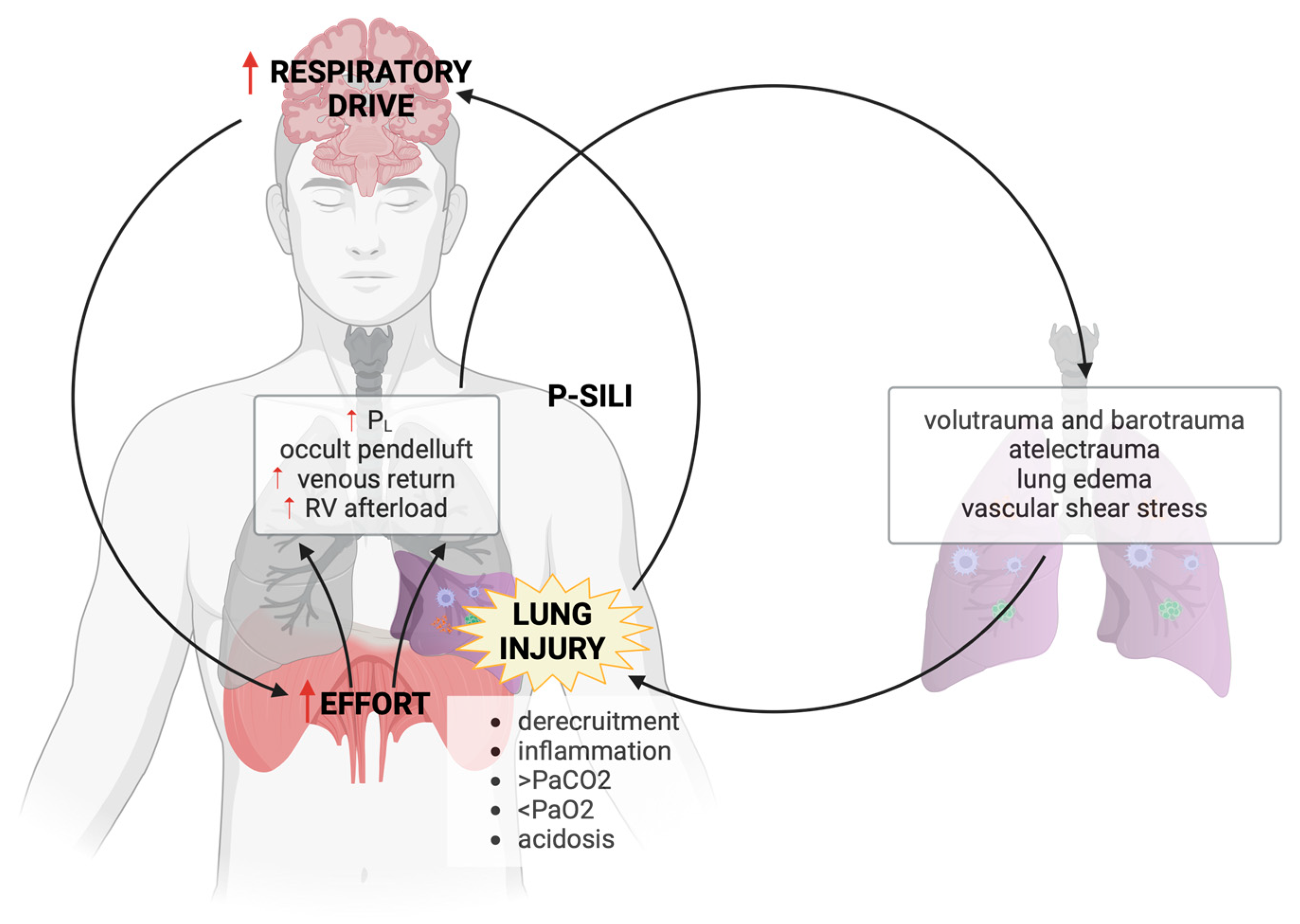
:1. Introduction: What Is P-SILI?
