The Immediate Effect of Dry Needling Electric Muscle Stimulation on the Position of Atlas
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
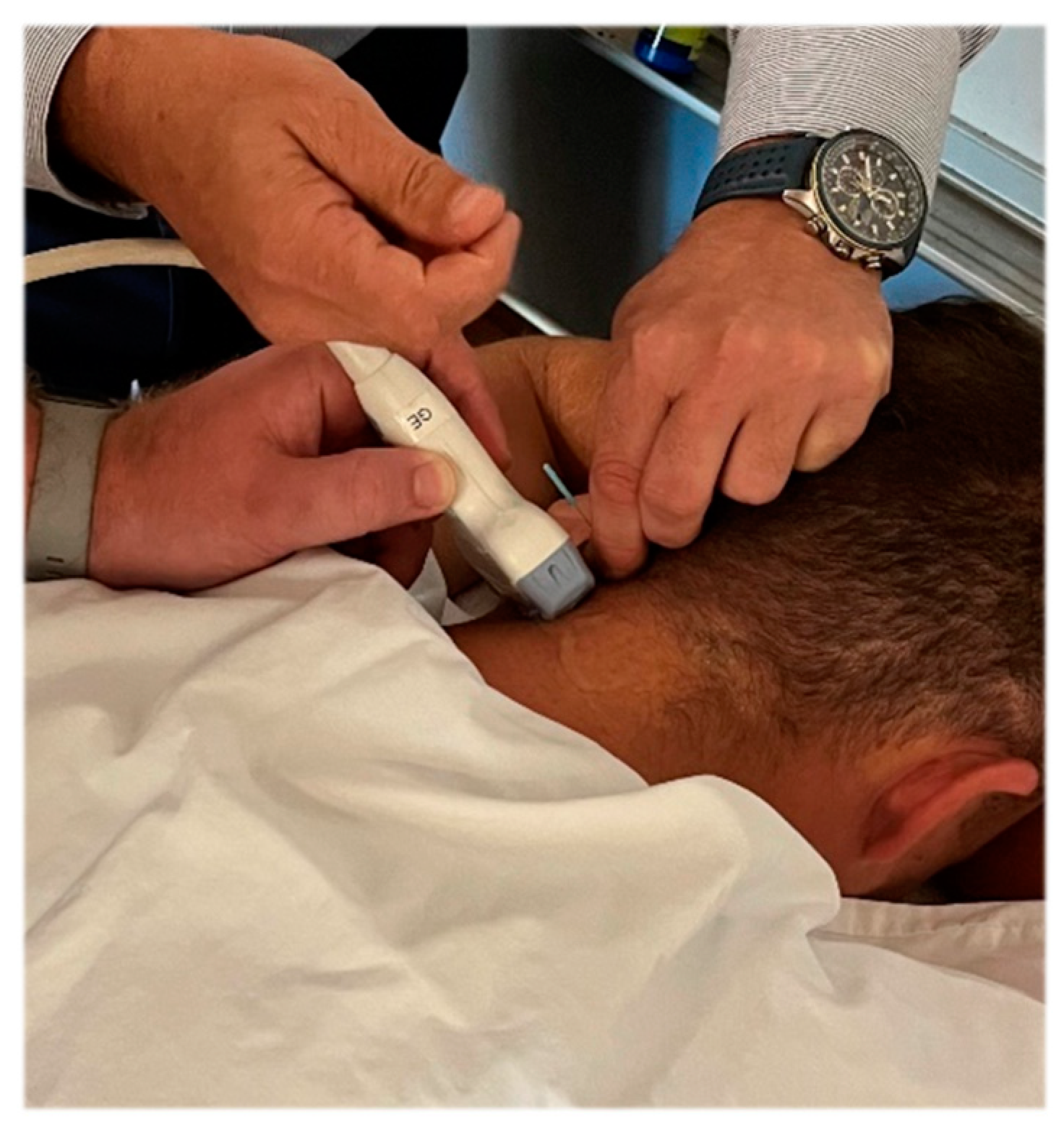
2.2. Study Protocol
2.3. Data Analysis
3. Results
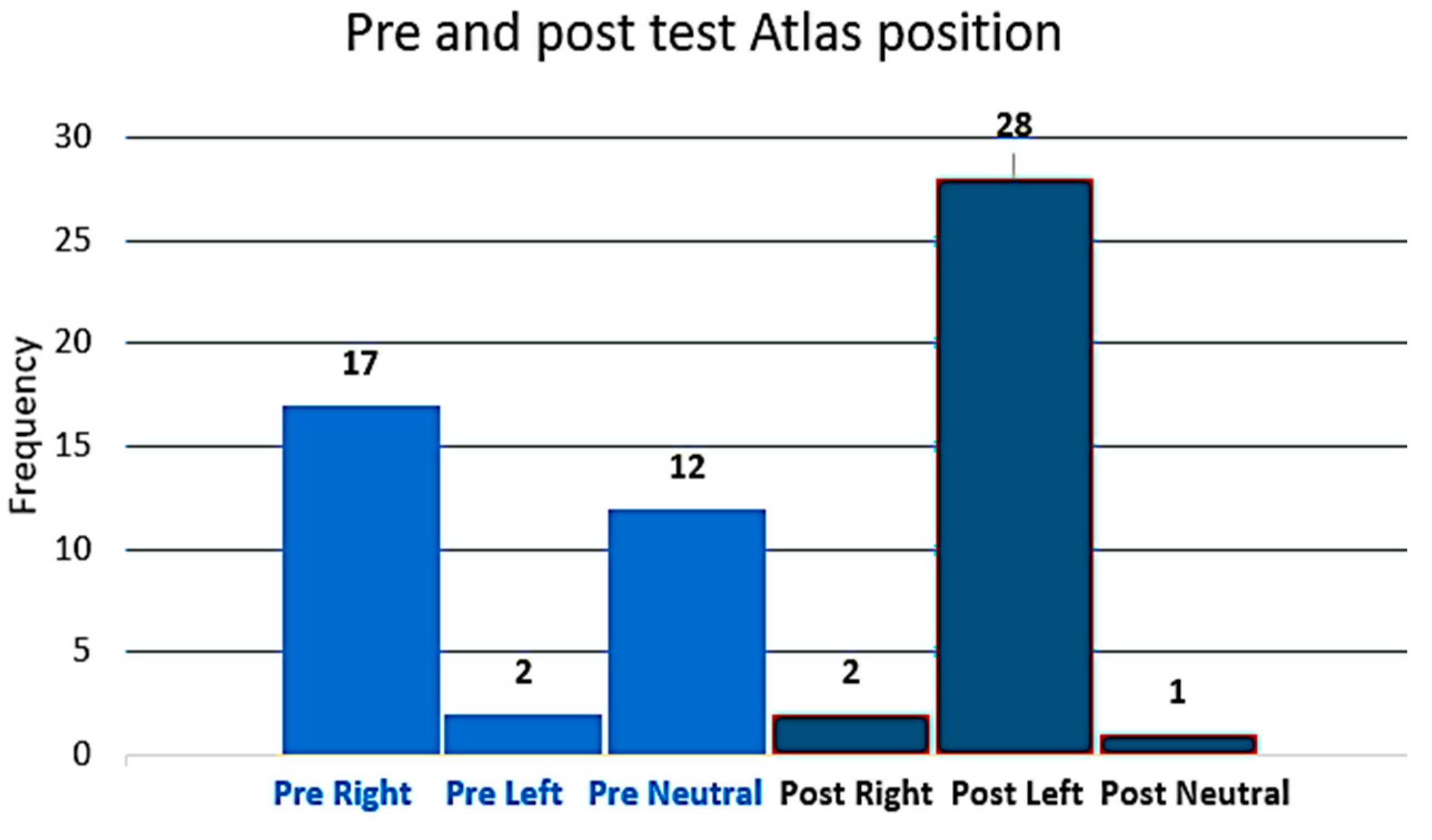
3.1. The Position of the Atlas Is Measured with Atlas Transverse Process Palpation
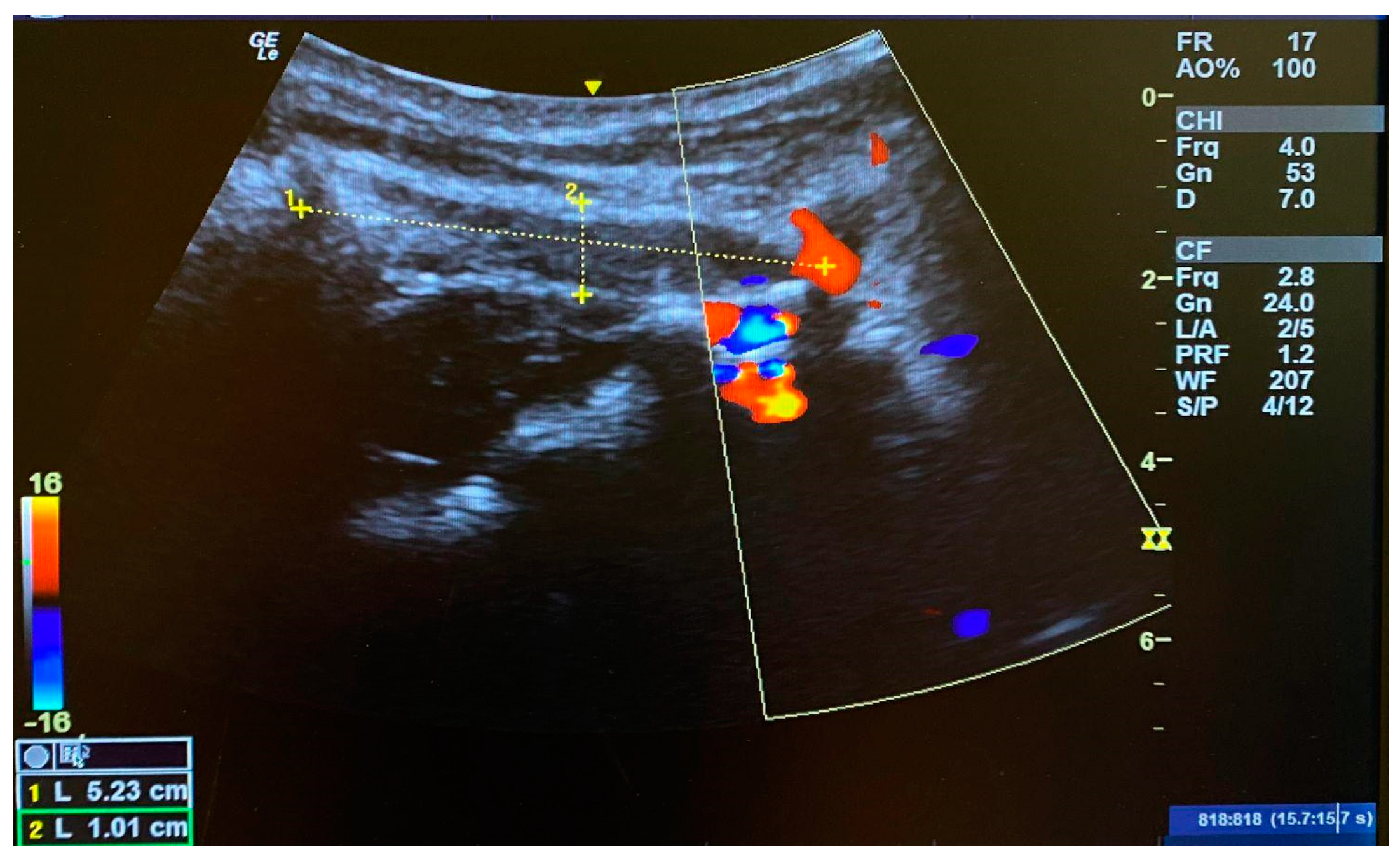
3.2. Muscle Length of the OCI Pre- and Post-Intervention
3.3. Correlation between Post-Intervention Muscle Length Test and FRT
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, K.M.; Beckerly, R.; Imani, F. Musculoskeletal Disorders a Universal Source of Pain and Disability Misunderstood and Mismanaged: A Critical Analysis Based on the U.S. Model of Care. Anesthesiol. Pain Med. 2018, 8, e85532. [Google Scholar] [CrossRef] [PubMed]
- Abaspour, O.; Akbari, M. Relationship between echogenicity of deep cervical muscles and pain laterality in subjects suffering from cervicogenic headache. Cranio 2023, 41, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Swanick, K. Musculoskeletal ultrasound imaging and clinical reasoning in the management of a patient with cervicogenic headache: A case report. Physiother. Theory Pract. 2019, 5, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Yang, H.; Li, X.; Lin, C.; Ke, S.; Wu, S.; Ma, C. Ultrasound-Guided versus Fluoroscopy-Guided Deep Cervical Plexus Block for the Treatment of Cervicogenic Headache. BioMed Res. Int. 2017, 2017, 4654803. [Google Scholar] [CrossRef] [PubMed]
- Page, P. Cervicogenic headaches: An evidence-led approach to clinical management. Int. J. Sports Phys. Ther. 2011, 6, 254–266. [Google Scholar] [PubMed]
- Bogduk, N.; Govind, J. Cervicogenic headache: An assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chai, H.M.; Wang, C.L.; Shau, Y.W.; Wang, S.F. Asymmetric Thickness of Oblique Capitis Inferior and Cervical Kinesthesia in Patients with Unilateral Cervicogenic Headache. J. Manip. Physiol. Ther. 2018, 41, 680–690. [Google Scholar] [CrossRef]
