Development of a Risk Prediction Model for Adverse Skin Events Associated with TNF-α Inhibitors in Rheumatoid Arthritis Patients
Abstract
:1. Introduction
2. Methods
2.1. Genotyping Methods
2.2. Statistical Analysis and Machine Learning Methods
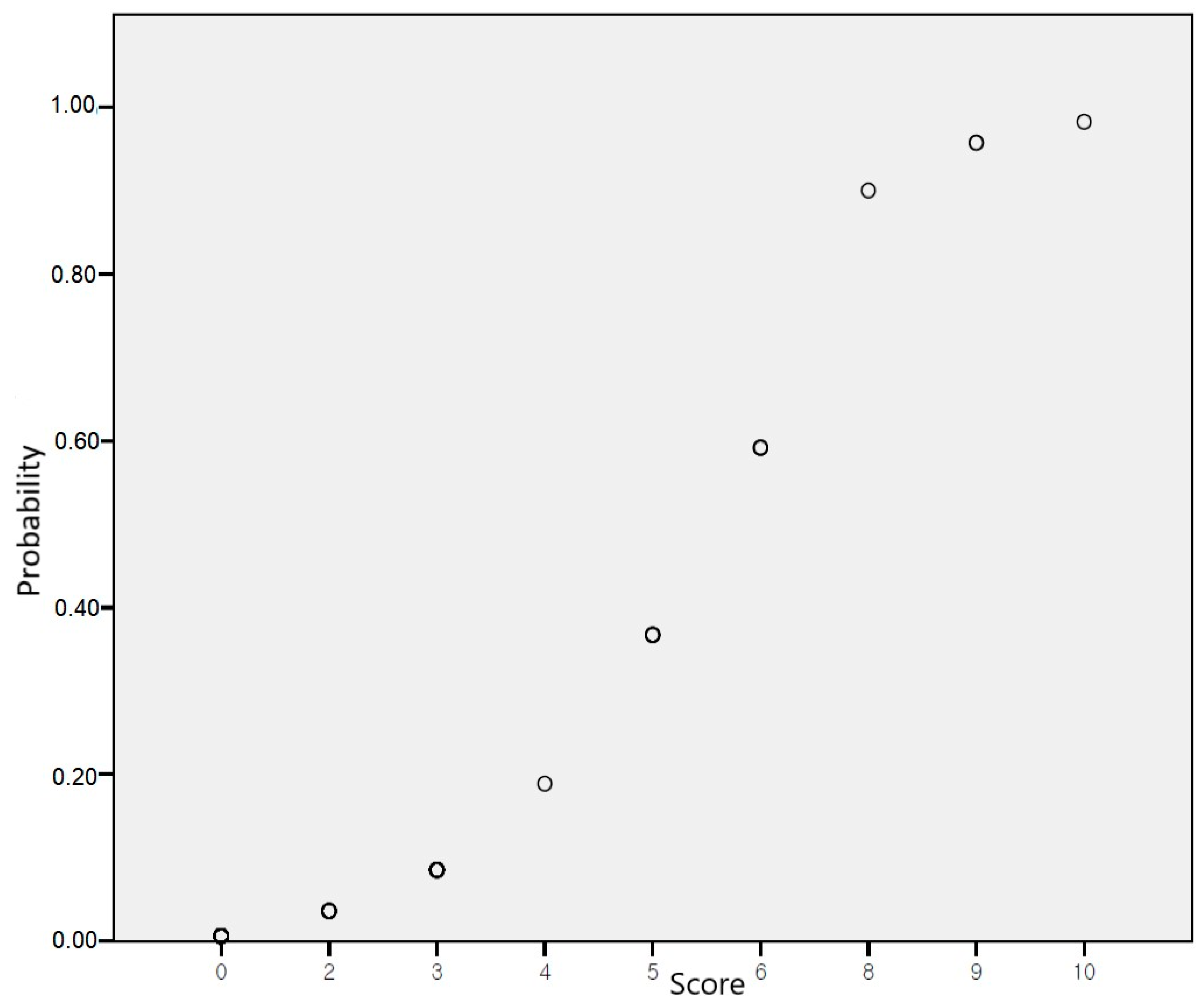
2.3. Development of a Risk-Scoring System
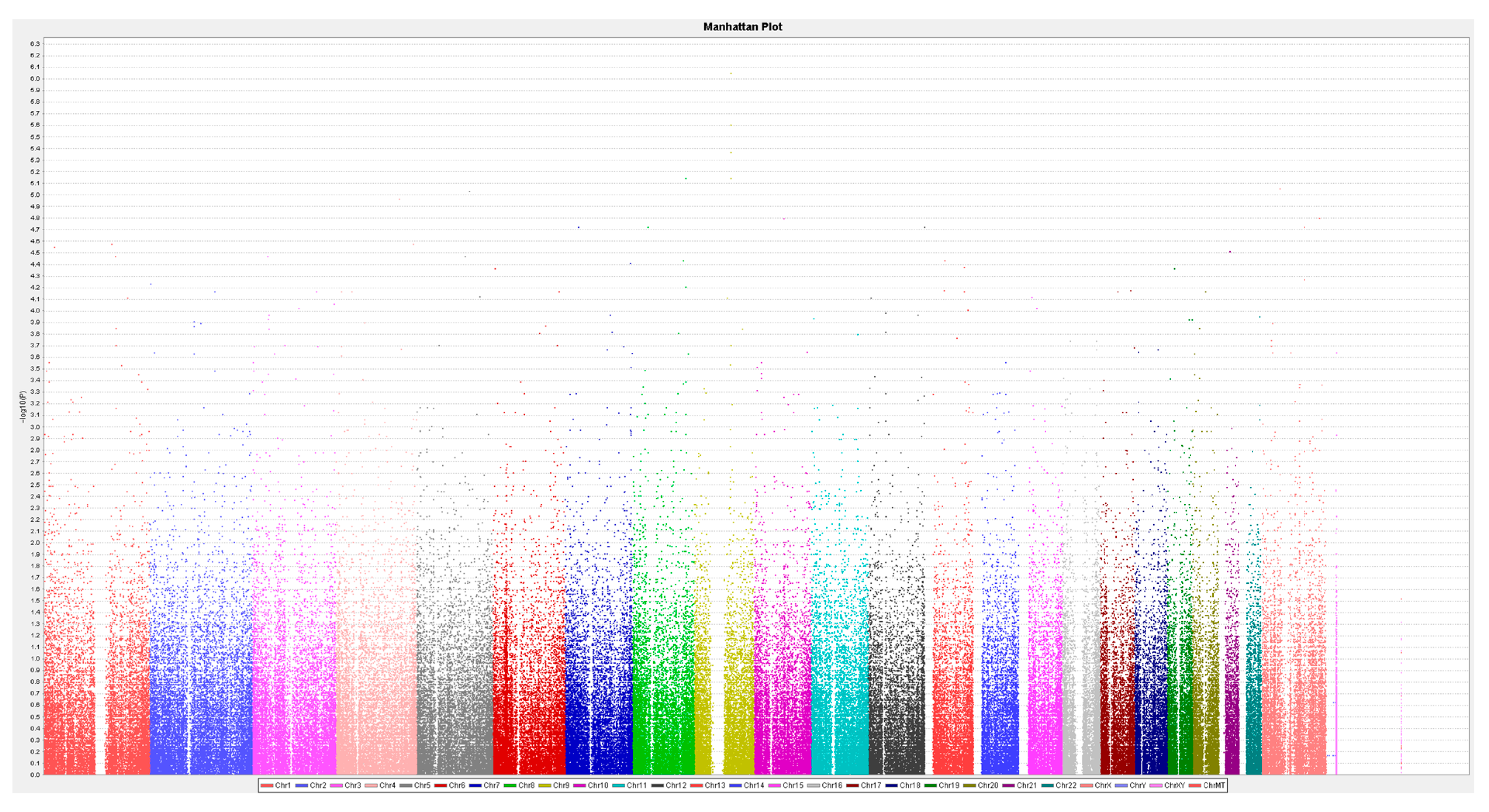
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malm, K.; Bergman, S.; Andersson, M.L.; Bremander, A.; Larsson, I. Quality of life in patients with established rheumatoid arthritis: A phenomenographic study. SAGE Open Med. 2017, 5, 2050312117713647. [Google Scholar] [CrossRef] [PubMed]
- Symmons, D.; Mathers, C.; Pfleger, B. The Global Burden of Rheumatoid Arthritis in the Year 2000 Global Burden of Disease; World Health Organization: Geneva, Switzerland, 2015; Volume 18, pp. 1–30. [Google Scholar]
- Kleinert, S.; Tony, H.P.; Krause, A.; Feuchtenberger, M.; Wassenberg, S.; Richter, C.; Röther, E.; Spieler, W.; Gnann, H.; Wittig, B.M. Impact of patient and disease characteristics on therapeutic success during adalimumab treatment of patients with rheumatoid arthritis: Data from a German noninterventional observational study. Rheumatol. Int. 2012, 32, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.; Brennan, F.M.; Chantry, D.; Turner, M.; Maini, R.N.; Feldmann, M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: Regulation by tumor necrosis factor-α. Eur. J. Immunol. 1991, 21, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. APLAR J. Rheumatol. 2007, 10, 270–274. [Google Scholar] [CrossRef]
- Kerbleski, J.F.; Gottlieb, A.B. Dermatological complications and safety of anti-TNF treatments. Gut 2009, 58, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Flendrie, M.; Vissers, W.H.; Creemers, M.C.; de Jong, E.M.; van de Kerkhof, P.C.; van Riel, P.L. Dermatological conditions during TNF-α-blocking therapy in patients with rheumatoid arthritis: A prospective study. Arthritis Res. Ther. 2005, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Song, I.H.; Friedrich, M.; Gauliard, A.; Detert, J.; Röwert, J.; Audring, H.; Kary, S.; Burmester, G.R.; Sterry, W. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-α antagonists. Br. J. Dermatol. 2007, 156, 486–491. [Google Scholar] [CrossRef]