- During SB in the setting of a healthy lung, changes in pleural pressure brought about by diaphragm activation are homogenously distributed across the surface of the lung. This leads to an even distribution of regional PL and lung inflation and has been referred to as the healthy lung exhibiting “fluid-like behavior” [11]. In contrast, the injured ARDS lung has been described as exhibiting “solid-like” behavior because intense diaphragm activation causes larger negative inspiratory pressure swings in the dorsal, collapsed lung region. As a result, significant regional variations in PL induce excessive deformation of some lung regions and can cause a redistribution of ventilation within the lung during a single respiratory cycle.
- The redistribution of ventilation within the lung occurring at the early onset of strenuous inspiratory effort is a distinct mechanism that differentiates P-SILI from classical VILI and has been termed the “occult pendelluft” phenomenon. “Occult pendelluft” is the shift of gas from non-dependent to dependent regions during inspiration, in addition to the dorsal tidal volume coming from outside (ventilator or non-invasive support). In lung injured pigs, Yoshida et al. demonstrated that SB was associated with an early redistribution of ventilation from the non-dependent to the dependent lung occurring before the initiation of inspiratory flow from the ventilator [12]. What was particularly striking about this report is that the investigators also demonstrated that the dorsal VT was nearly threefold higher during SB than during passive ventilation with neuromuscular blockade (NMB). The implication is that significant and potentially injurious levels of regional PL may develop during SB, even at low global VT and driving pressure.
- Another often-overlooked mechanism of injury specific to P-SILI is related to the hemodynamic changes that may result in pulmonary vascular injury. The fall in pleural pressure that occurs during inspiratory effort lowers right atrial pressure (referenced to atmosphere) and thereby decreases the downstream pressure that opposes venous return, favoring the return of blood to the right ventricle. At end-inflation, PL is maximal and, particularly in the setting of reduced lung compliance, this can dramatically increase right ventricular afterload due to the increase in West non-zone 3 lung units [13,14]. This cyclic increase in RV preload followed by the increase in RV afterload may increase shear stress within the pulmonary vasculature and contribute to lung injury. This was the conclusion of an experimental study in which a detailed hemodynamic analysis was performed during a reproduction of the classic study on VILI by Webb and Tierney [15]. Although this study was performed under passive conditions, the cyclic exaggeration and interruption of RV filling and ejection during inspiration are expected to be even more prominent in the presence of decreased lung compliance and vigorous negative pleural pressure swings during SB [13,14,15,16,17].
- Finally, the inspiratory decrease in alveolar pressure to levels lower than PEEP increases the transmural pressure within the pulmonary vasculature, favoring fluid extravasation into the interstitial space. The tidal change in extravascular pressure [18] and exaggeration of pulmonary blood flow at high intravascular pressures [17] have both been shown to be potentially important contributors to lung edema that may be exaggerated during vigorous SB.
2. Does P-SILI Exist? Clinical Evidence
| Clinical Studies | Clinical Setting | Type of Ventilatory Support | Sample Size | Main Results |
|---|---|---|---|---|
