The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature
Abstract
1. Introduction
2. Discussion
2.1. Currently Used Therapeutic Methods for Treating Rop
2.2. Hypoxia-Inducible Factor—HIF
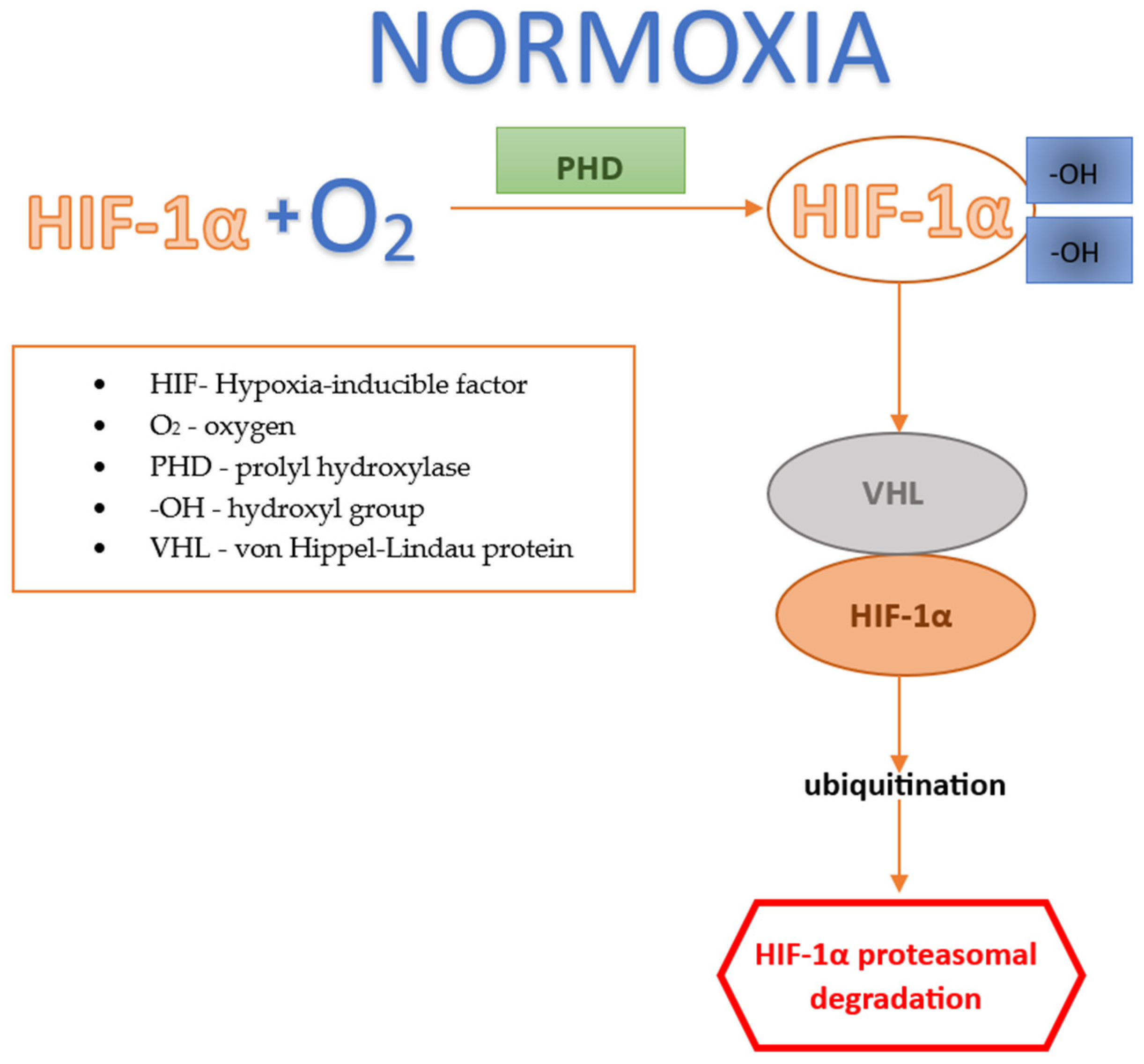
2.2.1. Factor HIF under Normoxic Conditions
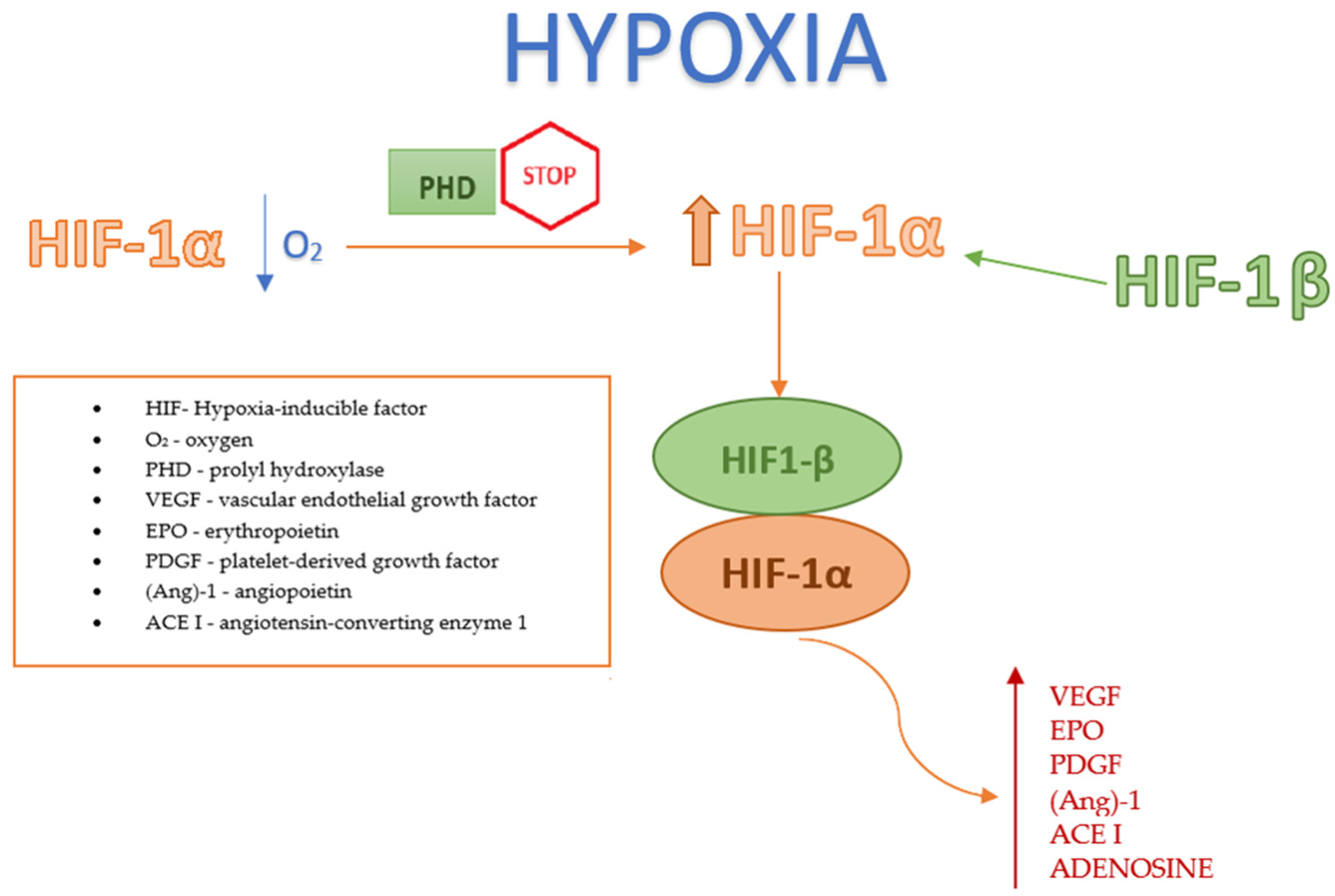
2.2.2. Factor HIF under Hypoxic Conditions
2.3. HIF in Therapeutic Management
2.3.1. Role of Systemic Metabolism in the Protective Function of the Retina
Serine and 1-Carbon Metabolism
Fibrates
2.3.2. HIF Inhibitors
Thrombomodulin 1
Topotecan, Doxorubicin
Decapterus Tabl Substrate
Celastrol (Tripterine)
3-Hydroxypyruvate
2.3.3. Apurinic/Apyrimidinic Endonuclease 1/Oxidation-Reduction Factor 1 (APE1/Ref-1)
2.3.4. MicroRNA
2.3.5. Lowering Intraocular Pressure Reduces HIF-1α
2.3.6. Bone Marrow Cells
2.4. HIF-1α in the Diagnostic Management of Rop
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatnagar, A.; Skrehot, H.C.; Bhatt, A.; Herce, H.; Weng, C.Y. Epidemiology of Retinopathy of Prematurity in the US From 2003 to 2019. JAMA Ophthalmol. 2023, 141, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Modrzejewska, M.; Bosy, W. Nine-Year Epidemiological Data on the Incidence of Retinopathy of Prematurity in Poland-A Literature Review for the 2012–2021 Period. Int. J. Environ. Res. Public Health 2022, 19, 15694. [Google Scholar] [CrossRef] [PubMed]
- Dammann, O.; Hartnett, M.E.; Stahl, A. Retinopathy of prematurity. Dev. Med. Child. Neurol. 2023, 65, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Webster, K.A.; Bhatt, A.; Tian, H.; Su, G.; Li, W. Concurrent Physiological and Pathological Angiogenesis in Reti-nopathy of Prematurity and Emerging Therapies. Int. J. Mol. Sci. 2021, 22, 4809. [Google Scholar] [CrossRef] [PubMed]
- Bonafiglia, E.; Gusson, E.; Longo, R.; Ficial, B.; Tisato, M.G.; Rossignoli, S.; Caltran, G.; Pedrotti, E.; Beghini, R.; Marchini, G. Early and late onset sepsis and retinopathy of prematurity in a cohort of preterm infants. Sci. Rep. 2022, 12, 11675. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Hellström, W.; Tomita, Y.; Fu, Z.; Liegl, R.; Winberg, A.; Hansen-Pupp, I.; Ley, D.; Hellström, A.; Löfqvist, C.; et al. IGF1, serum glucose, and retinopathy of prematurity in extremely preterm infants. JCI Insight 2020, 5, e140363. [Google Scholar] [CrossRef] [PubMed]
