Assessment of the Concentrations of Selected Aminothiols in Patients after COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Definitions Used to Classify Co-Existing Conditions
2.3. Biochemical Analyses of Aminothiols
2.4. Statistical Analyses
3. Results
3.1. General Characteristics of the Study Group
3.2. Levels of Analyzed Aminothiols in Sex Subgroups of Patients after COVID-19
3.3. Levels of Analyzed Aminothiols in the Subgroups of Post-COVID-19 Patients
3.4. Correlations between HCy and Cys Levels
3.5. Comparisons of Aminothiols Levels between COVID-19 Patients and the Reference Group of Patients with ASCVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azevedo, R.B.; Botelho, B.G.; Hollanda, J.V.G.; Ferreira, L.V.L.; Junqueira de Andrade, L.Z.; Oei, S.S.M.L.; Mello, T.S.; Muxfeldt, E.S. COVID-19 and the cardiovascular system: A comprehensive review. J. Hum. Hypertens. 2021, 35, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Bader, F.; Manla, Y.; Atallah, B.; Starling, R.C. Heart failure and COVID-19. Heart Fail. Rev. 2021, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W.; Venter, F.W.D. Long-COVID: An evolving problem with an extensive impact. S. Afr. Med. J. 2020, 111, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker, and imaging abnormalities following hospitalization for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: A prospective cohort in UK Biobank. Cardiovasc. Res. 2023, 119, 1718–1727. [Google Scholar] [CrossRef]
- Kounis, N.G.; Gogos, C.; de Gregorio, C.; Hung, M.Y.; Kounis, S.N.; Tsounis, E.P.; Assimakopoulos, S.F.; Pourmasumi, S.; Mplani, V.; Servos, G.; et al. “When,” “Where,” and “How” of SARS-CoV-2 Infection Affects the Human Cardiovascular System: A Narrative Review. Balk. Med. J. 2024, 41, 7–22. [Google Scholar] [CrossRef]
- Jud, P.; Gressenberger, P.; Muster, V.; Avian, A.; Meinitzer, A.; Strohmaier, H.; Sourij, H.; Raggam, R.B.; Stradner, M.H.; Demel, U.; et al. Evaluation of Endothelial Dysfunction and Inflammatory Vasculopathy After SARS-CoV-2 Infection-A Cross-Sectional Study. Front. Cardiovasc. Med. 2021, 8, 750887. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, M. Assessing cardiovascular disease: Looking beyond cholesterol. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 427–433. [Google Scholar] [CrossRef]
