Comparative Analysis of Serum Amino Acid Profiles in Patients with Myasthenia Gravis and Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Serum Samples
2.2. Chemicals and Reagents
2.3. Instrumentation and Conditions
2.4. Statistical Analysis
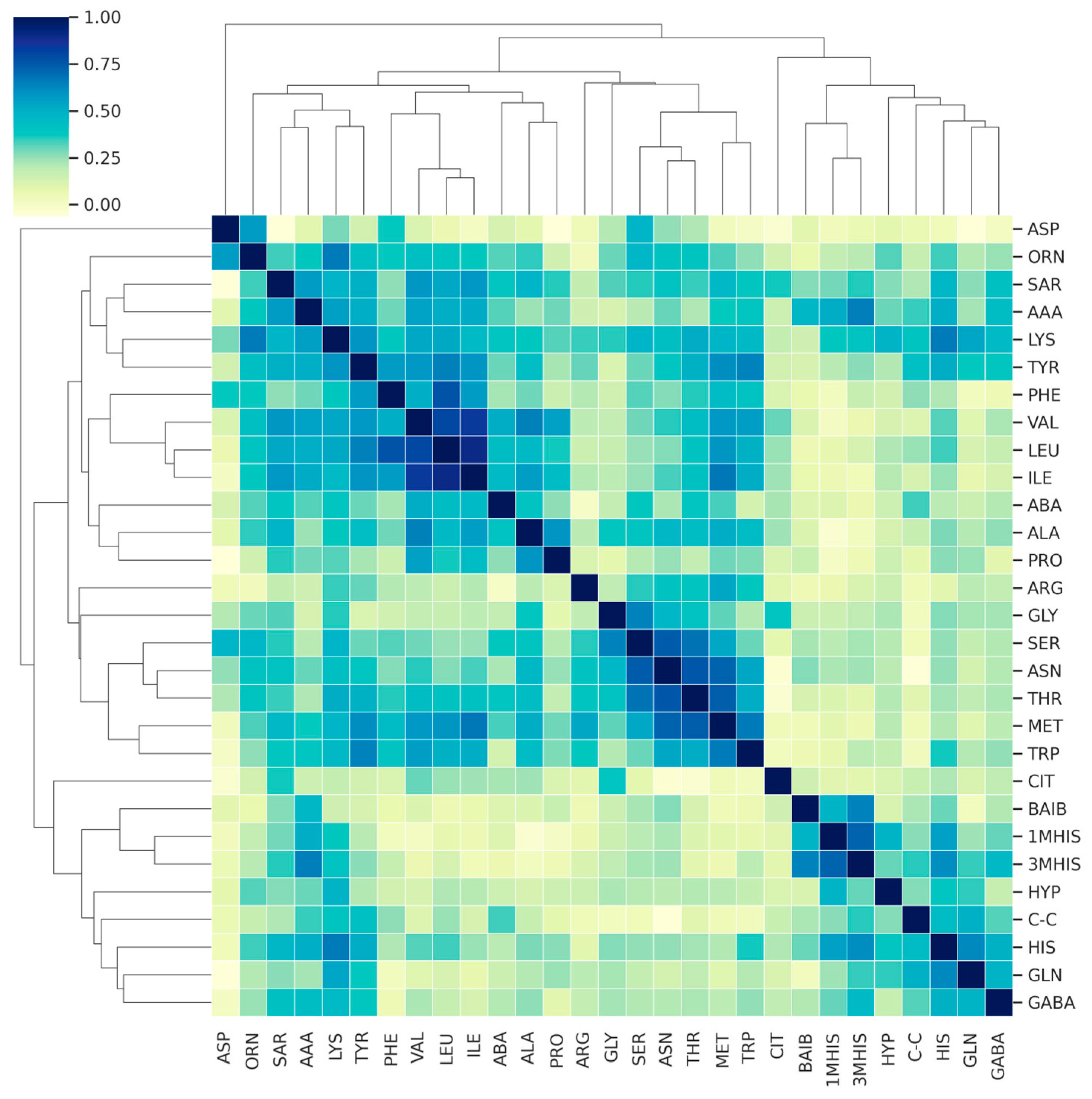
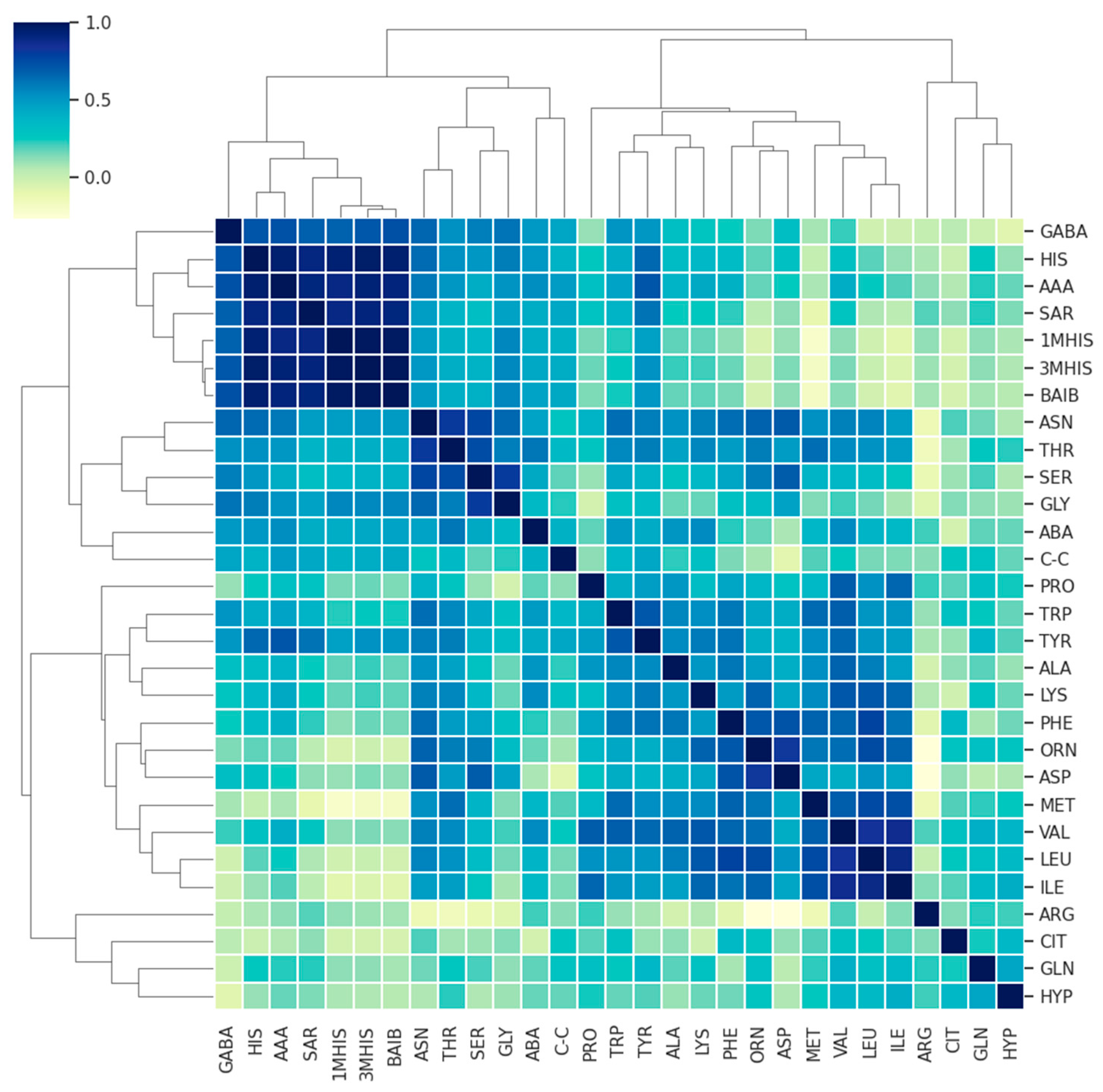
3. Results
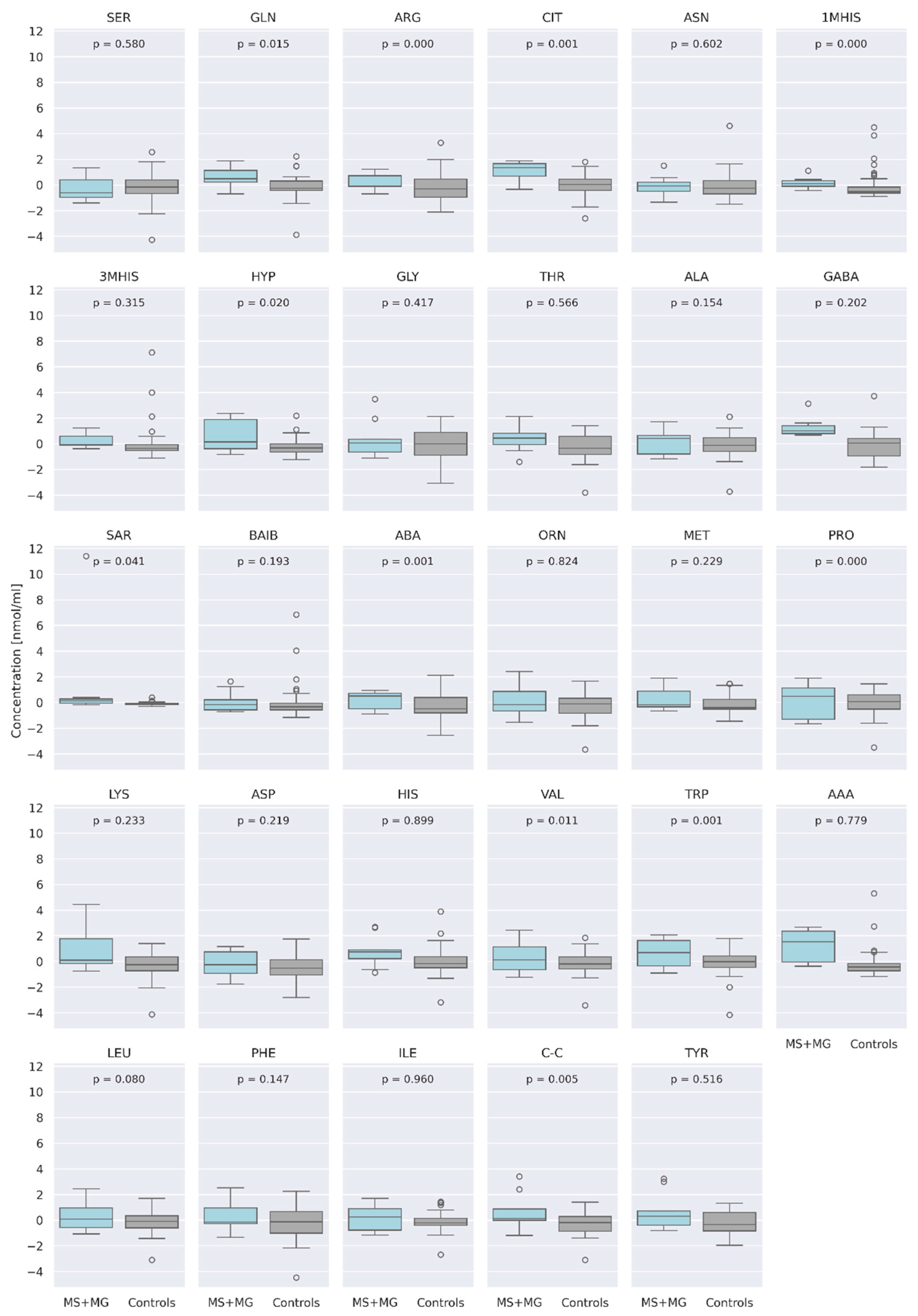
3.1. Comparison of AA Concentration between a Group of Patients (MS + MG Patients) and a Control Group of Healthy People
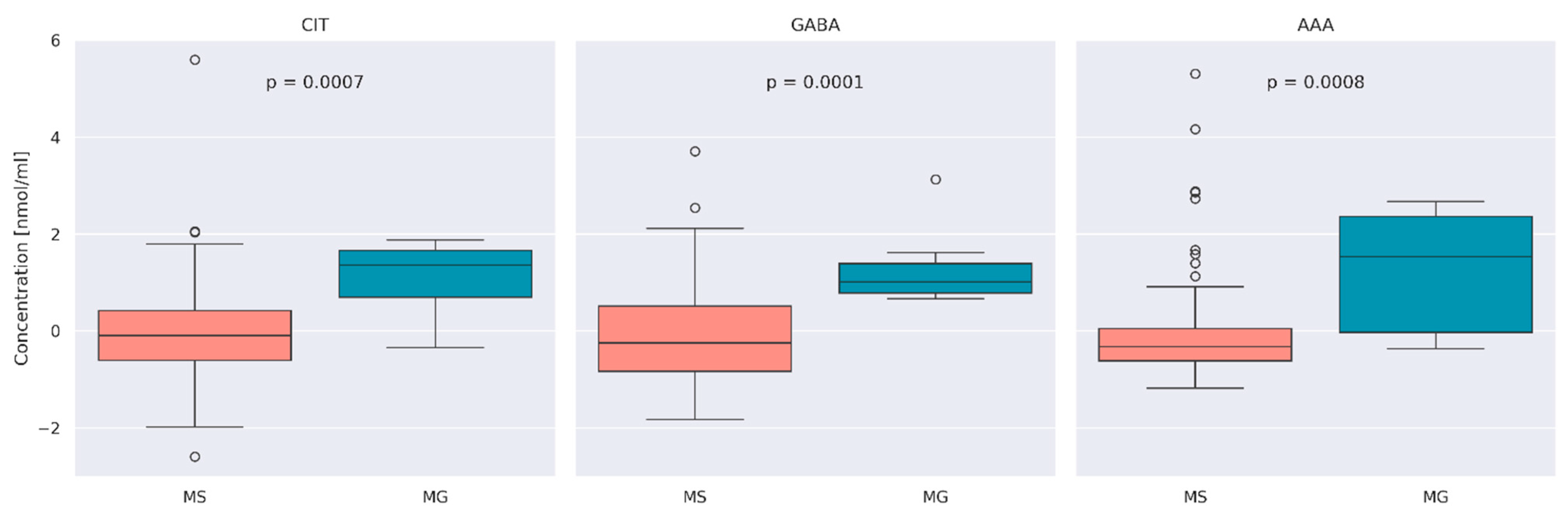
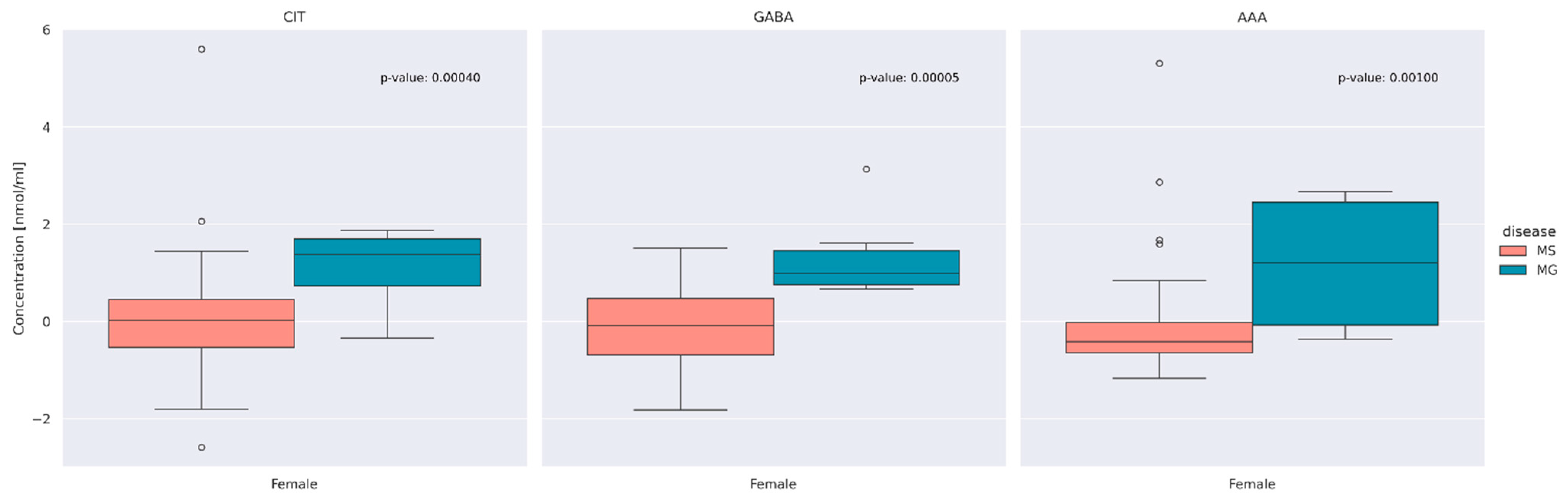
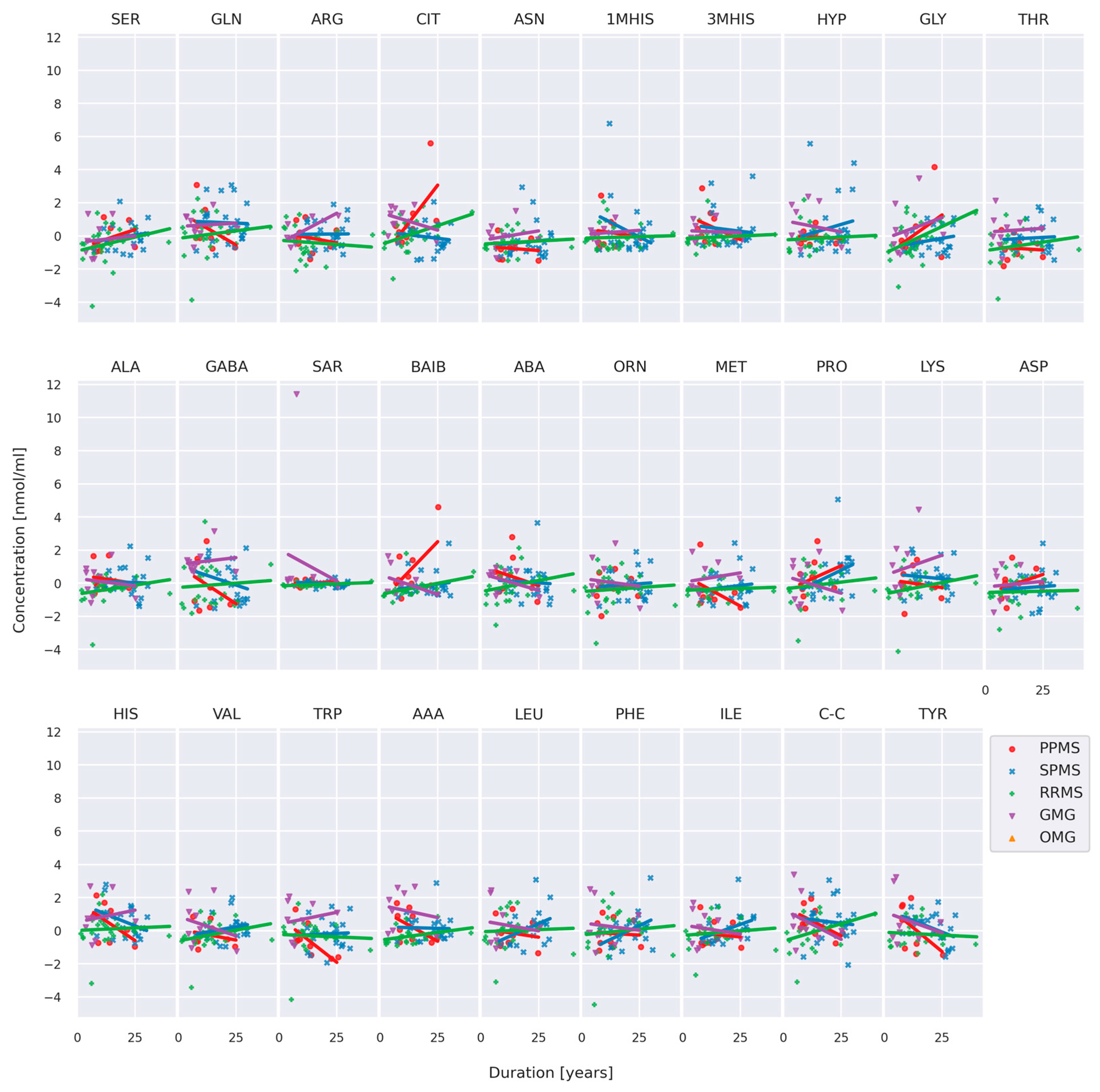
3.2. Comparison of AA Concentration between MS and MG Patients
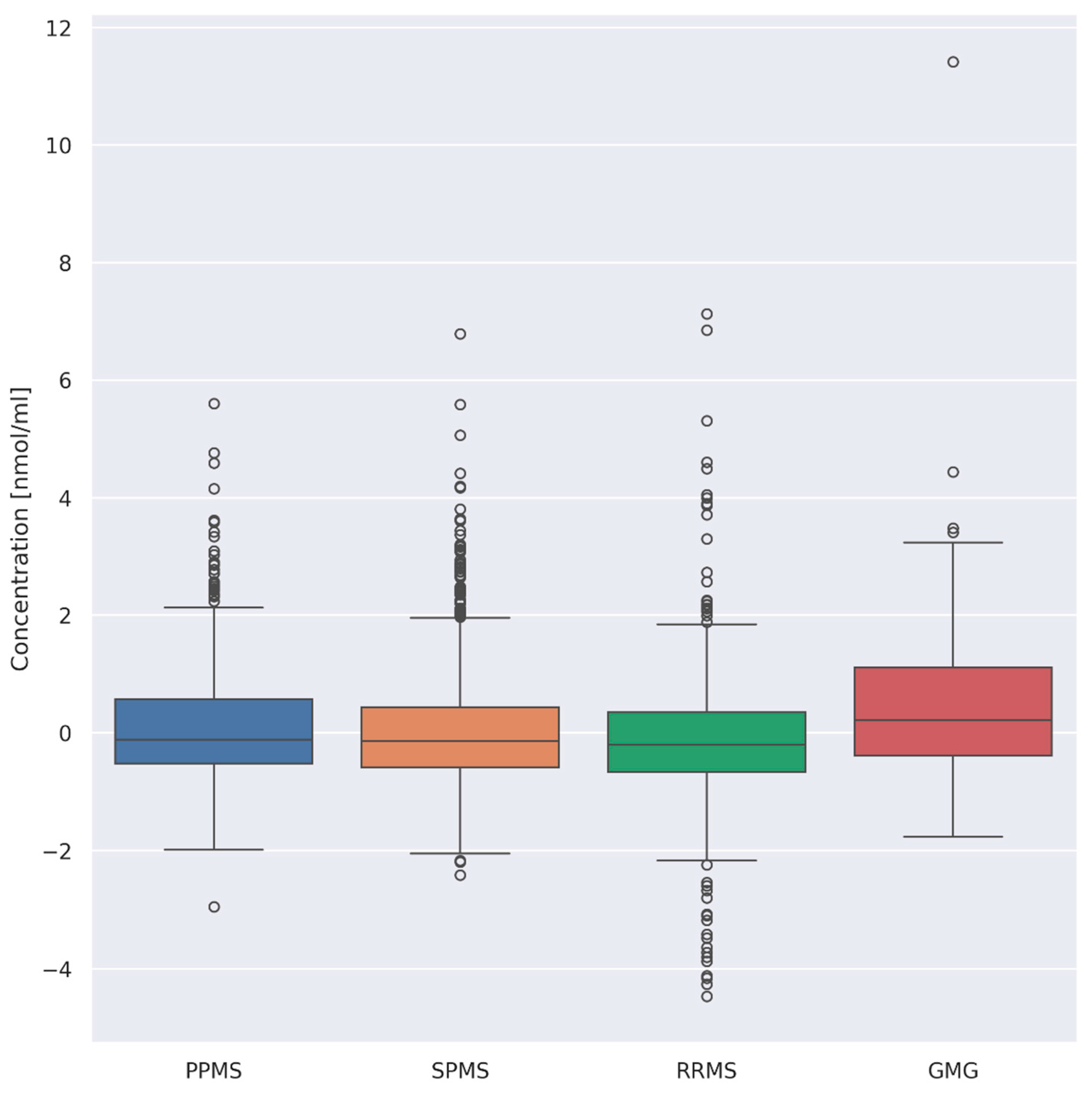
3.3. Evaluation of Differences between Overall AA Concentrations according to the Type of Disease
- (i)
- PPMS (n = 26) and RRMS (n = 41) (p = 0.008)
- (ii)
- PPMS (n = 26) and GMG (n = 25) (p = 0.03)
- (iii)
- RRMS (n = 41) and GMG (n = 25) (p = 0.00001)
- (iv)
- SPMS (n = 55) and GMG (n = 25) (p = 0.0008)
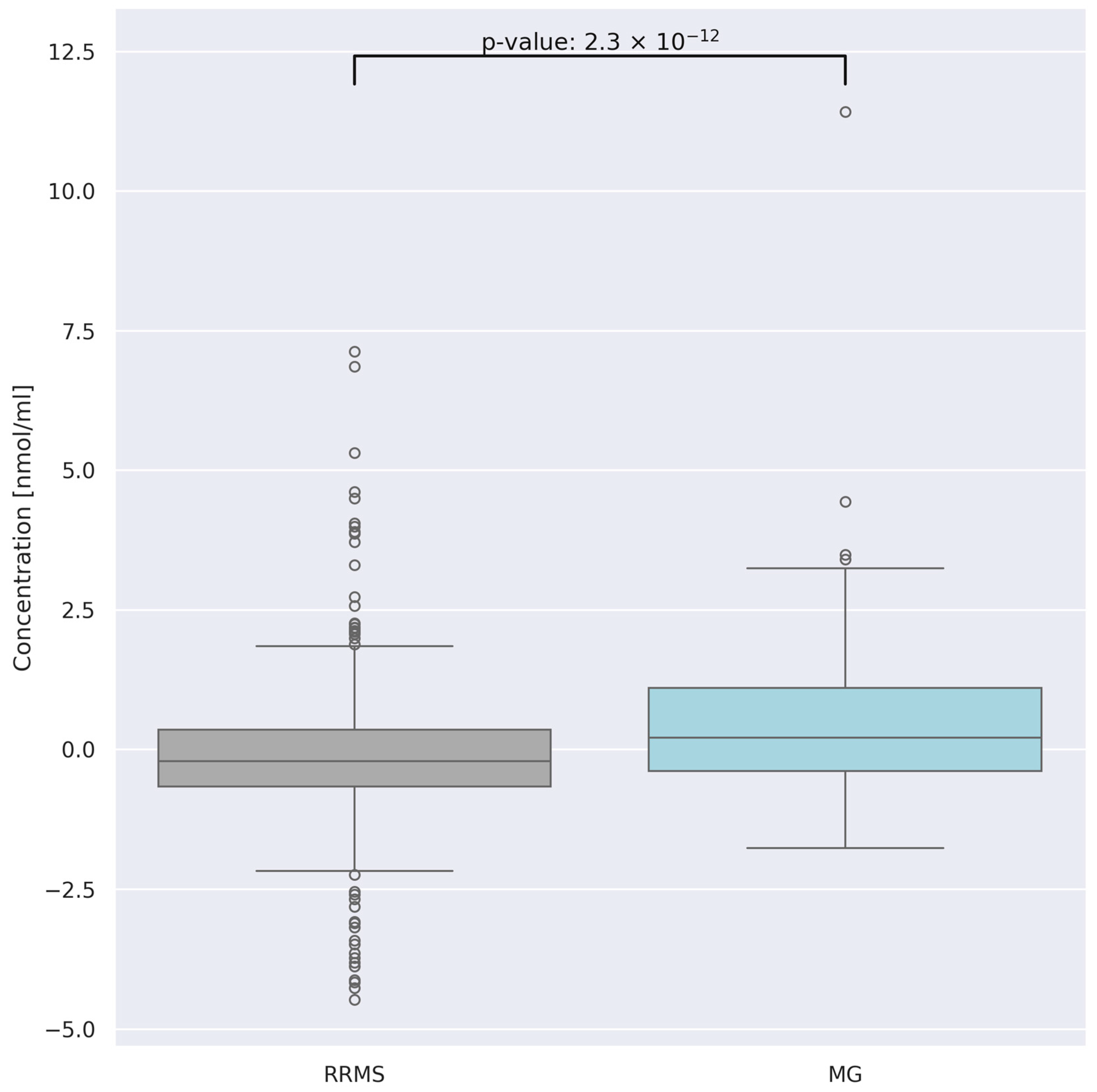
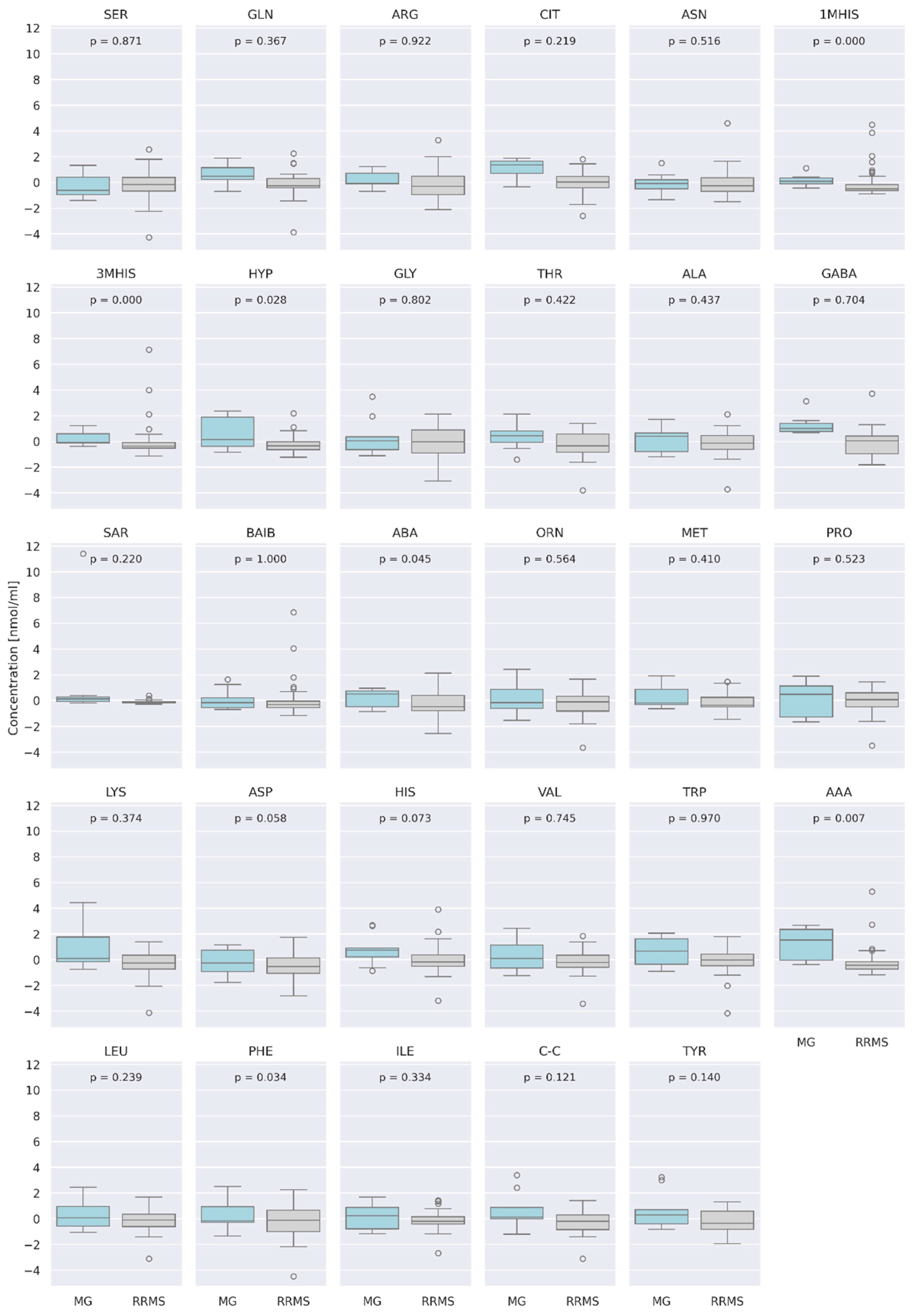
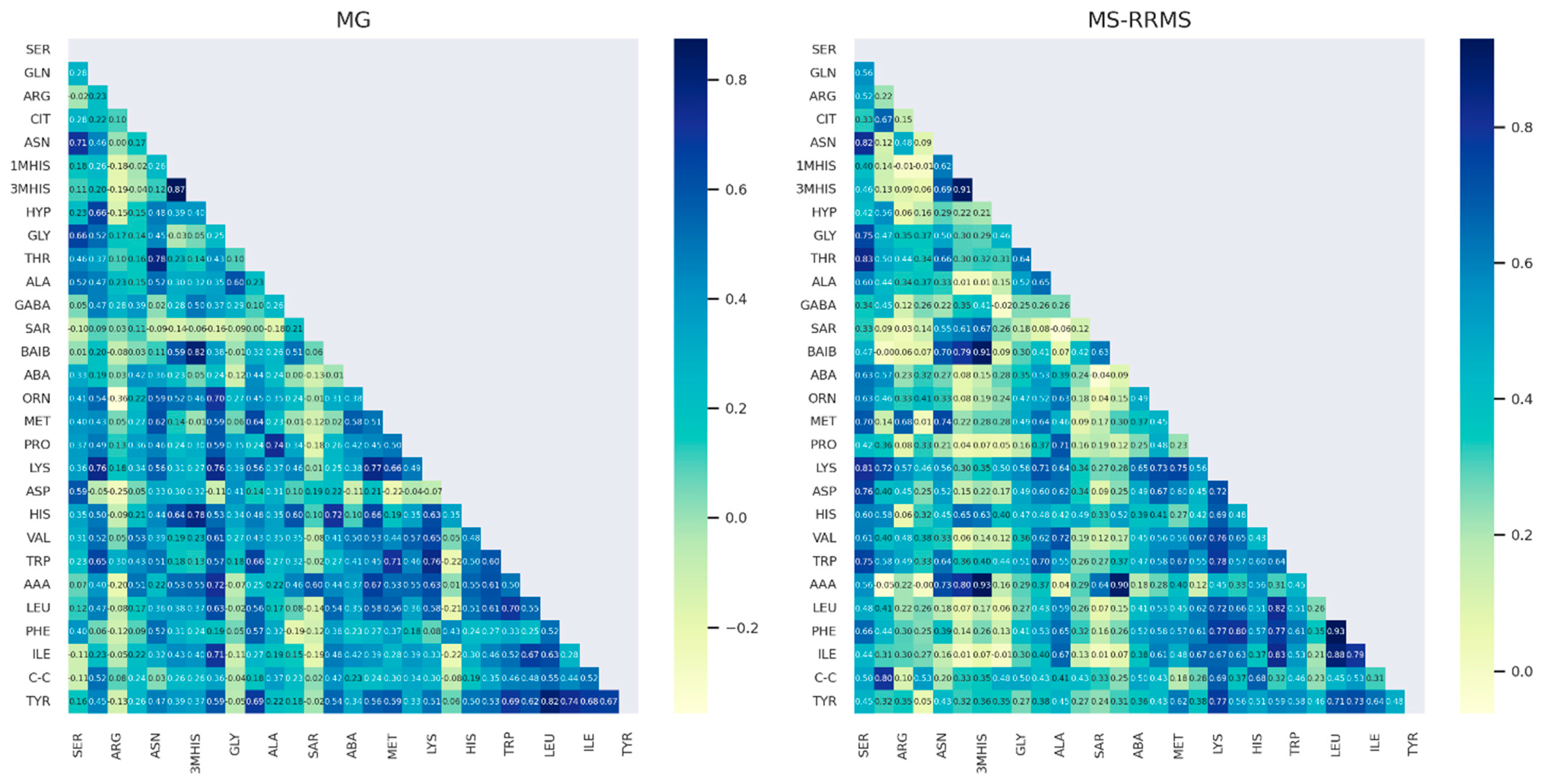
3.4. Comparison of AA Concentration between Two Groups of Patients: RRMS (n = 41) and MG (n = 28)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeMaio, A.; Mehrotra, S.; Sambamurti, K.; Husain, S. The Role of the Adaptive Immune System and T Cell Dysfunction in Neurodegenerative Diseases. J. Neuroinflamm. 