- Sillevis. Correlation and Reliability of the Atlas Position on X-ray in Subjects with and without Cervicogenic Headache: A Retrospective Study. 2021. Available online: https://www.semanticscholar.org/paper/Correlation-and-Reliability-of-the-Atlas-Position-A-Sillevis/2d444e843d5cb06d1e0442e42e3f86db5a7fcdbc (accessed on 9 July 2024).
- Forbes, J.; Das, J.M. Anatomy, Head and Neck: Atlantoaxial Joint. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sillevis, R.; Hogg, R. Anatomy and clinical relevance of sub occipital soft tissue connections with the dura mater in the upper cervical spine. PeerJ 2020, 8, e9716. [Google Scholar] [CrossRef]
- Ginszt, M.; Szkutnik, J.; Zieliński, G.; Bakalczuk, M.; Stodółkiewicz, M.; Litko-Rola, M.; Ginszt, A.; Rahnama, M.; Majcher, P. Cervical Myofascial Pain Is Associated with an Imbalance of Masticatory Muscle Activity. Int. J. Environ. Res. Public Health 2022, 19, 1577. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Cook, C.; Cleland, J.A.; Florencio, L.L. The cervical spine in tension type headache. Musculoskelet. Sci. Pract. 2023, 66, 102780. [Google Scholar] [CrossRef]
- Fakhoury, J.; Dowling, T.J. Cervical Degenerative Disc Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kekere, V.; Alsayouri, K. Anatomy, Head and Neck, Dura Mater. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Alix, M.E.; Bates, D.K. A proposed etiology of cervicogenic headache: The neurophysiologic basis and anatomic relationship between the dura mater and the rectus posterior capitis minor muscle. J. Manip. Physiol. Ther. 1999, 22, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shi, B.; Zheng, X.; Zhou, Z.; Jin, A.; Ding, Z.; Lv, H.; Zhang, H. Anatomic study and clinical significance of the dorsal meningovertebral ligaments of the thoracic dura mater. Spine 2015, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Enix, D.E.; Scali, F.; Pontell, M.E. The cervical myodural bridge, a review of literature and clinical implications. J. Can. Chiropr. Assoc. 2014, 58, 184–192. [Google Scholar] [PubMed]