- Fevereiro-Martins, M.; Marques-Neves, C.; Guimarães, H.; Bicho, M. Retinopathy of prematurity: A review of pathophysiology and signaling pathways. Surv. Ophthalmol. 2023, 68, 175–210. [Google Scholar] [CrossRef] [PubMed]
- Banjac, L.; Banjac, G.; Kotur-Stevuljević, J.; Spasojević-Kalimanovska, V.; Gojković, T.; Bogavac-Stanojević, N.; Jelić-Ivanović, Z.; Banjac, G. Pro-Oxidants and Antioxidants in Retinopathy of Prematurity. Acta Clin. Croat. 2018, 57, 458–463. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, Y.C. Retinal vascular development in an immature retina at 33–34 weeks postmenstrual age pre-dicts retinopathy of prematurity. Sci. Rep. 2020, 10, 18111. [Google Scholar] [CrossRef]
- Zhang, L.; Buonfiglio, F.; Fieß, A.; Pfeiffer, N.; Gericke, A. Retinopathy of Prematurity-Targeting Hypoxic and Redox Signaling Pathways. Antioxidants 2024, 13, 148. [Google Scholar] [CrossRef]
- Palmer, E.A.; Flynn, J.T.; Hardy, R.J.; Phelps, D.L.; Phillips, C.L.; Schaffer, D.B.; Tung, B. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991, 98, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Omri, S.; Sitaras, N.; Rivera, J.C.; Sapieha, P.; Chemtob, S. Neovascularization in retinopathy of prematurity: Opposing actions of neuronal factors GPR91 and semaphorins 3A. Acta Paediatr. 2012, 101, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Josan, A.S.; Purohit, R.; Patel, C.K.; Xue, K. A Network Meta-Analysis of Retreatment Rates following Bevacizumab, Ranibizumab, Aflibercept, and Laser for Retinopathy of Prematurity. Ophthalmology 2022, 129, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.D.; Cernichiaro-Espinosa, L.A.; Berrocal, A.M. Management of Retinopathy of Prematurity—Use of Anti-VEGF Therapy. Asia Pac. J. Ophthalmol. 2018, 7, 56–62. [Google Scholar] [CrossRef]