- Szoltysek-Boldys, I.; Zielinska-Danch, W.; Loboda, D.; Wilczek, J.; Gibinski, M.; Paradowska-Nowakowska, E.; Golba, K.S.; Sarecka-Hujar, B. Photoplethysmographic Measurement of Arterial Stiffness in Polish Patients with Long-COVID-19 Syndrome-The Results of a Cross-Sectional Study. Diagnostics 2022, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana-Carnerero, C.; Giner-Soriano, M.; Dominguez, À.; Morros, R.; Pericas, C.; Álamo-Junquera, D.; Toledo, D.; Gallego, C.; Redondo, A.; Grau, M. Atherosclerosis, Cardiovascular Disease, and COVID-19: A Narrative Review. Biomedicines 2023, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Dinavahi, R.; Falkner, B. Relationship of homocysteine with cardiovascular disease and blood pressure. J. Clin. Hypertens. 2004, 6, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Durand, P.; Prost, M.; Loreau, N.; Lussier-Cacan, S.; Blache, D. Impaired homocysteine metabolism and atherothrombotic disease. Lab. Investig. 2001, 81, 645–672. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Signorello, M.G.; Segantin, A.; Passalacqua, M.; Leoncini, G. Homocysteine decreases platelet NO level via protein kinase C activation. Nitric Oxide 2009, 20, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine thiolactone and N-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Baszczuk, A.; Kopczyński, Z.; Thielemann, A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postep. Hig. Med. Dosw. 2014, 68, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Mazurakova, A.; Samec, M.; Biringer, K.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Golubnitschaja, O. Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person. EPMA J. 2021, 12, 477–505. [Google Scholar] [CrossRef]
- Shih, C.C.; Shih, Y.L.; Chen, J.Y. The association between homocysteine levels and cardiovascular disease risk among middle-aged and elderly adults in Taiwan. BMC Cardiovasc. Disord. 2021, 21, 191. [Google Scholar] [CrossRef]
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120–138. [Google Scholar]
- Todua, F.; Akhvlediani, M.; Vorobiova, E.; Tsivtsivadze, G.; Baramidze, A. Homocysteine and D-dimer levels and multilayer computed tomography for diagnosing pulmonary artery thromboembolism. Vessel Plus 2017, 1, 38–42. [Google Scholar] [CrossRef]
- Mills, B.J.; Weiss, M.M.; Lang, C.A.; Liu, M.C.; Ziegler, C. Blood glutathione and cysteine changes in cardiovascular disease. J. Lab. Clin. Med. 2000, 135, 396–401. [Google Scholar] [CrossRef]