- Scali, F.; Marsili, E.S.; Pontell, M.E. Anatomical connection between the rectus capitis posterior major and the dura mater. Spine 2011, 36, E1612–E1614. [Google Scholar] [CrossRef] [PubMed]
- Pontell, M.E.; Scali, F.; Marshall, E.; Enix, D. The obliquus capitis inferior myodural bridge. Clin. Anat. 2013, 26, 450–454. [Google Scholar] [CrossRef]
- Talley, J.T.; Goto, K.K. Osteopathic Manipulative Treatment: Muscle Energy Procedure with Post-Isometric Relaxation—Thoracic Vertebrae. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bishop, M.D.; Torres-Cueco, R.; Gay, C.W.; Lluch-Girbes, E.; Beneciuk, J.M.; Bialosky, J.E. What effect can manual therapy have on a patient’s pain experience? Pain Manag. 2015, 5, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Wyss, K. The management of a positional default of atlas in a patient with cervicogenic headache: A case report. Clin. Case Rep. Rev. 2015, 1, 218–223. [Google Scholar] [CrossRef]
- Smith, J.; Finnoff, J.T. Diagnostic and interventional musculoskeletal ultrasound: Part 1. Fundamentals. PM&R 2009, 1, 64–75. [Google Scholar] [CrossRef]
- Smith, J.; Finnoff, J.T. Diagnostic and interventional musculoskeletal ultrasound: Part 2. Clinical applications. PM&R 2009, 1, 162–177. [Google Scholar] [CrossRef]
- Womack, A.; Butts, R.; Dunning, J. Dry needling as a novel intervention for cervicogenic somatosensory tinnitus: A case study. Physiother. Theory Pract. 2020, 38, 1319–1327. [Google Scholar] [CrossRef]
- Sillevis, R.; Van Duijn, J.; Shamus, E.; Hard, M. Time effect for in-situ dry needling on the autonomic nervous system, a pilot study. Physiother. Theory Pract. 2019, 37, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Briffa, K.; Hopper, D.; Robinson, K. Reliability of manual examination and frequency of symptomatic cervical motion segment dysfunction in cervicogenic headache. Comparative Study. Man Ther. 2010, 15, 542–546. [Google Scholar] [CrossRef]