2022, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.C.; Richman, D.P. Myasthenia Gravis and Related Disorders: Pathology and Molecular Pathogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 651–657. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar]
- Dong, D.; Chong, M.K.C.; Wu, Y.; Kaminski, H.; Cutter, G.; Xu, X.; Li, H.; Zhao, C.; Yin, J.; Yu, S.; et al. Gender Differences in Quality of Life among Patients with Myasthenia Gravis in China. Health Qual. Life Outcomes 2020, 18, 296. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintora, M. Sex and Gender Issues in Multiple Sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T Cells in Multiple Sclerosis and Myasthenia Gravis. J. Neuroinflamm. 2017, 14, 117. [Google Scholar] [CrossRef]
- Kohler, I.; Verhoeven, A.; Derks, R.J.; Giera, M. Analytical Pitfalls and Challenges in Clinical Metabolomics. Bioanalysis 2016, 8, 1509–1532. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, C.; Chen, Z.; Zhao, H.; Chen, J.; Liu, X.; Kwan, Y.; Lin, H.; Ngai, S. Serum Metabolomics for the Diagnosis and Classification of Myasthenia Gravis. Metabolomics 2012, 8, 704–713. [Google Scholar] [CrossRef]
- Blackmore, D.; Siddiqi, Z.; Li, L.; Wang, N.; Maksymowych, W. Beyond the Antibodies: Serum Metabolomic Profiling of Myasthenia Gravis. Metabolomics 2019, 15, 109. [Google Scholar] [CrossRef]