| Papazian: N. Engl. J. Med. 2010, 363, 1107–1116. [19] | Acute respiratory distress syndrome. | Invasive mechanical ventilation. | 340 | Administration of neuromuscular blockade decreased the occurrence of barotrauma and increased adjusted 90-day survival and number of ventilator-free days. |
| Carteaux: Crit. Care Med. 2016, 44, 282–290. [21] | Acute hypoxemic respiratory failure. | Non-invasive ventilation. | 62 | Expired tidal volume independently associated with failure of non-invasive ventilation. |
| Bellani: Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [20] | Acute respiratory distress syndrome. | Non-invasive ventilation. | 436 | Failure of non-invasive ventilation occurred in 47.1% of patients with severe ARDS and NIV use was independently associated with increased ICU mortality. |
| Tonelli: Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [22] | Acute hypoxemic respiratory failure. | Non-invasive ventilation. | 30 | Reduction in the esophageal pressure swing by 10 cm H2O or more after 2 h of non-invasive ventilation strongly associated with avoidance of intubation. |
| Coppola: Intensive Care Medicine 2021, 47, 1130–1139. [24] | COVID-19 pneumonia. | Continuous positive airway pressure or non-invasive ventilation | 140 | Total lung stress independently associated with failure of non-invasive respiratory support. |
| Xu: BMC Pulm. Med 2024, 24, 228. [23] | Acute hypoxemic respiratory failure. | Non-invasive ventilation. | 1029 | Lower PaCO2 non-linearly associated with increased intubation risk. |
| Le Marec: J. Respir. Crit. Care Med. 2024 [25]. | Patients receiving mechanical ventilation in the intensive care unit for more than 24 h. | Invasive mechanical ventilation. | 260 | Elevated P0.1 independently associated with increased mortality. |
3. Does P-SILI Exist? Experimental Evidence
4. Bedside Monitoring to Prevent P-SILI
4.1. How to Quantify the Respiratory Drive
4.1.1. Diaphragm Electrical Activity (EAdi)
4.1.2. P0.1
4.2. How to Quantify Respiratory Effort
4.2.1. ΔPes and Pmus
4.2.2. WOB and PTP
4.2.3. ΔPocc
4.2.4. Tidal Swing of CVP
4.2.5. Diaphragm Ultrasound
4.3. How to Monitor Dangerous Breathing Patterns
4.3.1. Tidal Volume and Respiratory Rate
4.3.2. Asynchronies
4.3.3. Distribution of Ventilation with EIT
5. Conclusions
Funding
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Kangelaris, K.N.M.; Ware, L.B.; Wang, C.Y.M.; Janz, D.R.M.; Zhuo, H.; Matthay, M.A.; Calfee, C.S.M. Timing of Intubation and Clinical Outcomes in Adults With Acute Respiratory Distress Syndrome. Crit. Care Med. 2016, 44, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Fujino, Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. Crit. Care Med. 2013, 41, 536–545. [Google Scholar] [CrossRef]
- Spinelli, E.; Pesenti, A.; Slobod, D.; Fornari, C.; Fumagalli, R.; Grasselli, G.; Volta, C.A.; Foti, G.; Navalesi, P.; Knafelj, R.; et al. Clinical risk factors for increased respiratory drive in intubated hypoxemic patients. Crit. Care 2023, 27, 138. [Google Scholar] [CrossRef]
- Baedorf-Kassis, E.; Murn, M.; Dzierba, A.L.; Serra, A.L.; Garcia, I.; Minus, E.; Padilla, C.; Sarge, T.; Goodspeed, V.M.; Matthay, M.A.; et al. Respiratory drive heterogeneity associated with systemic inflammation and vascular permeability in acute respiratory distress syndrome. Crit. Care 2024, 28, 136. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Mauri, T.; Beitler, J.R.; Pesenti, A.; Brodie, D. Respiratory drive in the acute respiratory distress syndrome: Pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020, 46, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Langer, T.; Zanella, A.; Grasselli, G.; Pesenti, A. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med. 2016, 42, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Grasselli, G.; Teggia-Droghi, M.; Mauri, T.; Coppadoro, A.; Brochard, L.; Pesenti, A. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit. Care 2016, 20, 142. [Google Scholar] [CrossRef]