- Scali, F.; Pontell, M.E.; Enix, D.E.; Marshall, E. Histological analysis of the rectus capitis posterior major’s myodural bridge. Spine J. 2013, 13, 558–563. [Google Scholar] [CrossRef]
- Sillevis, R.; Hansen, A. Upper cervical spine syndrome: A new perspective. Arch. Prev. Med. 2024, 9, 018–021. [Google Scholar]
- Kahkeshani, K.; Ward, P.J. Connection between the spinal dura mater and suboccipital musculature: Evidence for the myodural bridge and a route for its dissection—A review. Clin. Anat. 2012, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Shamus, E.; Wyss, K. Does Cervicogenic Headache Result in the Presence of Neural Tension, and Does this Affect the Position and Mobility of Atlas? Med. Clin. Res. 2021, 6, 344. [Google Scholar]
- Murakami, G.; Cho, K.H.; Kitamura, K.; Rodriguez-Vazquez, J.F.; Sato, T. Rectus capitis lateralis muscle revisited: A histological study using human fetuses. Surg. Radiol. Anat. 2023, 45, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lv, X.; Zhou, Y.; Li, Z.; She, H.; Bai, L.; Bao, J. Myofascial release for the treatment of pain and dysfunction in patients with chronic mechanical neck pain: Systematic review and meta-analysis of randomised controlled trials. Clin. Rehabil. 2023, 37, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, M.H.; Thomas, L.C.; Swan, A.A.; Lu, L.H.; Treleaven, J.M. Sub-occipital muscle pressure pain thresholds correlate to direction of symptomatic active comfortable sustained neck rotation testing in post-concussive headache: A retrospective observational cross-sectional study. J. Man Manip. Ther. 2023, 31, 124–129. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Vicenzino, B.; Yang, C.H.; Hu, M.H.; Yang, C. Mulligan’s mobilization with movement for the thumb: A single case report using magnetic resonance imaging to evaluate the positional fault hypothesis. Man Ther. 2002, 7, 44–49. [Google Scholar] [CrossRef]