- Rispoli, M.G.; Valentinuzzi, S.; De Luca, G.; Del Boccio, P.; Federici, L.; Di Ioia, M.; Digiovanni, A.; Grasso, E.A.; Pozzilli, V.; Villani, A.; et al. Contribution of Metabolomics to Multiple Sclerosis Diagnosis, Prognosis and Treatment. Int. J. Mol. Sci. 2021, 22, 11112. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Kurniawan, H.; Soriano-Baguet, L.; Brenner, D. Regulatory T Cell Metabolism at the Intersection between Autoimmune Diseases and Cancer. Eur. J. Immunol. 2020, 50, 1626–1642. [Google Scholar] [CrossRef]
- Antony, I.R.; Wong, B.H.S.; Kelleher, D.; Verma, N.K. Maladaptive T-Cell Metabolic Fitness in Autoimmune Diseases. Cells 2023, 12, 2541. [Google Scholar] [CrossRef] [PubMed]
- Socha, E.; Koba, M.; Kośliński, P. Amino Acid Profiling as a Method of Discovering Biomarkers for Diagnosis of Neurodegenerative Diseases. Amino Acids 2019, 51, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Cristofano, A.; Sapere, N.; La Marca, G.; Angiolillo, A.; Vitale, M.; Fratangelo, R.; Lombardi, T.; Porcile, C.; Intrieri, M.; et al. Serum Amino Acid Profiles in Normal Subjects and in Patients with or at Risk of Alzheimer Dementia. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 143–159. [Google Scholar] [CrossRef]