- Stahl, A.; Sukgen, E.A.; Wu, W.C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. J. Am. Med. Assoc. 2022, 328, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoi-etin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nam, H.J.; Lee, J.; Park, D.Y.; Kim, C.; Yu, Y.S.; Kim, D.; Park, S.W.; Bhin, J.; Hwang, D.; et al. Methylation-dependent regulation of HIF-1α stability restricts retinal and tumour angiogenesis. Nat. Commun. 2016, 7, 10347. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef]
- Murphy, M.P.; O’Neill, L.A.J. Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell 2018, 174, 780–784. [Google Scholar] [CrossRef]
- Singh, C.; Hoppe, G.; Tran, V.; McCollum, L.; Bolok, Y.; Song, W.; Sharma, A.; Brunengraber, H.; Sears, J.E. Serine and 1-carbon metabolism are required for HIF-mediated protection against retinopathy of prematurity. JCI Insight 2019, 4, e129398. [Google Scholar] [CrossRef]
- Hoppe, G.; Lee, T.J.; Yoon, S.; Yu, M.; Peachey, N.S.; Rayborn, M.; Zutel, M.J.; Trichonas, G.; Au, J.; Sears, J.E. Inducing a visceral organ to protect a peripheral capillary bed: Stabilizing hepatic HIF-1α prevents oxygen-induced retinopathy. Am. J. Pathol. 2014, 184, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Usui-Ouchi, A.; Nilsson, A.K.; Yang, J.; Ko, M.; Hellström, A.; Fu, Z. Metabolism in Retinopathy of Prematurity. Life 2021, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Ozawa, N.; Miwa, Y.; Ishida, A.; Ohta, M.; Tsubota, K.; Kurihara, T. Pemafibrate Prevents Retinal Pathological Neovascularization by Increasing FGF21 Level in a Murine Oxygen-Induced Retinopathy Model. Int. J. Mol. Sci. 2019, 20, 5878. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Kunimi, H.; Ibuki, M.; Shoda, C.; Nakai, A.; Kurihara, T. HIF Inhibition Therapy in Ocular Diseases. Keio J. Med. 2022, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Kuo, C.H.; Peng, I.C.; Chang, Y.S.; Tseng, S.H.; Conway, E.M.; Wu, H.L. Recombinant thrombomodulin domain 1 rescues pathological angiogenesis by inhibition of HIF-1α-VEGF pathway. Cell. Mol. Life Sci. 2021, 78, 7681–7692. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Hoshino, Y.; Shoda, C.; Jiang, X.; Tsubota, K.; Kurihara, T. Pharmacological HIF inhibition prevents retinal neovasculari-zation with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 2019, 128, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Miwa, Y.; Nimura, K.; Okamoto, K.; Yamagami, S.; Tsubota, K.; Kurihara, T. Hypoxia-Inducible Factor Inhibitors Derived from Marine Products Suppress a Murine Model of Neovascular Retinopathy. Nutrients 2020, 12, 1055. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Aguilar, E.; Murinello, S.; Prins, M.; Gantner, M.L.; Wright, P.E.; Berlow, R.B.; Friedlander, M. An allosteric peptide inhibitor of HIF-1α regulates hypoxia-induced retinal neovascularization. Proc. Natl. Acad. Sci. USA 2020, 117, 28297–28306. [Google Scholar] [CrossRef]
- Zhao, K.; Jiang, Y.; Zhang, J.; Shi, J.; Zheng, P.; Yang, C.; Chen, Y. Celastrol inhibits pathologic neovascularization in oxygen-induced retinopathy by targeting the miR-17-5p/HIF-1α/VEGF pathway. Cell Cycle 2022, 21, 2091–2108. [Google Scholar] [CrossRef]
- Padilla-Montaño, N.; de León Guerra, L.; Moujir, L. Antimicrobial Activity and Mode of Action of Celastrol, a Nortriterpen Quinone Isolated from Natural Sources. Foods 2021, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Shin, E.K.; Kim, Y.H.; Lee, B.W.; Jun, J.G.; Park, J.H.; Kim, J.K. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur. J. Clin. Investig. 2009, 39, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, D.S.; Meel, M.H.; Benedict, B.; Waranecki, P.; Koster, J.; Kaspers, G.J.L.; Hulleman, E. Celastrol-induced degradation of FANCD2 sensitizes pediatric high-grade gliomas to the DNA-crosslinking agent carboplatin. EBioMedicine 2019, 50, 81–92. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, K.J.; Seo, W.D.; Jang, S.Y.; Kim, M.; Lee, B.W.; Kim, J.Y.; Kang, S.; Park, K.H.; Lee, Y.S.; et al. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiedemann, R.E.; Schmidt, J.; Keats, J.J.; Shi, C.X.; Zhu, Y.X.; Palmer, S.E.; Mao, X.; Schimmer, A.D.; Stewart, A.K. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood 2009, 113, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, X.; Hu, Y.; Yang, B.; Tsui, C.K.; Yu, S.; Lu, L.; Liang, X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J. Pineal Res. 2018, 64, e12473. [Google Scholar] [CrossRef]