- Kulkarni, V.; Chandy, M.J.; Babu, K.S. Quantitative study of muscle spindles in suboccipital muscles of human foetuses. Neurol. India. 2001, 49, 355–359. [Google Scholar] [PubMed]
- Fernandez-de-Las-Penas, C.; Mesa-Jimenez, J.A.; Lopez-Davis, A.; Koppenhaver, S.L.; Arias-Buria, J.L. Cadaveric and ultrasonographic validation of needling placement in the obliquus capitis inferior muscle. Musculoskelet. Sci. Pract. 2020, 45, 102075. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Wyss, K. Effectiveness of dry needling to the sternocleidomastoid muscle. Manual Therapy, and Exercise to Reduce Pain and Improve Function in Subjects with Chronic Cervicogenic Headaches: A Retrospective Case Series. Ann. Case Rep. 2020, 14, 423. [Google Scholar]
- Rodriguez-Jimenez, J.; Ortega-Santiago, R.; Bonilla-Barba, L.; Falla, D.; Fernandez-de-Las-Penas, C.; Florencio, L.L. Immediate Effects of Dry Needing or Manual Pressure Release of Upper Trapezius Trigger Points on Muscle Activity During the Craniocervical Flexion Test in People with Chronic Neck Pain: A Randomized Clinical Trial. Pain Med. 2022, 23, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.F.; Frisina-Deyo, A.J. Dry needling for spine related disorders: A scoping review. Chiropr. Man Therap. 2020, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.; Treleaven, J.; Cagnie, B.; Peral, J.; Falla, D.; Lluch, E. Effects of dry needling of the obliquus capitis inferior on sensorimotor control and cervical mobility in people with neck pain: A double-blind, randomized sham-controlled trial. Braz. J. Phys. Ther. 2021, 25, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Overas, C.K.; Myhrvold, B.L.; Rosok, G.; Magnesen, E. Musculoskeletal diagnostic ultrasound imaging for thickness measurement of four