- Figura, M.; Kuśmierska, K.; Bucior, E.; Szlufik, S.; Koziorowski, D.; Jamrozik, Z.; Janik, P. Serum Amino Acid Profile in Patients with Parkinson’s Disease. PLoS ONE 2018, 13, e0191670. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Yoshida, N.; Tsokos, G.C. Amino Acid Metabolism in Lupus. Front. Immunol. 2021, 12, 623844. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino Acids in Cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Negrotto, L.; Correale, J. Amino Acid Catabolism in Multiple Sclerosis Affects Immune Homeostasis. J. Immunol. 2017, 198, 1900–1909. [Google Scholar] [CrossRef]
- Rzepiński, Ł.; Kośliński, P.; Kowalewski, M.; Koba, M.; Maciejek, Z. Serum Amino Acid Profiling in Differentiating Clinical Outcomes of Multiple Sclerosis. Neurol. I Neurochir. Pol. 2023, 57, 414–422. [Google Scholar] [CrossRef]
- Pashaei, S.; Yarani, R.; Mohammadi, P.; Emami Aleagha, M.S. The Potential Roles of Amino Acids and Their Major Derivatives in the Management of Multiple Sclerosis. Amino Acids 2022, 54, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Kośliński, P.; Rzepiński, Ł.; Daghir-Wojtkowiak, E.; Koba, M.; Maciejek, Z. Serum Amino Acid Profiles in Patients with Myasthenia Gravis. Amino Acids 2023, 55, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Rzepiński, Ł.; Kośliński, P.; Gackowski, M.; Koba, M.; Maciejek, Z. Amino Acid Levels as Potential Biomarkers of Multiple Sclerosis in Elderly Patients: Preliminary Report. J. Clin. Neurol. 