- Yoshida, T.; Uchiyama, A.; Fujino, Y. The role of spontaneous effort during mechanical ventilation: Normal lung versus injured lung. J. Intensive Care 2015, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Torsani, V.; Gomes, S.; De Santis, R.R.; Beraldo, M.A.; Costa, E.L.V.; Tucci, M.R.; Zin, W.A.; Kavanagh, B.P.; Amato, M.B.P. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2013, 188, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Slobod, D.; Assanangkornchai, N.; Magder, S. Comparison of Right Ventricular Loading by Lung Inflation During Passive and Assisted Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2023, 208, 108–110. [Google Scholar] [CrossRef]
- Spinelli, E.; Scaramuzzo, G.; Slobod, D.; Mauri, T. Understanding cardiopulmonary interactions through esophageal pressure monitoring. Front. Physiol. 2023, 14, 1221829. [Google Scholar] [CrossRef]
- Katira, B.H.; Giesinger, R.E.; Engelberts, D.; Zabini, D.; Kornecki, A.; Otulakowski, G.; Yoshida, T.; Kuebler, W.M.; McNamara, P.J.; Connelly, K.A.; et al. Adverse Heart–Lung Interactions in Ventilator-induced Lung Injury. Am. J. Respir. Crit. Care Med. 2017, 196, 1411–1421. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Loubieres, Y.; Schmitt, J.-M.; Page, B.; Dubourg, O.; Jardin, F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J. Appl. Physiol. 1999, 87, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- López-Aguilar, J.; Piacentini, E.; Villagrá, A.; Murias, G.; Pascotto, S.; Saenz-Valiente, A.; Fernández-Segoviano, P.; Hotchkiss, J.R.; Blanch, L. Contributions of vascular flow and pulmonary capillary pressure to ventilator-induced lung injury. Crit. Care Med. 2006, 34, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, J.R., Jr.; Blanch, L.; Naveira, A.; Adams, A.B.; Carter, C.; Olson, D.A.; Leo, P.H.; Marini, J.J. Relative roles of vascular and airspace pressures in ventilator-induced lung injury. Crit. Care Med. 2001, 29, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Forel, J.-M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.-M.; Perez, D.; Seghboyan, J.-M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Madotto, F.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Bumbasirevic, V.; Piquilloud, L.; et al. Noninvasive Ventilation of Patients with Acute Respiratory Distress Syndrome. Insights from the LUNG SAFE Study. Am. J. Respir. Crit. Care Med. 2017, 195, 67–77. [Google Scholar] [CrossRef]
- Carteaux, G.; Millan-Guilarte, T.; De Prost, N.; Razazi, K.; Abid, S.; Thille, A.W.; Schortgen, F.; Brochard, L.; Brun-Buisson, C.; Mekontso Dessap, A. Failure of Noninvasive Ventilation for De Novo Acute Hypoxemic Respiratory Failure: Role of Tidal Volume. Crit. Care Med. 2016, 44, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, R.; Fantini, R.; Tabbì, L.; Castaniere, I.; Pisani, L.; Pellegrino, M.R.; Della Casa, G.; D’amico, R.; Girardis, M.; Nava, S.; et al. Early Inspiratory Effort Assessment by Esophageal Manometry Predicts Noninvasive Ventilation Outcome in De. Novo Respiratory Failure. A Pilot Study. Am. J. Respir. Crit. Care Med. 2020, 202, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, M.; Min, Y.; Hu, W.; Bai, L.; Duan, J. PaCO2 is nonlinearly associated with NIV failure in patients with hypoxemic respiratory failure. BMC Pulm. Med. 2024, 24, 228. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Chiumello, D.; Busana, M.; Giola, E.; Palermo, P.; Pozzi, T.; Steinberg, I.; Roli, S.; Romitti, F.; Lazzari, S.; et al. Role of total lung stress on the progression of early COVID-19 pneumonia. Intensive Care Med. 2022, 48, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Le Marec, J.; Hajage, D.; Decavèle, M.; Schmidt, M.; Laurent, I.; Ricard, J.-D.; Jaber, S.; Azoulay, E.; Fartoukh, M.; Hraiech, S.; et al. High Airway Occlusion Pressure Is Associated with Dyspnea and Increased Mortality in Critically Ill Mechanically Ventilated Patients. Am. J. Respir. Crit. Care Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Slobod, D.; Assanangkornchai, N.; Samoukovic, G. Getting SILI between Two Extracorporeal Membrane Oxygenation Runs. Ann. Am. Thorac. Soc. 2021, 18, 167–171. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss, S.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar]