- van den Brandhof, W.E.; Haks, K.; Schouten, E.G.; Verhoef, P. The relationship between plasma cysteine, plasma homocysteine, and coronary atherosclerosis. Atherosclerosis 2001, 157, 403–409. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M.; Refsum, H.; Graham, I.M.; Vollset, S.E.; European Concerted Action Project. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation 2001, 103, 2544–2549. [Google Scholar] [CrossRef]
- Lima, A.; Ferin, R.; Fontes, A.; Santos, E.; Martins, D.; Baptista, J.; Pavão, M.L. Cysteine is a better predictor of coronary artery disease than conventional homocysteine in high-risk subjects under preventive medication. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1281–1288. [Google Scholar] [CrossRef]
- Musthafa, Q.A.; Abdul Shukor, M.F.; Ismail, N.A.S.; Mohd Ghazi, A.; Mohd Ali, R.; Nor, I.F.M.; Dimon, M.Z.; Wan Ngah, W.Z. Oxidative status and reduced glutathione levels in premature coronary artery disease and coronary artery disease. Free Radic. Res. 2017, 51, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, S.; Venkatachalam, R.; Perumal, S.M.; Ayyamkulamkara, S.S. Evaluation of Oxidative Stress Parameters and Antioxidant Status in Coronary Artery Disease Patients. Arch. Razi Inst. 2022, 77, 853–859. [Google Scholar] [CrossRef]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2021, 11, 605908. [Google Scholar] [CrossRef]
- Siregar, J.; Darmadi, D. Serum homocysteine level and severity of coronavirus disease-2019 (COVID-19). Rom. J. Intern. Med. 2023, 61, 106–111. [Google Scholar] [CrossRef]
- Fouda, E.M.; Wahba, N.S.; Elsharawy, A.I.M.; Ishak, S.R. Serum homocysteine level in pediatric patients with COVID-19 and its correlation with the disease severity. Pediatr. Pulmonol. 2022, 57, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Al-Sabah, S.; Hammad, M.M.; Hebbar, P.; Channanath, A.M.; John, S.E.; Taher, I.; Almaeen, A.; Ghazy, A.; Mohammad, A.; et al. Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A Focused Analysis on D-Dimer, Homocysteine and Thromboembolism. Front. Pharmacol. 2020, 11, 587451. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.M.; Seet, C.Y.H.; Koh, J.S.; Tham, C.H.; Chiew, H.J.; De Leon, J.A.; Chua, C.Y.K.; Hui, A.C.; Tan, S.S.Y.; Vasoo, S.S.; et al. Acute Ischemic Stroke During the Convalescent Phase of Asymptomatic COVID-2019 Infection in Men. JAMA Netw. Open 2021, 4, e217498. [Google Scholar] [CrossRef] [PubMed]