- Nigam, G.B.; Bhandare, A.P.; Antoniou, G.A.; Limdi, J.K. Systematic review and meta-analysis of dermatological reactions in patients with inflammatory bowel disease treated with anti-tumour necrosis factor therapy. Eur. J. Gastroenterol. Hepatol. 2021, 33, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Pasadyn, S.R.; Knabel, D.; Fernandez, A.P.; Warren, C.B. Cutaneous adverse effects of biologic medications. Cleve Clin. J. Med. 2020, 87, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Fréling, E.; Baumann, C.; Cuny, J.F.; Bigard, M.A.; Schmutz, J.L.; Barbaud, A.; Peyrin-Biroulet, L. Cumulative incidence of, risk factors for, and outcome of dermatological complications of anti-TNF therapy in inflammatory bowel disease: A 14-year experience. Am. J. Gastroenterol. 2015, 110, 1186–1196. [Google Scholar] [CrossRef]
- Tillack, C.; Ehmann, L.M.; Friedrich, M.; Laubender, R.P.; Papay, P.; Vogelsang, H.; Stallhofer, J.; Beigel, F.; Bedynek, A.; Wetzke, M.; et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut 2014, 63, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Buche, S.; Peyrin-Biroulet, L.; Bouhnik, Y.; Duclos, B.; Louis, E.; Papay, P.; Allez, M.; Cosnes, J.; Cortot, A.; et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin. Gastroenterol. Hepatol. 2010, 8, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Broge, T.; Nguyen, N.; Sacks, A.; Davis, M. Infliximab-associated psoriasis in children with Crohn’s disease may require withdrawal of anti-tumor necrosis factor therapy. Inflamm. Bowel Dis. 2013, 19, E75–E77. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0; National Cancer Institute: Rockville, MD, USA, 2017. [Google Scholar]
- Moustou, A.E.; Matekovits, A.; Dessinioti, C.; Antoniou, C.; Sfikakis, P.P.; Stratigos, A.J. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: A clinical review. J. Am. Acad. Dermatol. 2009, 61, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Lopes, S.; Gaspar, R.; Nunes, A.; Magina, S.; Macedo, G. Anti-Tumor Necrosis Factor-α-Induced Dermatological Complications in a Large Cohort of Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2018, 63, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Seneschal, J.; Milpied, B.; Vergier, B.; Lepreux, S.; Schaeverbeke, T.; Taïeb, A. Cytokine imbalance with increased production of interferon-alpha in psoriasiform eruptions associated with antitumour necrosis factor-alpha treatments. Br. J. Dermatol. 2009, 161, 1081–1088. [Google Scholar] [CrossRef]