- Morais, C.C.A.; Koyama, Y.; Yoshida, T.; Plens, G.M.; Gomes, S.; Lima, C.A.S.; Ramos, O.P.S.; Pereira, S.M.; Kawaguchi, N.; Yamamoto, H.; et al. High Positive End-Expiratory Pressure Renders Spontaneous Effort Noninjurious. Am. J. Respir. Crit. Care Med. 2018, 197, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Dzierba, A.L.; Khalil, A.M.; Derry, K.L.; Madahar, P.; Beitler, J.R. Discordance Between Respiratory Drive and Sedation Depth in Critically Ill Patients Receiving Mechanical Ventilation. Crit. Care Med. 2021, 49, 2090–2101. [Google Scholar] [CrossRef]
- von Bethmann, A.N.; Brasch, F.; Nüsing, R.; Vogt, K.; Volk, H.D.; Müller, K.-M.; Wendel, A.; Uhlig, S. Hyperventilation induces release of cytokines from perfused mouse lung. Am. J. Respir. Crit. Care Med. 1998, 157, 263–272. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Guo, X.; Song, L. Resistive spontaneous breathing exacerbated lipopolysaccharide-induced lung injury in mice. Biochem. Biophys. Rep. 2024, 38, 101726. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, D.; Kolobow, T.; Fumagalli, R.; Moretti, M.P.; Chen, V.; Buckhold, D. Acute respiratory failure following pharmacologically induced hyperventilation: An experimental animal study. Intensive Care Med. 1988, 15, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Uchiyama, A.; Matsuura, N.; Mashimo, T.; Fujino, Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: High transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit. Care Med. 2012, 40, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.C.; Cruces, P.; Díaz, F.; Oviedo, V.; Goich, M.; Fuenzalida, J.; Damiani, L.F.; Basoalto, R.; Jalil, Y.; Carpio, D.; et al. Spontaneous breathing promotes lung injury in an experimental model of alveolar collapse. Sci. Rep. 2022, 12, 12648. [Google Scholar] [CrossRef] [PubMed]
- Dubo, S.; Oviedo, V.; Garcia, A.; Alegría, L.; García, P.; Valenzuela, E.D.; Damiani, L.F.; Araos, J.; Medina, T.; Bachmann, M.C.; et al. Low Spontaneous Breathing Effort during Extracorporeal Membrane Oxygenation in a Porcine Model of Severe Acute Respiratory Distress Syndrome. Anesthesiology 2020, 133, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, A.H.; de Vries, H.J.; Heunks, L.M.A. Physiology of the Respiratory Drive in ICU Patients: Implications for Diagnosis and Treatment. Crit. Care 2024, 28, 94. [Google Scholar] [CrossRef]
- Luo, Y.M.; Moxham, J.; Polkey, M.I. Diaphragm electromyography using an oesophageal catheter: Current concepts. Clin. Sci. 2008, 115, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Piquilloud, L.; Beloncle, F.; Richard, J.-C.M.; Mancebo, J.; Mercat, A.; Brochard, L. Information conveyed by electrical diaphragmatic activity during unstressed, stressed and assisted spontaneous breathing: A physiological study. Ann. Intensive Care 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, H.; Yang, Y.; Huang, Y.; Liu, S.; Beck, J.; Slutsky, A.S.; Sinderby, C.; Qiu, H. Neuroventilatory efficiency and extubation readiness in critically ill patients. Crit. Care 2012, 16, R143. [Google Scholar] [CrossRef]
- Bellani, G.; Mauri, T.; Coppadoro, A.; Grasselli, G.; Patroniti, N.; Spadaro, S.; Sala, V.; Foti, G.; Pesenti, A. Estimation of Patient’s Inspiratory Effort From the Electrical Activity of the Diaphragm. Crit. Care Med. 2013, 41, 1483–1491. [Google Scholar] [CrossRef]