principal muscles of the cervical spine—A reliability and agreement study. Chiropr. Man Ther. 2017, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Ashir, A.; Jerban, S.; Barrère, V.; Wu, Y.; Shah, S.B.; Andre, M.P.; Chang, E.Y. Skeletal Muscle Assessment Using Quantitative Ultrasound: A Narrative Review. Sensors 2023, 23, 4763. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.E.; Walker, B.F.; Young, K.J. The accuracy of diagnostic ultrasound imaging for musculoskeletal soft tissue pathology of the extremities: A comprehensive review of the literature. Chiropr. Man. Ther. 2015, 23, 31. [Google Scholar] [CrossRef]
- Mouratev, G.; Howe, D.; Hoppmann, R.; Poston, M.B.; Reid, R.; Varnadoe, J.; Smith, S.; McCallum, B.; Rao, V.; DeMarco, P. Teaching medical students ultrasound to measure liver size: Comparison with experienced clinicians using physical examination alone. Teach. Learn. Med. 2013, 25, 84–88. [Google Scholar] [CrossRef]
- Ferreira, A.P.A.; Zanier, J.F.C.; Santos, E.B.G.; Ferreira, A.S. Accuracy of Palpation Procedures for Locating the C1 Transverse Process and Masseter Muscle as Confirmed by Computed Tomography Images. J. Manip. Physiol. Ther. 2022, 45, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.-F.; Wu, L.-P.; Fan, J.-H.; Li, Y.-K.; Das, M. Morphological asymmetry of the atlas and its clinical implications. J. Manip. Physiol. Ther. 2011, 34, 463–467. [Google Scholar] [CrossRef]
- Filippucci, E.; Unlu, Z.; Farina, A.; Grassi, W. Sonographic training in rheumatology: A self teaching approach. Ann. Rheum. Dis. 2003, 62, 565–567. [Google Scholar] [CrossRef] [PubMed]

| One-Sample Test | |||||||
| Test Value = 0 | |||||||
| t | df | Significance | Mean Difference | 95% Confidence Interval of the Difference | |||
| One-Sided p | Two-Sided p | Lower | Upper | ||||
| Post muscle length | 45.563 | 31 | <0.001 | <0.001 | 4.08656 | 3.9036 | 4.2695 |
| Pre muscle length | 38.364 | 31 | <0.001 | <0.001 | 4.06406 | 3.8480 | 4.2801 |