- Collamer, A.N.; Battafarano, D.F. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: Clinical features and possible immunopathogenesis. Semin. Arthritis Rheum. 2010, 40, 233–240. [Google Scholar] [CrossRef] [PubMed]
- de Gannes, G.C.; Ghoreishi, M.; Pope, J.; Russell, A.; Bell, D.; Adams, S.; Shojania, K.; Martinka, M.; Dutz, J.P. Psoriasis and pustular dermatitis triggered by TNF-{alpha} inhibitors in patients with rheumatologic conditions. Arch. Dermatol. 2007, 143, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Hansel, G.; Koch, A.; Schönlebe, J.; Köstler, E.; Haroske, G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: First 120 cases from the literature including a series of six new patients. Am. J. Clin. Dermatol. 2008, 9, 1–14. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: A single-centre cohort study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef]
- Askling, J.; Fahrbach, K.; Nordstrom, B.; Ross, S.; Schmid, C.H.; Symmons, D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: Meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol. Drug Saf. 2011, 20, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Pezzolesi, M.G.; Poznik, G.D.; Mychaleckyj, J.C.; Paterson, A.D.; Barati, M.T.; Klein, J.B.; Ng, D.P.; Placha, G.; Canani, L.H.; Bochenski, J.; et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 2009, 58, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.; Malley, C.; Taylor, P.; Preethi Boorgula, M.; Chavan, S.; Daya, M.; Mathias, M.; Shankar, G.; Rafaels, N.; Vergara, C.; et al. Whole genome sequencing identifies novel genetic mutations in patients with eczema herpeticum. Allergy 2021, 76, 2510–2523. [Google Scholar] [CrossRef] [PubMed]
- Hellwege, J.N.; Russell, S.B.; Williams, S.M.; Edwards, T.L.; Velez Edwards, D.R. Gene-based evaluation of low-frequency variation and genetically-predicted gene expression impacting risk of keloid formation. Ann. Hum. Genet. 2018, 82, 206–215. [Google Scholar] [CrossRef] [PubMed]