- Di Mussi, R.; Spadaro, S.; Volta, C.A.; Bartolomeo, N.; Trerotoli, P.; Staffieri, F.; Pisani, L.; Iannuzziello, R.; Dalfino, L.; Murgolo, F.; et al. Continuous assessment of neuro-ventilatory drive during 12 h of pressure support ventilation in critically ill patients. Crit. Care 2020, 24, 652. [Google Scholar] [CrossRef] [PubMed]
- Whitelaw, W.A.; Derenne, J.-P.; Milic-Emili, J. Occlusion pressure as a measure of respiratory center output cm conscious man. Respir. Physiol. 1975, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Telias, I.; Junhasavasdikul, D.; Rittayamai, N.; Piquilloud, L.; Chen, L.; Ferguson, N.D.; Goligher, E.C.; Brochard, L. Airway Occlusion Pressure As an Estimate of Respiratory Drive and Inspiratory Effort during Assisted Ventilation. Am. J. Respir. Crit. Care Med. 2020, 201, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Yoshida, T.; Bellani, G.; Goligher, E.C.; Carteaux, G.; Rittayamai, N.; Mojoli, F.; Chiumello, D.; Piquilloud, L.; Grasso, S.; et al. Esophageal and transpulmonary pressure in the clinical setting: Meaning, usefulness and perspectives. Intensiv. Care Med. 2016, 42, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, C.S.H.; Light, R.W.; Lodia, R.; Sieck, G.C.; Mahutte, C.K. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and t-piece during weaning from mechanical ventilation. Am. Rev. Respir. Dis. 1991, 143, 469–475. [Google Scholar] [CrossRef]
- Bertoni, M.; Telias, I.; Urner, M.; Long, M.; Del Sorbo, L.; Fan, E.; Sinderby, C.; Beck, J.; Liu, L.; Qiu, H.; et al. A novel non-invasive method to detect excessively high respiratory effort and dynamic transpulmonary driving pressure during mechanical ventilation. Crit. Care 2019, 23, 346. [Google Scholar] [CrossRef] [PubMed]
- Roesthuis, L.; van den Berg, M.; van der Hoeven, H. Non-invasive method to detect high respiratory effort and transpulmonary driving pressures in COVID-19 patients during mechanical ventilation. Ann. Intensive Care 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Walling, P.T.; Savege, T.M. A Comparison of oesophageal and central venous pressures in the measurement of transpulmonary pressure change. Br. J. Anaesth. 1976, 48, 475–479. [Google Scholar] [CrossRef]
- Colombo, J.; Spinelli, E.; Grasselli, G.; Pesenti, A.M.; Protti, A. Detection of strong inspiratory efforts from the analysis of central venous pressure swings: A preliminary clinical study. Minerva Anestesiol. 2020, 86, 1296–1304. [Google Scholar] [CrossRef]
- Verscheure, S.; Massion, P.; Gottfried, S.; Goldberg, P.; Samy, L.; Damas, P.; Magder, S. Measurement of pleural pressure swings with a fluid-filled esophageal catheter vs pulmonary artery occlusion pressure. J. Crit. Care 2017, 37, 65–71. [Google Scholar] [CrossRef]
- Vivier, E.; Dessap, A.M.; Dimassi, S.; Vargas, F.; Lyazidi, A.; Thille, A.W.; Brochard, L. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012, 38, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Lassola, S.; Miori, S.; Sanna, A.; Cucino, A.; Magnoni, S.; Umbrello, M. Central venous pressure swing outperforms diaphragm ultrasound as a measure of inspiratory effort during pressure support ventilation in COVID-19 patients. J. Clin. Monit. Comput. 2022, 36, 461–471. [Google Scholar] [CrossRef]
- Yang, K.L.; Tobin, M.J. A Prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N. Engl. J. Med. 1991, 324, 1445–1450. [Google Scholar] [CrossRef]
- Hey, E.; Lloyd, B.; Cunningham, D.; Jukes, M.; Bolton, D. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir. Physiol. 1966, 1, 193–205. [Google Scholar] [CrossRef]
- Bulleri, E.; Fusi, C.; Bambi, S.; Pisani, L. Patient-ventilator asynchronies: Types, outcomes and nursing detection skills. Acta Biomed. 2018, 89, 6–18. [Google Scholar]