2022, 18, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Rzepiński, Ł.; Zawadka-Kunikowska, M.; Newton, J.L.; Zalewski, P. Cardiac Autonomic Dysfunction in Myasthenia Gravis and Relapsing-Remitting Multiple Sclerosis—A Pilot Study. J. Clin. Med. 2021, 10, 2173. [Google Scholar] [CrossRef] [PubMed]
- Dehbashi, S.; Hamouda, D.; Shanina, E. Co-Occurrence of Multiple Sclerosis and Myasthenia Gravis: A Case Report and Review of Immunological Theories. Mult. Scler. Relat. Disord. 2019, 34, 135–136. [Google Scholar] [CrossRef]
- Hope, H.C.; Salmond, R.J. The Role of Non-Essential Amino Acids in T Cell Function and Anti-Tumour Immunity. Arch. Immunol. Ther. Exp. 2021, 69, 29. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Bröer, A. Amino Acid Homeostasis and Signalling in Mammalian Cells and Organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Sellebjerg, F.; Giovannoni, G.; Hand, A.; Madsen, H.O.; Jensen, C.V.; Garred, P. Cerebrospinal Fluid Levels of Nitric Oxide Metabolites Predict Response to Methylprednisolone Treatment in Multiple Sclerosis and Optic Neuritis. J. Neuroimmunol. 2002, 125, 198–203. [Google Scholar] [CrossRef]
- Virarkar, M.; Alappat, L.; Bradford, P.G.; Awad, A.B. L-Arginine and Nitric Oxide in CNS Function and Neurodegenerative Diseases. Crit. Rev. Food Sci. Nutr. 2013, 53, 1157–1167. [Google Scholar] [CrossRef]
