High Medial Longitudinal Arch of the Foot and Latent Trigger Points in Lower Limb Muscles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. Intra-Rater Reliability
2.4. Participants and Settings
2.5. Variables
- Demographics: sex, height, weight, and body mass index.
- Type of MLA: standard (NDT from 5 to 9 mm) or high (NDT ≤ 4 mm) [24].
- Total number of LTrPs in all muscles assessed.
- Prevalence of LTrPs in each of the muscles assessed.
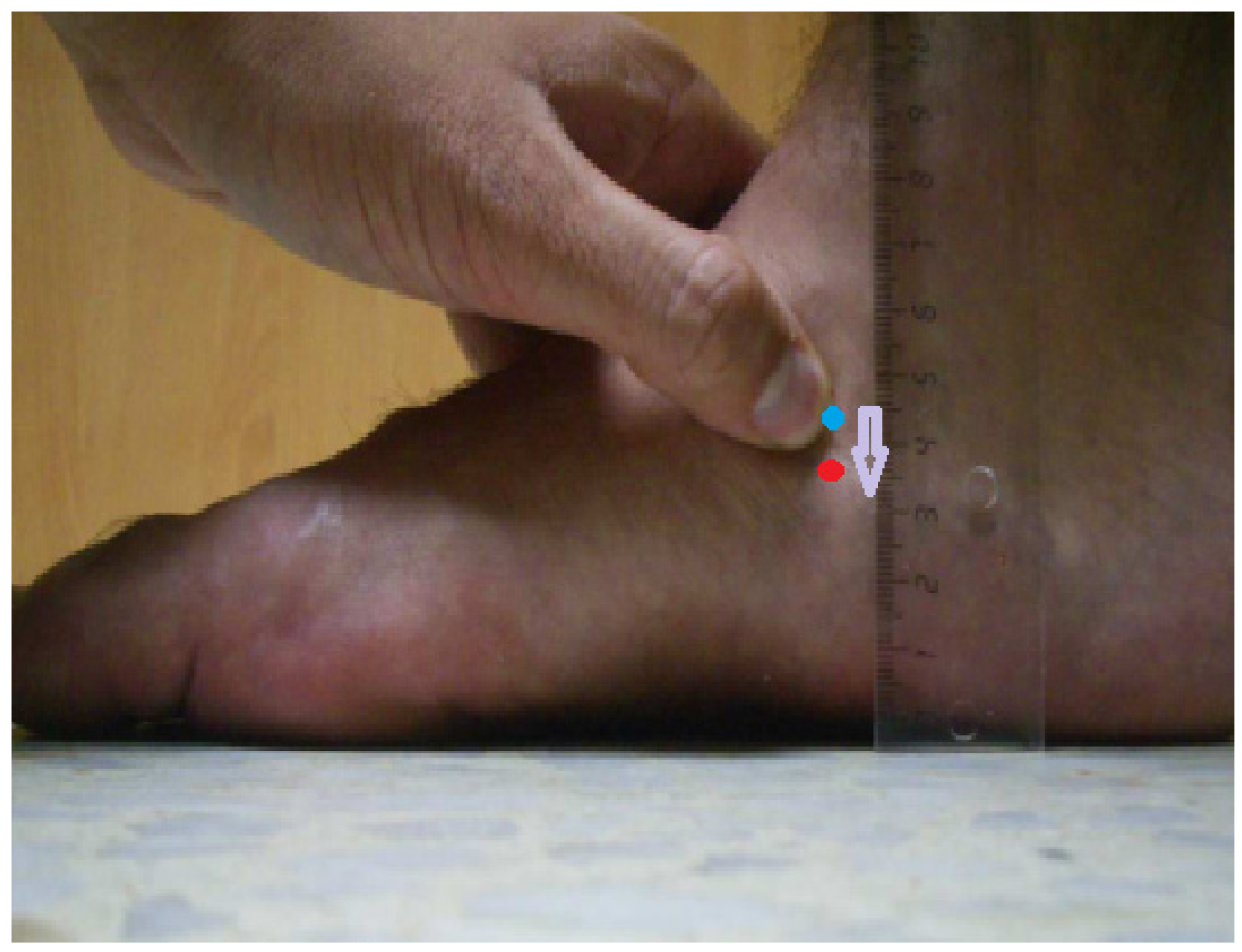
2.6. Measurement
- A palpable taut band in skeletal muscle.