- Singh, C.; Sharma, A.; Hoppe, G.; Song, W.; Bolok, Y.; Sears, J.E. 3-Hydroxypyruvate Destabilizes Hypoxia Inducible Factor and induces Angiostasis. Invest. Ophthalmol. Vis. Sci. 2018, 59, 3440–3448. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Sardar Pasha, S.P.B.; Sishtla, K.; Sulaiman, R.S.; Park, B.; Shetty, T.; Shah, F.; Fishel, M.L.; Wikel, J.H.; Kelley, M.R.; Corson, T.W. Ref-1/APE1 Inhibition with Novel Small Molecules Blocks Ocular Neovascularization. J. Pharmacol. Exp. Ther. 2018, 367, 108–118. [Google Scholar] [CrossRef]
- Hartman, G.D.; Lambert-Cheatham, N.A.; Kelley, M.R.; Corson, T.W. Inhibition of APE1/Ref-1 for Neovascular Eye Diseases: From Biology to Therapy. Int. J. Mol. Sci. 2021, 22, 10279. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Calvo, J.; Esquiva, G.; Gómez-Vicente, V.; Valor, L.M. MicroRNAs in the Mouse Developing Retina. Int. J. Mol. Sci. 2023, 24, 2992. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.T.; Li, X.X.; Peng, D.W.; Zhang, W.M.; Qu, J.; Lu, F.; D’Amato, R.J.; Chi, Z.L. MicroRNA-18a-5p Administration Suppresses Retinal Neovascularization by Targeting FGF1 and HIF1A. Front. Pharmacol. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ni, B.; Zhou, T.; Huang, Z.; Zhou, H.; Zhou, Y.; Lin, S.; He, C.; Liu, X. HIF-1α Reduction by Lowering Intraocular Pressure Alleviated Retinal Neovascularization. Biomolecules 2023, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; Alibabrdel, M.; Asadian, S.; Siavashi, V.; Jabarpour, M.; Nassiri, S.M. Increased circulation mobilization of endothelial progenitor cells in preterm infants with retinopathy of prematurity. J. Cell Biochem. 2018, 119, 6575–6583. [Google Scholar] [CrossRef] [PubMed]
- Villacampa, P.; Liyanage, S.E.; Klaska, I.P.; Cristante, E.; Menger, K.E.; Sampson, R.D.; Barlow, M.; Abelleira-Hervas, L.; Duran, Y.; Smith, A.J.; et al. Stabilization of myeloid-derived HIFs promotes vascular regeneration in retinal ischemia. Angiogenesis 2020, 23, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.I.; Kilburn, T.C.; Duvall, C.L.; Penn, J.S. Visualizing HIF-1α mRNA in a Subpopulation of Bone Marrow-Derived Cells to Predict Retinal Neovascularization. ACS Chem. Biol. 2020, 15, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.R.; Banin, E.; Moreno, S.K.; Aguilar, E.; Dorrell, M.I.; Friedlander, M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J. Clin. Invest. 2006, 116, 3266–3276. [Google Scholar] [CrossRef]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef]