- Desine, S.; Gabriel, C.L.; Smith, H.M.; Antonetti, O.R.; Wang, C.; Calcutt, M.W.; Doran, A.C.; Silver, H.J.; Nair, S.; Terry, J.G.; et al. Association of Alpha-Aminoadipic Acid with Cardiometabolic Risk Factors in Healthy and High-Risk Individuals. Front. Endocrinol. 2023, 14, 1122391. [Google Scholar] [CrossRef]
- da Silva, J.C.; Amaral, A.U.; Cecatto, C.; Wajner, A.; dos Santos Godoy, K.; Ribeiro, R.T.; de Mello Gonçalves, A.; Zanatta, Â.; da Rosa, M.S.; Loureiro, S.O.; et al. α-Ketoadipic Acid and α-Aminoadipic Acid Cause Disturbance of Glutamatergic Neurotransmission and Induction of Oxidative Stress In Vitro in Brain of Adolescent Rats. Neurotox. Res. 2017, 32, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Vyver, M.V.; Beelen, R.; De Keyser, J.; Nagels, G.; Van Binst, A.M.; Verborgh, C.; D’haeseleer, M. Plasma Citrulline Levels Are Increased in Patients with Multiple Sclerosis. J. Neurol. Sci. 2018, 387, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Crenn, P.; Coudray-Lucas, C.; Thuillier, F.; Cynober, L.; Messing, B. Postabsorptive Plasma Citrulline Concentration Is a Marker of Absorptive Enterocyte Mass and Intestinal Failure in Humans. Gastroenterology 2000, 119, 1496–1505. [Google Scholar] [CrossRef]
- Breuillard, C.; Cynober, L.; Moinard, C. Citrulline and Nitrogen Homeostasis: An Overview. Amino Acids 2015, 47, 685–691. [Google Scholar] [CrossRef]