- A hypersensitive tender spot.
- The reproduction of referred pain in response to compression.
- The jump sign.
- A local twitch response provoked by palpation of the taut band.
2.7. Statistical Methods
3. Results
3.1. Prevalence of LTrPs
3.1.1. Participants and Descriptive Data
3.1.2. Main Results
3.2. Intra-Rater Reliability
4. Discussion
4.1. Intra-Rater Reliability
4.2. Prevalence of LTrPs
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buldt, A.K.; Murley, G.S.; Butterworth, P.; Levinger, P.; Menz, H.B.; Landorf, K.B. The relationship between foot posture and lower limb kinematics during walking: A systematic review. Gait Posture 2013, 38, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, F.; Bdua, R.; Mosca, M.; Calabrò, A.; Giannini, S. Foot and lower limb diseases in runners: Assessment of risk factors. Sports Sci. Med. 2010, 9, 587–596. [Google Scholar]
- Subotnick, S.I. The biomechanics of running: Implications for the prevention of foot injuries. Sports Med. 1985, 2, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Davis, I.M.; Scholz, J.P.; Hamill, J.; Buchanan, T.S. High-arched runners exhibit increased leg stiffness compared to low-arched runners. Gait Posture 2004, 19, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S., 3rd; McClay, I.S.; Hamill, J. Arch structure and injury patterns in runners. Clin. Biomech. 2001, 16, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Crosbie, J.; Hunt, A.; Ouvrier, R. The effect of pes cavus on foot pain and plantar pressure. Clin. Biomech. 2005, 20, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.R.; Morag, E.; Boulton, A.J.; Young, M.J.; Deffner, K.T.; Pammer, S.E. The relationship of static foot structure to dynamic foot function. J. Biomech. 1997, 30, 243–250. [Google Scholar] [PubMed]
- Rosenbaum, D.; Hautmann, S.; Gold, M.; Claes, L. Effects of walking speed on plantar pressure patterns and hindfoot angular motion. Gait Posture 1994, 2, 191–197. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Stoltenberg, B.E.; Collinsworth, K.M.; Giesel, C.L.; Williams, D.G.; Kardouni, C.H.; Molloy, J.M.; Goffar, S.L.; Christie, D.S.; McPoil, T. Dynamic plantar pressure parameters associated with static arch height index during gait. Clin. Biomech. 2009, 29, 172–189. [Google Scholar] [CrossRef]
- Powell, D.W.; Long, B.; Milner, C.E.; Zhang, S. Frontal plane multi-segment foot kinematics in high-an low-arched females during dynamic loading tasks. Hum. Mov. Sci. 2011, 30, 105–114. [Google Scholar] [CrossRef]
- Hreljac, A.; Marshall, R.N.; Hume, P.A. Evaluation of lower extremity overuse injury potential in runners. Med. Sci. Sports Exerc. 2000, 32, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.R.; Brodine, S.K.; Shaffer, R.A.; Johnson, C.W.; Cullison, T.R. The effect of foot structure and range of motion on musculoskeletal overuse injuries. Am. J. Sports Med. 1999, 27, 585–593. [Google Scholar] [CrossRef]
- Cowan, D.N.; Jones, B.H.; Robinson, J.R. Foot morphologic characteristics and risk of exercise-related injury. Arch. Fam. Med. 1993, 2, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.; Trombini-Souza, F.; Tessutti, V.; Rodrigues Lima, F.; Sacco Ide, C.; João, S.M. Rearfoot alignment and medial longitudinal arch configurations of runners with symptoms and histories of plantar fasciitis. Clinics 2011, 66, 1027–1033. [Google Scholar] [CrossRef]
- de César, P.C.; Alves, J.A.; Gomes, J.L. Height of the foot longitudinal arch and anterior cruciate ligament injuries. Acta Ortop. Bras. 2014, 22, 312–314. [Google Scholar] [CrossRef]
- Morrison, K.E.; Kaminski, T.W. Foot characteristics in association with inversion ankle injury. J. Athl. Train. 2007, 42, 135–142. [Google Scholar]