- Petelina, T.; Musikhina, N.; Avdeeva, K.; Leonovich, S.; Gapon, L.; Gorbatenko, E.; Sharoyan, Y.; Zueva, E.; Yaroslavskaya, E.; Gultyaeva, E.; et al. Prospective analysis of inflammatory response markers, endothelial dysfunction and hemostasis parameters in COVID-19 associated pneumonia patients with and without type 2 diabetes mellitus. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724.3394. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, R.; Kim, S.H.; Shah, H.; Zhang, S.; Liang, J.H.; Fang, Y.; Gentili, M.; Leary, C.N.O.; Elledge, S.J.; et al. SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication. Nat. Commun. 2021, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Nowakowska, E.; Łoboda, D.; Gołba, K.; Sarecka-Hujar, B. Long COVID-19 Syndrome Severity According to Sex, Time from the Onset of the Disease, and Exercise Capacity-The Results of a Cross-Sectional Study. Life 2023, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 487–496. [Google Scholar] [CrossRef]
- Sarecka-Hujar, B.; Szołtysek-Bołdys, I.; Kopyta, I. Serum Levels of Lipids and Selected Aminothiols in Epileptic Children—A Pilot Case-Control Study. Brain Sci. 2022, 12, 120. [Google Scholar] [CrossRef]
- Rezel-Potts, E.; Douiri, A.; Sun, X.; Chowienczyk, P.J.; Shah, A.M.; Gulliford, M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022, 19, e1004052. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiang, M.; Jing, H.; Wang, C.; Novakovic, V.A.; Shi, J. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis 2024, 27, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.K.; Imperatore, G.; Rolka, D.B.; Lundeen, E.; Rutkowski, R.E.; Jackson, S.L.; He, S.; Kuklina, E.V.; Park, S.; Pavkov, M.E. Risk of Cardiovascular Disease After COVID-19 Diagnosis Among Adults with and Without Diabetes. J. Am. Heart Assoc. 2023, 12, e029696. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, C.; Chowdhury, R.; Sharma, S.; Bhandari, N.; Taneja, S.; Ueland, P.M.; Strand, T.A. Association of Plasma Total Cysteine and Anthropometric Status in 6-30 Months Old Indian Children. Nutrients 2020, 12, 3146. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, M.; Gao, J.; Ji, X.; He, J.; Zhang, J.; Zhao, W. A series of BODIPY-based probes for the detection of cysteine and homocysteine in living cells. Talanta 2019, 195, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, E.V.; Ivanov, A.V.; Karpov, V.O.; Vasil’evich Alexandrin, V.; Dygai, A.M.; Kruglova, M.P.; Kostiuchenko, G.I.; Kazakov, S.P.; Kubatiev, A.A. Association of Low Molecular Weight Plasma Aminothiols with the Severity of Coronavirus Disease 2019. Oxidative Med. Cell. Longev. 2021, 2021, 9221693. [Google Scholar] [CrossRef] [PubMed]