| Outcome (n = 11) | No Outcome (n = 102) | p-Value | ||
|---|---|---|---|---|
| Sex | 0.69 | |||
| Male | 1 (9.1) | 21 (20.6) | ||
| Female | 10 (90.9) | 81 (79.4) | ||
| Age, years | 1.00 | |||
| <65 | 9 (81.8) | 84 (82.4) | ||
| ≥65 | 2 (18.2) | 18 (17.6) | ||
| Body Mass Index, m2/kg | 1.00 | |||
| <23 | 6 (54.5) | 58 (56.9) | ||
| ≥23 | 5 (45.5) | 44 (43.1) | ||
| Duration of rheumatoid arthritis, months | 117.36 ± 67.686 | 103.02 ± 78.518 | 0.81 | |
| Rheumatoid factor | 0.12 | |||
| Positive | 11 (100.0) | 77 (75.5) | ||
| Negative | 0 (0.0) | 25 (24.5) | ||
| ACPA | 0.73 | |||
| Positive | 7 (63.6) | 73 (71.6) | ||
| Negative | 4 (36.4) | 29 (28.4) | ||
| Medications | ||||
| Hydroxychloroquine | 0.042 | |||
| No | 2 (18.2) | 51 (50.5) | ||
| Yes | 9 (81.8) | 50 (49.5) | ||
| Leflunomide | 0.20 | |||
| No | 4 (36.4) | 57 (56.4) | ||
| Yes | 7 (63.6) | 44 (43.6) | ||
| Methotrexate | 0.12 | |||
| No | 3 (27.3) | 10 (9.9) | ||
| Yes | 8 (72.7) | 91 (90.1) | ||
| Sulfasalazine | 0.08 | |||
| No | 11 (100) | 78 (77.2) | ||
| Yes | 0 (0) | 23 (22.8) | ||
| Tacrolimus | 0.39 | |||
| No | 8 (72.7) | 85 (84.2) | ||
| Yes | 3 (27.3) | 16 (15.8) | ||
| Comorbidities | ||||
| Gastroduodenitis | 0.27 | |||
| No | 10 (90.9) | 99 (98) | ||
| Yes | 1 (9.1) | 2 (2) | ||
| Gastroesophageal reflux disease | 0.59 | |||
| No | 11 (100) | 91 (90.1) | ||
| Yes | 0 (0) | 10 (9.9) | ||
| Headache | 1.00 | |||
| No | 11 (100) | 95 (94.1) | ||
| Yes | 0 (0) | 6 (5.9) | ||
| Hyperlipidemia | 0.63 | |||
| No | 9 (81.8) | 90 (88.2) | ||
| Yes | 2 (18.2) | 12 (11.8) | ||
| Hypertension | 0.69 | |||
| No | 9 (81.8) | 86 (84.3) | ||
| Yes | 2 (18.2) | 16 (15.7) | ||
| Insomnia | 0.47 | |||
| No | 10 (90.9) | 96 (95) | ||
| Yes | 1 (9.1) | 5 (5) | ||
| Osteoporosis | 0.35 | |||
| No | 11 (100) | 88 (86.3) | ||
| Yes | 0 (0) | 14 (13.7) | ||
| Rheumatoid lung disease | 0.27 | |||
| No | 10 (90.9) | 99 (98) | ||
| Yes | 1 (9.1) | 2 (2) | ||
| Tuberculosis | 1.00 | |||
| No | 11 (100) | 99 (98) | ||
| Yes | 0 (0) | 2 (2) | ||
| Type 2 diabetes mellitus | 1.00 | |||
| No | 11 (100) | 96 (94.1) | ||
| Yes | 0 (0) | 6 (5.9) | ||