- Simkin, A.; Leichter, I.; GIladi, M.; Stein, M.; Milgrom, C. Combined effect of foot arch structure and an orthotic device on stress fractures. Foot Ankle Int. 1989, 10, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.W.; Kong, P.W. Association between foot type and lower extremity injuries: Systematic literature review with meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 700–714. [Google Scholar] [CrossRef]
- Aydog, S.T.; Özçakar, L.; Tetik, O.; Demirel, H.A.; Hasçelik, Z.; Doral, M.N. Foot arch indices and ankle strength in gymnasts. Br. J. Sports Med. 2005, 39, e13. [Google Scholar] [CrossRef] [PubMed]
- Lizis, P.; Posadzki, P.; Smith, T. Relationship between explosive muscle strength and medial longitudinal arch of the foot. Foot Ankle Int. 2010, 31, 815–822. [Google Scholar] [CrossRef]
- Razeghi, M.; Batt, M.E. Foot type classification: A critical review of current methods. Gait Posture 2002, 15, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Brody, D. Techniques in the evaluation and treatment of the injured runner. Orthop. Clin. N. Am. 1982, 13, 542–558. [Google Scholar] [CrossRef]
- Barton, C.J.; Bonanno, D.; Levinger, P.; Menz, H.B. Foot and ankle characteristics in patellofemoral pain syndrome: A case control and reliability study. J. Orthop. Sports Phys. Ther. 2010, 40, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, M.D.; Carcia, C.R.; Gansneder, B.M.; Shultz, S.J. Subtalar pronation does not influence impact forces or rate of loading during a single-leg landing. J. Athl. Train. 2003, 38, 18–23. [Google Scholar] [PubMed]
- Shrader, J.A.; Popovich, J.M., Jr.; Gracey, G.C.; Danoff, J.V. Navicular drop measurement in people with rheumatoid arthritis: Interrater and intrarater reliability. Phys. Ther. 2005, 85, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Billis, E.; Katsakri, E.; Kapodistrias, C.; Kapreli, E. Assessment of foot posture: Correlation between different clinical techniques. Foot 2007, 17, 26–72. [Google Scholar] [CrossRef]
- Queen, R.B.; Mall, N.A.; Hardaker, W.M.; Nunley, J.A., 2nd. Describing the medial longitudinal arch using footprint indices and a clinical grading system. Foot Ankle Int. 2007, 28, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Shiang, T.Y.; Lee, S.H.; Lee, S.J.; Chu, W.C. Eavluating different footprint parameters as a predictor of arch height. IEEE Eng. Med. Biol. Mag. 1998, 17, 62–66. [Google Scholar] [CrossRef]
- Cote, K.P.; Brunett, M.E.; Gansneder, B.M.; Shultz, S.J. Effects of pronated and supinated foot postures on static and dynamic postural stability. J. Athl. Train. 2005, 40, 41–46. [Google Scholar]
- Shakibi, B.; Mimar, R.; Shakibi, V.; Mohammadi, H. The effects of foot type and heritability on balance and plantar pressure distribution of female twins. J. Sports Med. Phys. Fit. 2015, 55, 969–977. [Google Scholar]
- Simons, D.G.; Travell, J.; Simons, L. Myofascial Pain and Dysfuction: The Trigger Point Manual. Vol. 1, 2nd ed.; William & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Simons, D.G. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J. Electromyogr. Kinesiol. 2004, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Shah, J.P.; Gebreab, T.; Yen, R.H.; Gilliams, E.; Danoff, J.; Gerber, L.H. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch. Phys. Med. Rehabil. 2009, 90, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Phillips, T.M.; Danoff, J.V.; Gerber, L.H. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal. J. Appl. Physiol. 