- Behringer, S.; Wingert, V.; Oria, V.; Schumann, A.; Grünert, S.; Cieslar-Pobuda, A.; Kölker, S.; Lederer, A.K.; Jacobsen, D.W.; Staerk, J.; et al. Targeted Metabolic Profiling of Methionine Cycle Metabolites and Redox Thiol Pools in Mammalian Plasma, Cells and Urine. Metabolites 2019, 9, 235. [Google Scholar] [CrossRef]
- Hortin, G.L.; Sullivan, P.; Csako, G. Relationships among plasma homocysteine, cysteine, and albumin concentrations: Potential utility of assessing the cysteine/homocysteine ratio. Clin. Chem. 2001, 47, 1121–1124. [Google Scholar] [CrossRef]
- Keskin, A.; Ustun, G.U.; Aci, R.; Duran, U. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomark. Med. 2022, 16, 559–568. [Google Scholar] [CrossRef]
- Kalan Sarı, I.; Keskin, O.; Seremet Keskin, A.; Elli Dağ, H.Y.; Harmandar, O. Is Homocysteine Associated with the Prognosis of COVID-19 Pneumonia. Int. J. Clin. Pract. 2023, 2023, 9697871. [Google Scholar] [CrossRef] [PubMed]
- Rafii, M.; Elango, R.; Courtney-Martin, G.; House, J.D.; Fisher, L.; Pencharz, P.B. High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography-electrospray tandem mass spectrometry. Anal. Biochem. 2007, 371, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.; Wardas, W.; Zielinska-Danch, W.; Pawlicki, K. The influence of smoking on plasma homocysteine and cysteine levels in passive and active smokers. Clin. Chem. Lab. Med. 2004, 42, 408–414. [Google Scholar] [CrossRef]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and Over: United States, 2017–2018; NCHS Data Brief, no. 364; National Center for Health Statistics: Hyattsville, MD, USA, 2020. [Google Scholar]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R.I.; Freeman, P.R.; Bruzzese, J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respir. Med. Case Rep. 2020, 30, 101063. [Google Scholar] [CrossRef]
- Özdemir, K.; Saruhan, E.; Kaya, G.; Kaya Benli, T.; Meral, O.; Bozoğlan, H.; Demir, H.; Demir, C.; Kavak, S. Determination of hemoglobin a1c, lipid profiles, homocysteine, oxidative stress and physical activity levels in diabetic and/or nondiabetic COVID-19 patients. Karya J. Health Sci. 2023, 4, 85–91. [Google Scholar] [CrossRef]
| Total COVID-19 Group N = 212 | Female Subgroup N = 123 | Male Subgroup N = 89 | p-Value | |
|---|---|---|---|---|
| General parameters | ||||