| Vitamin D deficiency | 0.26 | |||
| No | 9 (81.8) | 93 (92.1) | ||
| Yes | 2 (18.2) | 8 (7.9) | ||
| Genotype | Outcome (n = 11) | No Outcome (n = 102) | p-Value | |
|---|---|---|---|---|
| FRMD3 | ||||
| rs12551103 | 0.001 | |||
| CC, CT | 7 (63.6) | 100 (98) | ||
| TT | 4 (36.4) | 2 (2) | ||
| RP11-1082L8.3 | ||||
| rs13265933 | 0.000166 | |||
| GG | 2 (18.2) | 79 (77.5) | ||
| GA, AA | 9 (81.8) | 23 (22.5) | ||
| RP11-245M24.1 | ||||
| rs73210737 | 0.000419 | |||
| TT | 4 (36.4) | 89 (87.3) | ||
| CT, CC | 7 (63.6) | 13 (12.7) | ||
| 33kb 3′ of RP11-425E13.1 | ||||
| rs920388 | 0.003 | |||
| GG, GT | 8 (72.7) | 101 (99) | ||
| TT | 3 (27.3) | 1 (1) |
| Crude OR (95% CI) | Adjusted OR (95% CI) | Score | |
|---|---|---|---|
| Sex (female) | 2.593 (0.314–21.404) | - | - |
| Age ≥ 65 | 1.037 (0.206–5.212) | - | - |
| Hydroxychloroquine | 4.590 (0.944–22.308) | - | - |
| rs12551103 TT | 28.571 (4.437–183.964) | 20.137 (1.129–359.238) | 4 |
| rs13265933 A | 15.457 (3.117–76.633) | 14.336 (1.468–140.013) | 3 |
| rs73210737 C | 11.981 (3.077–46.649) | 10.140 (1.502–68.435) | 2 |
| rs920388 TT | 37.875 (3.523–407.155) | 15.916 (0.874–289.792) | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.; Oh, S.-J.; Kim, H.-J.; Kim, J.-H.; Gil, J.-Y.; Ku, Y.-S.; Kim, J.-H.; Kim, H.-A.; Jung, J.-Y.; Choi, I.-A.; et al. Development of a Risk Prediction Model for Adverse Skin Events Associated with TNF-α Inhibitors in Rheumatoid Arthritis Patients. J. Clin. Med. 2024, 13, 4050. https://doi.org/10.3390/jcm13144050
Kim W, Oh S-J, Kim H-J, Kim J-H, Gil J-Y, Ku Y-S, Kim J-H, Kim H-A, Jung J-Y, Choi I-A, et al. Development of a Risk Prediction Model for Adverse Skin Events Associated with TNF-α Inhibitors in Rheumatoid Arthritis Patients. Journal of Clinical Medicine. 2024; 13(14):4050. https://doi.org/10.3390/jcm13144050
Chicago/Turabian StyleKim, Woorim, Soo-Jin Oh, Hyun-Jeong Kim, Jun-Hyeob Kim, Jin-Yeon Gil, Young-Sook Ku, Joo-Hee Kim, Hyoun-Ah Kim, Ju-Yang Jung, In-Ah Choi, and et al. 2024. "Development of a Risk Prediction Model for Adverse Skin Events Associated with TNF-α Inhibitors in Rheumatoid Arthritis Patients" Journal of Clinical Medicine 13, no. 14: 4050. https://doi.org/10.3390/jcm13144050