- Watanabe, M.; Maernura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA Receptors in the Central Nervous System and Other Organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [PubMed]
- Gao, F.; Yin, X.; Edden, R.A.E.; Evans, A.C.; Xu, J.; Cao, G.; Li, H.; Li, M.; Zhao, B.; Wang, J.; et al. Altered Hippocampal GABA and Glutamate Levels and Uncoupling from Functional Connectivity in Multiple Sclerosis. Hippocampus 2018, 28, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Nantes, J.C.; Proulx, S.; Zhong, J.; Holmes, S.A.; Narayanan, S.; Brown, R.A.; Hoge, R.D.; Koski, L. GABA and Glutamate Levels Correlate with MTR and Clinical Disability: Insights from Multiple Sclerosis. NeuroImage 2017, 157, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Yoshizawa, F.; Nishizawa, N. Plasma N-Methylhistidine Concentration Is a Sensitive Index of Myofibrillar Protein Degradation during Starvation in Rats. Biosci. Biotechnol. Biochem. 1996, 60, 501–502. [Google Scholar] [CrossRef]
- Sjijlin, J.; Hjort, G.; Friman, G.; Hambraeus, L. Urinary Excretion of L-Methylhistidine: A Qualitative Indicator of Exogenous 3-Methylhistidine and Intake of Meats From Various Sources. Metabolism 1987, 36, 1175–1184. [Google Scholar] [CrossRef]
- Mary, P.; Servais, L.; Vialle, R. Neuromuscular Diseases: Diagnosis and Management. Orthop. Traumatol. Surg. Res. 2018, 104, S89–S95. [Google Scholar] [CrossRef]
| MS | MG | |
|---|---|---|