2005, 99, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Arendt-Nielsen, L. Latent myofascial trigger points. Curr. Pain. Headache Rep. 2011, 15, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.M.; Ge, H.Y.; Wang, C.; Martínez Vizcaíno, V.; Graven-Nielsen, T.; Arendt-Nielsen, L. Latent myofascial tigger points are associated with an increased antagonistic muscle activity during agonist muscle contraction. J. Pain. 2011, 12, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Zhang, Y.; Boudreau, S.; Yue, S.W.; Arendt-Nielsen, L. Induction of muscle cramps by nociceptive stimulation of latent myofascial trigger points. Exp. Brain Res. 2008, 187, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Celik, D.; Yedaln, P. The relationship between latent trigger point and muscle strength in healthy subjects: A double-blind study. J. Back. Musculoskelet. Rehabil. 2011, 24, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.R.; Polus, B.I.; Rich, P.A. Latent myofascial trigger points: Their effects on muscles activation and movement efficiency. J. Bodyw. Mov. Ther. 2004, 8, 160–166. [Google Scholar] [CrossRef]
- Bron, C.; Dommerholt, J.; Stegenga, B.; Wensing, M.; Oostendorp, R.A. High prevalence of shoulder girdle muscles with myofascial trigger points in patients with shoulder pain. BMC Musculoskel Disord. 2011, 12, 139. [Google Scholar] [CrossRef]
- Fernández-Carnero, J.; Fernández-de-las-Peñas, C.; de la Llave-Rincón, A.I.; Ge, H.Y.; Arendt-Nielsen, L. Prevalence of and referred pain from myofascial trigger points in the forearm muscles in patients with lateral epicondylalgia. Clin. J. Pain. 2007, 2, 353–360. [Google Scholar] [CrossRef]
- Sola, A.E.; Roderberger, M.I.; Gettys, B.B. Incidence of hypersensitive areas in posterior shoulder muscles. Am. J. Phys. Med. 1955, 34, 585–590. [Google Scholar] [PubMed]
- Lucas, K.R.; Rich, P.; Polus, B.I. How common are latent myofascial trigger points in the scapular positioning muscles? J. Musculoskelet. Pain. 2008, 16, 279–286. [Google Scholar] [CrossRef]
- Zuil-Escobar, J.C.; Martínez-Cepa, C.B.; Martín-Urrialde, J.A.; Gómez-Conesa, A. The Prevalence of latent trigger points in lower limb muscles in asymptomatic subjects. PM&R 2016, 8, 1055–1064. [Google Scholar]
- Grieve, R.; Barnett, S.; Coghill, N.; Cramp, F. The prevalence of latent myofascial trigger points and diagnostic criteria of the triceps surae and upper trapezius: A cross sectional study. Physiotherapy 2013, 99, 278–284. [Google Scholar] [CrossRef]
- Hoyle, J.A.; Marras, W.S.; Sheedy, J.E.; Hart, D.E. Effects of postural and visual stressors on myofascial trigger point development and motor unit rotation during computer work. J. Electromyogr. Kinesiol. 2011, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, G.N.; Chaves, T.C.; Dach, F.; Bevilaqua-Grossi, D.; Fernández-de-Las-Peñas, C.; Speciali, J.G. Relationship Between Active Trigger Points and Head/Neck Posture in Patients with Migraine. Am. J. Phys. Med. Rehabil. 2016, 95, 831–839. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia. 2006, 26, 1061–1070. [Google Scholar] [CrossRef]
- Zuil-Escobar, J.C.; Martínez-Cepa, C.B.; Martín-Urrialde, J.A.; Gómez-Conesa, A. Prevalence of myofascial trigger points and diagnostic criteria of different muscles in function of the medial longitudinal arch. Arch. Phys. Med. Rehabil. 2015, 96, 1123–1130. [Google Scholar] [CrossRef]
- Zuil-Escobar, J.C.; Martínez-Cepa, C.B.; Martín-Urrialde, J.A.; Gómez-Conesa, A. Correlation between navicular drop test and footprints parameters. J. Manip. Physiol. Ther. 2018, 41, 672–679. [Google Scholar] [CrossRef]