| One-Sample Effect Sizes | |||||||
| Standardizer | Point Estimate | 95% Confidence Interval | |||||
| Lower | Upper | ||||||
| Pre muscle length | Cohen’s d | 0.50737 | 8.054 | 6.026 | 10.075 | ||
| Hedges’ correction | 0.52007 | 7.858 | 5.879 | 9.829 | |||
| Post muscle length | Cohen’s d | 0.59925 | 6.782 | 5.063 | 8.493 | ||
| Hedges’ correction | 0.61425 | 6.616 | 4.940 | 8.285 | |||
| Post Muscle Length | Post Flex Rotation Right | Post Flex Rotation Left | ||
|---|---|---|---|---|
| Post muscle length | Pearson’s correlation | 1 | −0.405 * | 0.038 |
| Sig. (2-tailed) | 0.022 | 0.835 | ||
| N | 32 | 32 | 32 | |
| Post flex rotation right | Pearson’s correlation | −0.405 * | 1 | 0.475 ** |
| Sig. (2-tailed) | 0.022 | 0.006 | ||
| N | 32 | 32 | 32 | |
| Post flex rotation left | Pearson’s correlation | 0.038 | 0.475 ** | 1 |
| Sig. (2-tailed) | 0.835 | 0.006 | ||
| N | 32 | 32 | 32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillevis, R.; Cerdeira, D.; Yankovich, J.; Hansen, A.W. The Immediate Effect of Dry Needling Electric Muscle Stimulation on the Position of Atlas. J. Clin. Med. 2024, 13, 4097. https://doi.org/10.3390/jcm13144097
Sillevis R, Cerdeira D, Yankovich J, Hansen AW. The Immediate Effect of Dry Needling Electric Muscle Stimulation on the Position of Atlas. Journal of Clinical Medicine. 2024; 13(14):4097. https://doi.org/10.3390/jcm13144097
Chicago/Turabian StyleSillevis, Rob, Daniel Cerdeira, Jared Yankovich, and Anne Weller Hansen. 2024. "The Immediate Effect of Dry Needling Electric Muscle Stimulation on the Position of Atlas" Journal of Clinical Medicine 13, no. 14: 4097. https://doi.org/10.3390/jcm13144097
APA StyleSillevis, R., Cerdeira, D., Yankovich, J., & Hansen, A. W. (2024). The Immediate Effect of Dry Needling Electric Muscle Stimulation on the Position of Atlas. Journal of Clinical Medicine, 13(14), 4097. https://doi.org/10.3390/jcm13144097