| Age (years), M ± SD | 58.95 ± 8.22 | 59.42 ± 8.01 | 58.30 ± 8.51 | 0.33 |
| BMI (kg/m2), M ± SD | 29.28 ± 4.77 | 29.17 ± 5.21 | 29.43 ± 4.13 | 0.69 |
| Smoking status, n (%) | <0.001 | |||
| Former smokers | 78 (36.79) | 38 (30.89) | 40 (44.94) | |
| Smokers | 13 (6.13) | 3 (2.44) | 10 (11.24) | |
| Nonsmokers | 121 (57.08) | 82 (66.67) | 39 (43.82) | |
| Biochemical parameters | ||||
| TC (mg/dL), M ± SD | 238.12 ± 62.42 | 243.87 ± 59.78 | 230.17 ± 65.40 | 0.11 |
| LDL (mg/dL), M ± SD | 145.99 ± 40.47 | 149.76 ± 39.43 | 140.77 ± 41.53 | 0.11 |
| HDL (mg/dL), M ± SD | 69.66 ± 28.06 | 76.24 ± 26.72 | 60.56 ± 27.47 | <0.001 |
| TG (mg/dL), M ± SD | 192.35 ± 112.71 | 170.29 ± 71.22 | 222.85 ± 147.68 | <0.001 |
| Non-HDL 1 (mg/dL), M ± SD | 168.46 ± 50.58 | 167.63 ± 51.00 | 169.62 ± 50.27 | 0.70 |
| Fasting glucose (mg/dL), M ± SD | 92.46 ± 22.68 | 89.26 ± 17.67 | 96.88 ± 27.69 | 0.015 |
| CRP (mg/L), M ± SD | 3.62 ± 4.31 | 3.88 ± 5.09 | 3.25 ± 2.92 | 0.29 |
| D-dimers, (mg/L), M ± SD | 1.16 ± 7.13 | 1.54 ± 0.31 | 0.62 ± 0.73 | 0.35 |
| Troponin (ng/mL), M ± SD | 0.49 ± 0.30 | 0.46 ± 0.28 | 0.52 ± 0.32 | 0.18 |
| Comorbidities | ||||
| CAD, n (%) | 22 (10.38) | 7 (5.69) | 15 (16.85) | 0.009 |
| Hypertension, n (%) | 127 (59.91) | 65 (52.85) | 62 (69.66) | 0.013 |
| Diabetes, n (%) | 41 (19.34) | 21 (17.07) | 20 (22.47) | 0.33 |
| Hypercholesterolemia, n (%) | 103 (48.58) | 54 (43.90) | 49 (55.06) | 0.11 |
| History of ischemic stroke, n (%) | 1 (0.47) | 1 (0.81) | 0 (0.00) | 0.99 * |
| Chronic kidney disease, n (%) | 1 (0.47) | 1 (0.81) | 0 (0.00) | 0.99 * |
| Venous thrombosis, n (%) | 5 (2.36) | 3 (2.44) | 2 (2.25) | 0.99 * |
| Aminothiols | Total COVID-19 Patients N = 212 | Females N = 123 | Males N = 89 | p-Value |
|---|---|---|---|---|
| HCy (µmol/L), M ± SD | 9.50 ± 3.21 | 9.17 ± 3.14 | 9.96 ± 3.26 | 0.08 |
| Cys (µmol/L), M ± SD | 218.56 ± 47.08 | 210.35 ± 41.90 | 229.92 ± 51.54 | 0.003 |
| Glutathione (µmol/L), M ± SD | 2.60 ± 0.91 | 2.69 ± 0.90 | 2.48 ± 0.91 | 0.11 |
| Cys/HCy ratio, M ± SD | 24.81 ± 8.19 | 24.82 ± 7.83 | 24.80 ± 8.70 | 0.98 |
| Aminothiols | COVID-19 Patients <12 Weeks from Disease Onset N = 27 | COVID-19 Patients 12–24 Weeks from Disease Onset N = 71 | COVID-19 Patients >24 Weeks from Disease Onset N = 91 | p-Value |
|---|---|---|---|---|
| HCy (µmol/L), M ± SD | 9.95 ± 2.66 | 9.85 ± 2.79 | 9.13 ± 3.78 | 0.06 |
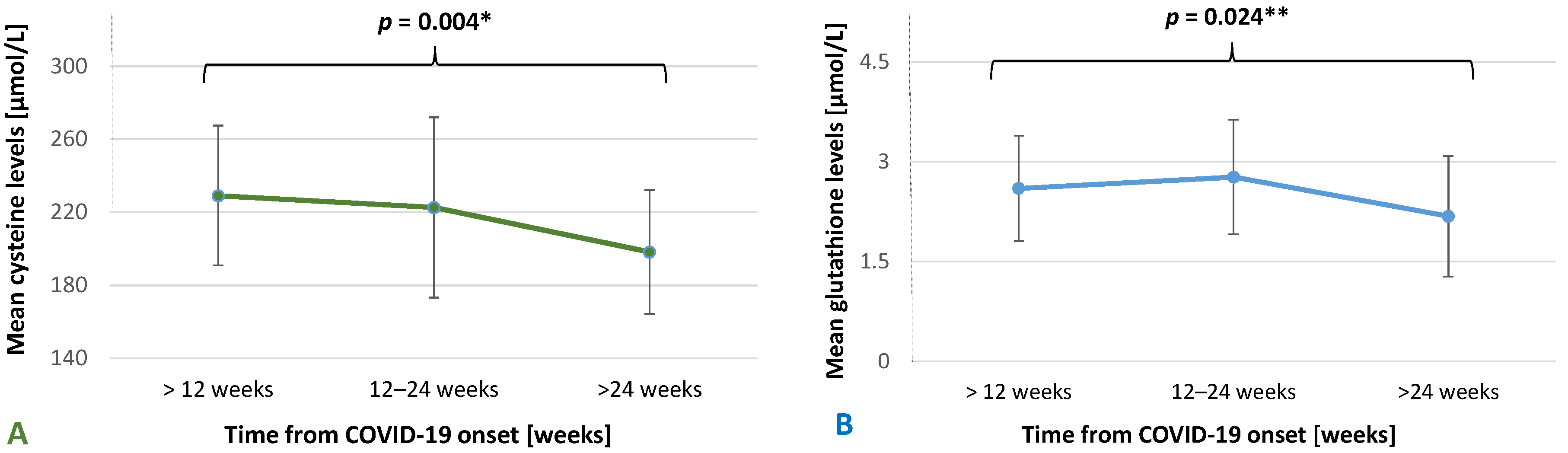