- Rodrigues, A.; Telias, I.; Damiani, L.F.; Brochard, L. Reverse Triggering during Controlled Ventilation: From Physiology to Clinical Management. Am. J. Respir. Crit. Care Med. 2023, 207, 533–543. [Google Scholar] [CrossRef]
- Slobod, D.; Leali, M.; Spinelli, E.; Grieco, D.L.; Spadaro, S.; Mauri, T. Integrating electrical impedance tomography and transpulmonary pressure monitoring to personalize PEEP in hypoxemic patients undergoing pressure support ventilation. Crit. Care 2022, 26, 314. [Google Scholar] [CrossRef]
- Cornejo, R.A.; Arellano, D.H.; Ruiz-Rudolph, P.; Guiñez, D.V.; Morais, C.C.A.; Gajardo, A.I.J.; Lazo, M.T.; Brito, R.E.; Cerda, M.A.; González, S.J.; et al. Inflammatory biomarkers and pendelluft magnitude in ards patients transitioning from controlled to partial support ventilation. Sci. Rep. 2022, 12, 20233. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Z.; Yun, P.-J.; Frerichs, I.; Möller, K.; Fu, F.; Liu, X.; Zhong, N.; Li, Y. Qualitative and quantitative assessment of pendelluft: A simple method based on electrical impedance tomography. Ann. Transl. Med. 2020, 8, 1216. [Google Scholar] [CrossRef]
- Protti, A.; Tonelli, R.; Corte, F.D.; Grieco, D.; Spinelli, E.; Spadaro, S.; Piovani, D.; Menga, L.; Schifino, G.; Pittao, M.V.; et al. Development of clinical tools to estimate the breathing effort during high-flow oxygen therapy: A multicenter cohort study. Pulmonology 2024. [Google Scholar] [CrossRef]

| Experimental Studies | Clinical Setting | Type of Ventilatory Support | Sample Size | Main Results |
|---|---|---|---|---|
| von Bethmann: Am. J. Respir. Crit. Care Med. 1998, 157, 263–272. [30] | Isolated hyperventilated and perfused mouse lung. | Positive pressure ventilation (PPV) or negative pressure ventilation (NPV). | 12 | Hyperventilation resulted in an increased expression of TNFα and IL-6 mRNA, and prostacyclin release into the perfusate. |
| Cai: Biochem. Biophys. Rep. 2024, 38, 101726. [31] | LPS induced ARDS + tracheal banding in female mice. | Resistive spontaneous breathing (RSB). | 60 | RSB exacerbated lung injury in ARDS: more congestion and edema, more severe inflammatory cell infiltration, and increased IL-1β, IL-6, TNF-α, and total protein levels in BALF. |
| Mascheroni: Intensive Care Med. 1988, 15, 8–14. [32] | Hyperventilation induced by sodium salicylate infusion in the cisterna magna of adult sheep. | Spontaneous breathing. | 31 | Hyperventilation by SB induced alterations in gas exchange, a decrease in the static compliance of the respiratory system, and atelectasis. |
| Yoshida: Crit Care Med. 2012, 40, 1578–1585. [33] | Acute lung injury induced by lung lavage with 25 mL/kg of normal saline in rabbits. | Invasive mechanical ventilation + spontaneous breathing. | 32 | High PL generated by strong spontaneous breathing effort worsened lung injury. |
| Yoshida: Crit Care Med 2013, 41, 536–545. [4] | Mild lung injury induced by lung lavage and severe lung injury induced by lung lavage + injurious mechanical ventilation in rabbits. | Invasive mechanical ventilation + spontaneous breathing. | 28 | SB worsened lung injury in the severe lung injury group, while muscle paralysis was protective. |
| Yoshida: Am. J. Respir. Crit. Care Med. 2013, 188, 1420–1427. [12] | Acute lung injury in pigs. | Invasive mechanical ventilation + spontaneous breathing. | 7 | Spontaneous breathing effort during mechanical ventilation caused pendelluft and overstretch during early inflation, with more negative local Ppl in dependent regions. |
| Morais: Am. J. Respir. Crit. Care Med. 2018, 197, 1285–1296. [28] | Lung injury induced by lung lavage + injurious mechanical ventilation in rabbits. | Invasive mechanical ventilation + spontaneous breathing. | 28 | Strong spontaneous effort at low PEEP injured the dependent lung, while high PEEP was protective. |