- Zuil-Escobar, J.C.; Martínez-Cepa, C.B.; Martín-Urrialde, J.A.; Gómez-Conesa, A. Evaluating the medial longitudinal arch of the foot: Correlations, reliability and accuracy in people with a low arch. Phys. Ther. 2019, 99, 364–372. [Google Scholar] [CrossRef]
- Andrade, C. The Inconvenient Truth About Convenience and Purposive Samples. Indian. J. Psychol. Med. 2021, 43, 86–88. [Google Scholar] [CrossRef]
- Hoffman, M.; Schrader, J.; Applegate, T.; Koceja, D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J. Athl. Train. 1998, 33, 319–322. [Google Scholar] [PubMed]
- Simons, D.G.; Travell, J.; Simons, L. Myofascial Pain and Dysfuction: The Trigger Point Manual. Vol. 2, 2nd ed.; William & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Kirmizi, M.; Cakiroglu, M.A.; Elvan, A.; Simsek, I.E.; Angin, S. Reliability of different clinical techniques for assessing foot posture. J. Manip. Physiol. Ther. 2020, 43, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J.; Nguyen, A.D.; Windley, T.C.; Kulas, A.S.; Botic, T.L.; Beynnond, B.C. Intratester and intertester reliability of clinical measures of lower extremity anatomic characteristics: Implications for multicenter studies. Clin. J. Sport. Med. 2006, 16, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.K.; Glasoe, W.M. Metrecom measurement of navicular drop in subjects with anterior cruciate ligament injury. J. Athl. Train. 2000, 35, 403–406. [Google Scholar] [PubMed]
- Rodríguez-Sanz, D.; Calvo-Lobo, C.; López-López, D.; Romero-Morales, C.; Sosa-Marín, C.; Sanz-Corbalán, I. Interrater reliability in the clinical evaluation of myofascial trigger points in three ankle muscles. J. Manip. Physiol. Ther. 2016, 39, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.; Kinsella, S.; McEvoy, J. The intra-rater reliability of locating and measuring the severity of latent trigger points in the quadriceps. J. Bodyw. Mov. Ther. 2017, 21, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Hong, C.Z.; Adams, A.H.; Platt, K.J.; Danielson, C.D.; Hoehler, F.K.; Tobis, J.S. Interexaminer reliability of the palpation of trigger points in the trunk and lower limb muscles. Arch. Phys. Med. Rehabil. 2000, 81, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, E.; Strinkovsky, A.; Finestone, A.S.; Kalichman, L. Reliability of Trigger Point Evaluation in the Lower Leg Muscles. Pain. Med. 2021, 22, 2283–2289. [Google Scholar] [CrossRef]
- Torres-Chica, B.; Núñez-Samper-Pizarroso, C.; Ortega-Santiago, R.; Cleland, J.A.; Salom-Moreno, J.; Laguarta-Val, S.; Fernández-de-las-Peñas, C. Trigger points and pressure pain hypersensitivity in people with post-meniscectomy pain. Clin. J. Pain. 2015, 31, 265–272. [Google Scholar] [CrossRef]
- Bajab, P.; Bajab, P.; Graven-Nielsen, T.; Arendt-Nielsen, T. Trigger points in patients with lower limb osteoarthritis. J. Musculoskelet. Pain. 2001, 9, 17–33. [Google Scholar]
- Sánchez Romero, E.A.; Fernández Carnero, J.; Villafañe, J.H.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Laguarta Val, S.; Pedersini, P.; Pecos Martín, D. Prevalence of Myofascial Trigger Points in Patients with Mild to Moderate Painful Knee Osteoarthritis: A Secondary Analysis. J. Clin. Med. 2020, 9, 2561. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, E.; Finestone, A.S.; Moran, U.; Damri, E.; Kalichman, L. The prevalence of myofascial trigger points in hip and thigh areas in anterior knee pain patients. J. Bodyw. Mov. Ther. 2020, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque-García, A.; Rodrigues-de-Souza, D.P.; Fernández-de-las-Peñas, C.; Alburquerque-Sendín, F. Association between muscle trigger points, ongoing pain, function, and sleep quality in elderly women with bilateral painful knee osteoarthritis. J. Manipulative Physiol. Ther. 2015, 38, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J. The importance of postural habits in perpetuating myofascial trigger point pain. Acupunct. Med. 2005, 23, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Justine, M.; Ruzali, D.; Hazidin, E.; Said, A.; Bukry, S.A.; Manaf, H. Range of motion, muscle length, and balance performance in older adults with normal, pronated, and supinated feet. J. Phys. Ther. Sci. 2016, 28, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Milner, C.E. Fore-and rearfoot kinematics in high-and low-arched individuals during running. Foot Ankle Int. 2011, 32, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.; Barker, K.; Shamley, D.; Newman, M.; Oskorchi, G.R.; Sandall, S. The role of foot and ankle assessment of patients with lower limb osteoarthritis. Physiotherapy 2009, 95, 164–169. [Google Scholar] [CrossRef]
- Burns, J.; Crosbie, J. Weight bearing ankle dorsiflexion range of motion in idiopathic pes cavus compared to normal and pes planus feet. Foot 2005, 15, 91–94. [Google Scholar] [CrossRef]
- Murley, G.S.; Tran, J.M.; Edwards, R.M.; De Luca, J.; Munteany, S.E.; Cook, J.L. Foot posture is associated with morphometry of the peroneus longus muscle, tibialis anterior tendon, and Achilles tendon. Scan J. Med. Sci. Sports 2014, 24, 535–541. [Google Scholar] [CrossRef]
- Yokozuka, M.; Okazaki, K.; Sakamoto, Y.; Takahashi, K. Relationship between foot morphology and toe muscle strength in female university students. J. Phys. Ther. Sci. 2019, 31, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Nagano, K.; Okuyama, R.; Taniguchi, N.; Yoshida, T. Gender difference in factors affecting the medial longitudinal arch height of the foot in healthy young adults. J. Phys. Ther. Sci. 2018, 6, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Buldt, A.K.; Forghany, S.; Landorf, K.B.; Murley, G.S.; Levinger, P.; Menz, H.B. Centre of pressure characteristics in normal, planus and cavus feet. J. Foot Ankle Res. 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Landorf, K.B.; Menz, H.B.; Bird, A.R. Effects of foot posture, foot orthoses and footwear on lower limb muscle activity during walking and running: A systematic review. Gait Posture 2009, 29, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Buldt, A.K.; Levinger, P.; Murley, G.S.; Menz, H.B.; Nester, C.H.; Landorf, K.B. Foot posture is associated with kinematics of the foot during gait: A comparion of normal, planus and cavus feet. Gait Posture 2015, 42, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. Foot posture influences the electromyographic activity of selected lower limb muscles during gait. J. Foot Ankle Res. 2009, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Findlow, A.H.; Nester, C.J.; Bowker, P. Foot kinematics in patients with two patterns of pathological plantar hyperkeratosis. J. Foot Ankle Res. 2011, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.G.; Rathleff, M.S.; Simonsen, O.H.; Langberg, H. Determination of normal values for navicular drop during walking: A new model correcting for foot length and gender. J. Foot Ankle Res. 2009, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Dicharry, J.M.; Franz, J.R.; Della Croce, U.; Wilder, R.P.; Riley, P.O.; Kerrigan, D.C. Differences in static and dynamic measures in evaluation of talonavicular mobility in gait. J. Orthop. Sports Phys. Ther. 2009, 39, 628–634. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Tillman, M.D.; Smith, A.N.; Borsa, P.A. A new force-plate technology measure ofdynamic postural stability: The dynamic posturalstability index. J. Athl. Train. 2005, 40, 305–309. [Google Scholar]