| Cys (µmol/L), M ± SD | 226.82 ± 40.57 | 232.23 ± 47.99 | 208.08 ± 48.43 | 0.005 1 |
| Glutathione (µmol/L), M ± SD | 2.75 ± 0.88 | 2.78 ± 0.83 | 2.50 ± 0.95 | 0.11 |
| Cys/HCy ratio, M ± SD | 24.26 ± 7.56 | 25.60 ± 9.72 | 24.61 ± 7.18 | 0.68 |
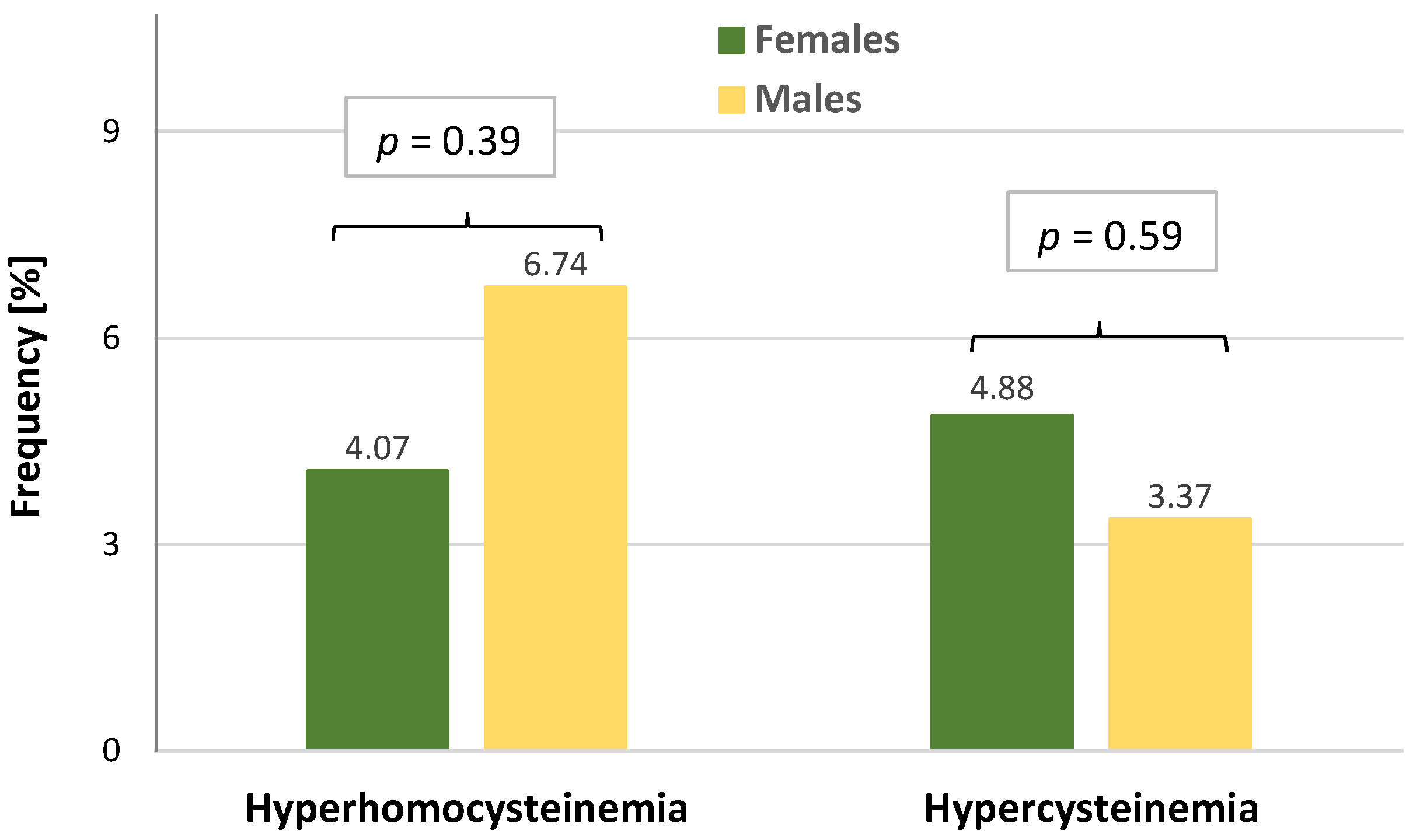
| Hyperhomocysteinemia, n (%) | 2 (7.41) | 4 (5.63) | 2 (2.20) | 0.28 * |
| Hypercysteinemia, n (%) | 1 (3.70) | 8 (11.27) | 2 (2.20) | 0.039 * |
| Aminothiols | Mild/Moderate N = 163 | Severe/Critical N = 45 | p-Value |
|---|---|---|---|
| HCy (µmol/L), M ± SD | 9.39 ± 2.95 | 9.91 ± 4.08 | 0.64 |
| Cys (µmol/L), M ± SD | 217.73 ± 44.47 | 224.06 ± 56.55 | 0.28 |
| Glutathione (µmol/L), M ± SD | 2.65 ± 0.92 | 2.45 ± 0.83 | 0.33 |
| Cys/HCy ratio, M ± SD | 24.88 ± 8.22 | 24.87 ± 8.25 | 0.54 |
| Hyperhomocysteinemia, n (%) | 6 (3.68) | 3 (6.25) | 0.65 |
| Hypercysteinemia, n (%) | 9 (5.52) | 2 (4.44) | 0.93 |
| Aminothiols | COVID-19 Patients N = 212 | ASCVD Group N = 95 | p-Value |
|---|---|---|---|
| HCy (µmol/L), M ± SD | 9.50 ± 3.21 | 10.22 ± 4.81 | 0.79 |
| Cys (µmol/L), M ± SD | 218.56 ± 47.08 | 151.00 ± 55.76 | <0.001 |
| Glutathione (µmol/L), M ± SD | 2.60 ± 0.91 | 1.77 ± 0.76 | <0.001 |
| Cys/HCy ratio, M ± SD | 24.81 ± 8.19 | 16.75 ± 6.97 | <0.001 |
| Hyperhomocysteinemia, n (%) | 9 (4.25) | 11 (11.58) | 0.021 |
| Hypercysteinemia, n (%) | 11 (5.19) | 2 (2.11) | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szołtysek-Bołdys, I.; Zielińska-Danch, W.; Łoboda, D.; Gołba, K.S.; Sarecka-Hujar, B. Assessment of the Concentrations of Selected Aminothiols in Patients after COVID-19. J. Clin. Med. 2024, 13, 4108. https://doi.org/10.3390/jcm13144108
Szołtysek-Bołdys I, Zielińska-Danch W, Łoboda D, Gołba KS, Sarecka-Hujar B. Assessment of the Concentrations of Selected Aminothiols in Patients after COVID-19. Journal of Clinical Medicine. 2024; 13(14):4108. https://doi.org/10.3390/jcm13144108
Chicago/Turabian StyleSzołtysek-Bołdys, Izabela, Wioleta Zielińska-Danch, Danuta Łoboda, Krzysztof S. Gołba, and Beata Sarecka-Hujar. 2024. "Assessment of the Concentrations of Selected Aminothiols in Patients after COVID-19" Journal of Clinical Medicine 13, no. 14: 4108. https://doi.org/10.3390/jcm13144108
APA StyleSzołtysek-Bołdys, I., Zielińska-Danch, W., Łoboda, D., Gołba, K. S., & Sarecka-Hujar, B. (2024). Assessment of the Concentrations of Selected Aminothiols in Patients after COVID-19. Journal of Clinical Medicine, 13(14), 4108. https://doi.org/10.3390/jcm13144108