| Number of subjects (n) | 121 | 28 |
| Sex (male/female) | 42/80 (34%/66%) | 3/25 (10.7%/89.3%) |
| Age, years | 52.5 ± 11.61 Min–Max: 23–77 | 48.92 ± 12.61 Min–Max: 29–75 |
| Disease duration (years) [range] | 16 ± 8.4 | 7.85 ± 6.53 |
| Median EDSS score (IQR) | 6 (4.0–6.5) | n.a. |
| MS type n (%) | RRMS: 41 (34%) | n.a. |
| SPMS: 55 (45%) | ||
| PPMS: 25 (21%) | ||
| MG type n (%) | GMG: 25 (89.3%) | |
| OMG: 3 (10.7%) | ||
| AChRAb | Yes: 21 (75%) | |
| No: 7 (25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kośliński, P.; Rzepiński, Ł.; Koba, M.; Maciejek, Z.; Kowalewski, M.; Daghir-Wojtkowiak, E. Comparative Analysis of Serum Amino Acid Profiles in Patients with Myasthenia Gravis and Multiple Sclerosis. J. Clin. Med. 2024, 13, 4083. https://doi.org/10.3390/jcm13144083
Kośliński P, Rzepiński Ł, Koba M, Maciejek Z, Kowalewski M, Daghir-Wojtkowiak E. Comparative Analysis of Serum Amino Acid Profiles in Patients with Myasthenia Gravis and Multiple Sclerosis. Journal of Clinical Medicine. 2024; 13(14):4083. https://doi.org/10.3390/jcm13144083
Chicago/Turabian StyleKośliński, Piotr, Łukasz Rzepiński, Marcin Koba, Zdzisław Maciejek, Mariusz Kowalewski, and Emilia Daghir-Wojtkowiak. 2024. "Comparative Analysis of Serum Amino Acid Profiles in Patients with Myasthenia Gravis and Multiple Sclerosis" Journal of Clinical Medicine 13, no. 14: 4083. https://doi.org/10.3390/jcm13144083