- Grieve, R.; Barnett, S.; Coghill, N.; Cramp, F. Myofascial trigger point therapy for triceps surae dysfunctions: A case series. Man. Ther. 2013, 19, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Roach, S.; Sorensen, E.; Headley, B.; San Juan, J.G. Prevalence of myofascial trigger points in the hip in patellofemoral pain. Arch. Phys. Med. Rehabil. 2013, 94, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Tough, E.A.; White, A.R.; Richards, S.; Campbell, J. Variability of criteria used to diagnose myofascial trigger point pain syndrome-evidence from a review of the literature. Clin. J. Pain. 2007, 23, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Monterde, S.; Graven-Nielsen, T.; Arendt-Nielsen, L. Latent myofascial trigger points are associated with an increased intramuscular elecromyographic activity during synergistic muscle activation. J. Pain. 2014, 15, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.Y.; Arendt-Nielsen, L.; Madeleine, P. Accelerated muscle fatigability of latent myofascial trigger points in humans. Pain. Med. 2013, 13, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Grieve, R.; Cranston, A.; Henderson, A.; John, R.; Malone, G.; Mayall, C. The immediate effect of tríceps surae myofascial trigger point therapy on restricted active ankle joint dorsiflexion in recreational runners: A crossover randomized controlled trial. J. Body Mov. Ther. 2013, 17, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mølgaard, C.; Lundbye-Christensen, S.; Simonsen, O. High prevalence of foot problems in the Danish population: A survey of causes and associations. Foot 2010, 20, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.I.I.I.; McClay, I.S.; Hamill, J.; Buchanan, T.S. Lower extremity kinematic and kinetic differences in runners with high and low arches. J. Appl. Biomech. 2001, 17, 153–163. [Google Scholar] [CrossRef]
- Buldt, A.K.; Forghany, S.; Landorf, K.B.; Levinger, P.; Murley, G.S.; Menz, H.B. Foot posture is associated with plantar pressure during gait: A comparison of normal, planus and cavus feet. Gait Posture 2018, 62, 235–240. [Google Scholar] [CrossRef]
- Hollander, K.; Zech, A.; Rahlf, A.L.; Orendurff, M.S.; Stebbins, J.; Heidt, C. The relationship between static and dynamic foot posture and running biomechanics: A systematic review and meta-analysis. Gait Posture 2019, 72, 109–122. [Google Scholar] [CrossRef]
- Franettovich, M.; Chapman, A.; Vicenzino, B. Tape that increases medial longitudinal arch height also reduces leg muscle activity: A preliminary study. Med. Sci. Sports Exerc. 2008, 40, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Franettovich, M.M.; Murley, G.S.; David, B.S.; Bird, A.R. A comparison of augmented low-Dye taping and ankle bracing on lower limb muscle activity during walking in adults with flat-arched foot posture. J. Sci. Med. Sport. 2012, 15, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Bird, A.R. The effect of three levels of foot orthotic wedging on the surface electromyographic activity of selected lower limb muscles during gait. Clin. Biomech. 2006, 21, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gomez, R.; Gomez-Carrion, A.; Martinez-Sebastian, C.; Alou, L.; Sevillano, D.; Nuñez-Fernandez, A.; Sanz-Wozniak, P.; de la Cruz-Torres, B. Innovative Medial Cushioning Orthoses Affect Peroneus Longus Electromyographic Activity during Running. J. Clin. Med. 2022, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sebastián, C.; Ramos-Petersen, L.; Gámez-Guijarro, M.; Alabau-Dasi, R.; Banwell, G.; Núñez-Fernández, A.; Sánchez-Gómez, R.; Gómez-Carrión, Á. Effects of Low-Dye Tape on Arch Height and Its Impact on the Medial Gastrocnemius Electromyographic Activity in Structurally Differentiable Foot Types: A Cross-Sectional Observational Study. Life 2023, 13, 2309. [Google Scholar] [CrossRef]