| Bachmann: Sci. Rep. 2022, 12, 12648. [34] | Acute lung injury induced by lung lavage with 30 mL/kg of isotonic saline in pigs. | Pressure support ventilation or controlled mechanical ventilation. | 18 | Prolonged SB caused progression of lung injury, while early muscle paralysis and controlled mechanical ventilation could be beneficial. |
| Dubo: Anesthesiology 2020, 133. [35] | Lung injury induced by lung lavage + injurious mechanical ventilation in pigs. | Invasive mechanical ventilation + spontaneous breathing during ECMO. | 12 | SB during ECMO in severe ARDS did not result in worsened lung injury if compared to controlled mechanical ventilation. |
| Monitoring Method | Main Measures | Physiological Range | Advantages | Limitations |
|---|---|---|---|---|
| Neural activity of the diaphragm | EAdiPEAK | Lack of absolute values. In SB healthy subjects 13–21 μV [38]. | Close to the neural drive, useful to assess change of the neural drive over time, EAdi does not require intubation. | Interindividual variability (no reference values), cannot detect activation of respiratory muscles apart from diaphragm. |
| TiNEUR | In SB healthy subjects 1.5–2 ms [38]. | |||
| NVE = EAdiPEAK/Vt | Lack of absolute values [39]. | |||
| NME = EAdiPEAK/ΔPaw | Lack of absolute values [40]. | |||
| EAdiPEAK ∗ NME | Lack of absolute values [40]. | |||
| Airway occlusion pressure | P0.1 | 1.0–3.5 cmH2O [43] | Not affected by muscle weakness or flow resistance | Requires intubation, breath-to-breath variability, can change according to the ventilator mode |
| Monitoring Method | Main Measures | Physiological Range | Advantages | Limitations |
|---|---|---|---|---|
| Esophageal pressure swings | ΔPes | 5–8 cmH2O [44] | Good indicator of effort, easy to obtain at the bedside and in non-intubated patients | Cannot discriminate the effort required to expand the chest wall. |
| Pmus | 5–10 cmH2O [44] | Best indicators of effort | Requires intubation and measurement of elastic chest wall recoil pressure under passive conditions. | |
| WOB | 0.35–2.4 j min−1 [44] | |||
| PTP | 80–200 cmH2O s min−1 [43] | |||
| Negative airway pressure swing during end-expiratory occlusion | ΔPocc | 6–13 cmH2O [46] | Good correlation with ΔPes and Pmus, easy to obtain in intubated patients. | Requires intubation and collaboration of the patient. |
| Tidal swing of CVP | ΔCVP | 0–8 mmHg [49] | Good correlation with ΔPes, useful in the absence of an esophageal balloon catheter | Depends on volemic state of the patient. |
| Diaphragm ultrasound | Thickening fraction | 15–30% [51] | Easy to obtain at the bedside and in non-intubated patients, cheap. | Does not account for inspiratory and expiratory muscle activation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marongiu, I.; Slobod, D.; Leali, M.; Spinelli, E.; Mauri, T. Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring. J. Clin. Med. 2024, 13, 4018. https://doi.org/10.3390/jcm13144018
Marongiu I, Slobod D, Leali M, Spinelli E, Mauri T. Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring. Journal of Clinical Medicine. 2024; 13(14):4018. https://doi.org/10.3390/jcm13144018
Chicago/Turabian StyleMarongiu, Ines, Douglas Slobod, Marco Leali, Elena Spinelli, and Tommaso Mauri. 2024. "Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring" Journal of Clinical Medicine 13, no. 14: 4018. https://doi.org/10.3390/jcm13144018
APA StyleMarongiu, I., Slobod, D., Leali, M., Spinelli, E., & Mauri, T. (2024). Clinical and Experimental Evidence for Patient Self-Inflicted Lung Injury (P-SILI) and Bedside Monitoring. Journal of Clinical Medicine, 13(14), 4018. https://doi.org/10.3390/jcm13144018