| Authors | Year of Publication | Pharmacological Agent/Molecular Pathway | Animal Model Used during Research | Key Findings for Each Potential Therapeutic Agent |
|---|---|---|---|---|
| Singh et al. [21] | 2019 | Newborn mice models of OIR |
| |
| Tomita et al. [23] | 2019 | Pemafibrate | Mouse models of OIR |
|
| Huang et al. [27] | 2021 | Recombinant Thrombomodulin 1 | Mouse models of OIR |
|
| Miwa et al. [28] | 2019 | Topotecan, Doxorubicin | Mouse model of OIR |
|
| Shoda et al. [29] | 2020 | Components of Decapterus tablets | Mouse model of OIR |
|
| Usui-Ouchi et al. [30] | 2020 | CITED2 peptide | Mouse model of OIR |
|
| Zhao et al. [31] | 2022 | Celastrol | Mouse model of OIR |
|
| Singh et al. [38] | 2018 | 3-hydroxypyruvate | Mouse model of OIR |
|
| Hartman et al. [41] | 2021 | Apurinic/Apyrimidinic Endonuclease 1/Reduction-Oxidation Factor 1 (APE1/Ref-1) | Mouse model of OIR |
|
| Guan et al. [43] | 2020 | miRNA mimic, agomiR-18a-5p |
| |
| Yang et al. [44] | 2023 | Reduced intraocular pressure (IOP) | Mouse model of OIR |
|
| Villacampa et al. [46] | 2020 | Stabilizing HIF through VHL deletion | Mouse model of OIR |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrzejewska, M.; Zdanowska, O.; Połubiński, P. The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature. J. Clin. Med. 2024, 13, 4034. https://doi.org/10.3390/jcm13144034
Modrzejewska M, Zdanowska O, Połubiński P. The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature. Journal of Clinical Medicine. 2024; 13(14):4034. https://doi.org/10.3390/jcm13144034
Chicago/Turabian StyleModrzejewska, Monika, Oliwia Zdanowska, and Piotr Połubiński. 2024. "The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature" Journal of Clinical Medicine 13, no. 14: 4034. https://doi.org/10.3390/jcm13144034
APA StyleModrzejewska, M., Zdanowska, O., & Połubiński, P. (2024). The Role of HIF-1α in Retinopathy of Prematurity: A Review of Current Literature. Journal of Clinical Medicine, 13(14), 4034. https://doi.org/10.3390/jcm13144034