- Ortega-Santiago, R.; Ríos-León, M.; Martín-Casas, P.; Fernández-de-Las-Peñas, C.; Plaza-Manzano, A.G. Active Muscle Trigger Points Are Associated with Pain and Related Disability in Patients with Plantar Heel Pain: A Case-Control Study. Pain. Med. 2020, 21, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.I.I.D.S.B.; Tiernes, R.N.; Butler, R.J. Increased medial longitudinal arch mobility, lower extremity kinematics, and ground reaction forces in high-arched runners. J. Athl. Train. 2014, 49, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Vinicombre, A.; Raspovic, A.; Menz, H.B. Reliability of navicular displacement measurement as a clinical indicator of foot posture. J. Am. Podiatr. Med. Assoc. 2001, 91, 262–268. [Google Scholar] [CrossRef]
- Levinger, P.; Menz, H.B.; Fottohabadi, M.R.; Feller, J.A.; Bartlett, J.R.; Bergman, N.R. Foot posture in people with medial compartment knee osteoarthritis. J. Foot Ankle Res. 2010, 3, 29. [Google Scholar] [CrossRef]

| Control Group (n = 37) | High MLA Group (n = 37) | p Value | |
|---|---|---|---|
| Age | 22.84 ± 2.57 | 23.24 ± 2.63 | 0.411 |
| Height | 169.78 ± 10.70 | 168.99 ± 10.32 | 0.794 |
| Weight | 67.34 ± 12.88 | 64.99 ± 11.05 | 0.133 |
| BMI | 23.12 ± 1.97 | 22.85 ± 1.67 | 0.288 |
| Control Group (n = 37) | Higher MLA Group (n = 37) | |
|---|---|---|
| Gastrocnemius LTrP1 | 14 (37.8%) | 15 (40.5%) |
| Gastrocnemius LTrP2 | 10 (27%) | 13 (35.1%) |
| Soleus LTrP | 9 (24.3%) | 11 (29.7%) |
| Peroneus longus LTrP | 11 (29.7%) | 13 (35.1%) |
| Peroneus brevis LTrP | 8 (21.6%) | 8 (21.6%) |
| Tibialis anterior LTrP * | 8 (21.6%) | 18 (48.6%) |
| Extensor digitorum longus LTrP * | 8 (21.6%) | 18 (48.6%) |
| Flexorum digitorum longus LTrP | 7 (18.9%) | 9 (24.3%) |
| Rectus femoris LTrP | 8 (21.6%) | 8 (21.6%) |
| Vastus mediales LTrP1 | 12 (32.4%) | 12 (32.4%) |
| Vastus mediales LTrP2 | 10 (27%) | 10 (27%) |
| Vastus lateralis LTrP1 * | 8 (21.6%) | 16 (43.2%) |
| Vastus lateralis LTrP2 * | 7 (18.9%) | 15 (40.5%) |
| Control Group (n = 37) | Higher MLA Group (n = 37) | |
|---|---|---|
| Gastrocnemius LTrP1 | 1 | 0.894 |
| Gastrocnemius LTrP2 | 1 | 1 |
| Soleus LTrP | 0.875 | 0.886 |
| Peroneus longus LTrP | 1 | 1 |
| Peroneus brevis LTrP | 0.875 | 0.828 |
| Tibialis anterior LTrP | 0.828 | 1 |
| Extensor digitorum longus LTrP | 0.828 | 1 |
| Flexorum digitorum longus LTrP | 0.828 | 0.875 |
| Rectus femoris LTrP | 1 | 0.857 |
| Vastus mediales LTrP1 | 0.875 | 0.886 |
| Vastus mediales LTrP2 | 0.875 | 0.875 |
| Vastus lateralis LTrP1 | 0.828 | 0.898 |
| Vastus lateralis LTrP2 | 0.828 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuil-Escobar, J.C.; Martín-Urrialde, J.A.; Gómez-Conesa, A.; Martínez-Cepa, C.B. High Medial Longitudinal Arch of the Foot and Latent Trigger Points in Lower Limb Muscles. J. Clin. Med. 2024, 13, 4049. https://doi.org/10.3390/jcm13144049
Zuil-Escobar JC, Martín-Urrialde JA, Gómez-Conesa A, Martínez-Cepa CB. High Medial Longitudinal Arch of the Foot and Latent Trigger Points in Lower Limb Muscles. Journal of Clinical Medicine. 2024; 13(14):4049. https://doi.org/10.3390/jcm13144049
Chicago/Turabian StyleZuil-Escobar, Juan Carlos, José Antonio Martín-Urrialde, Antonia Gómez-Conesa, and Carmen Belén Martínez-Cepa. 2024. "High Medial Longitudinal Arch of the Foot and Latent Trigger Points in Lower Limb Muscles" Journal of Clinical Medicine 13, no. 14: 4049. https://doi.org/10.3390/jcm13144049





