Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Prediction of Potential Drug Targets
2.2. Drugs
2.3. Cell Lines and Cell Culture Conditions
2.4. Cell Viability Assay
2.5. Wound Healing Assay
2.6. Clonogenic Assay
2.7. Statistical Analysis
2.8. In Silico ADMET Modeling
3. Results
3.1. Targets of Sildenafil, Tezosentan, and Levosimendan
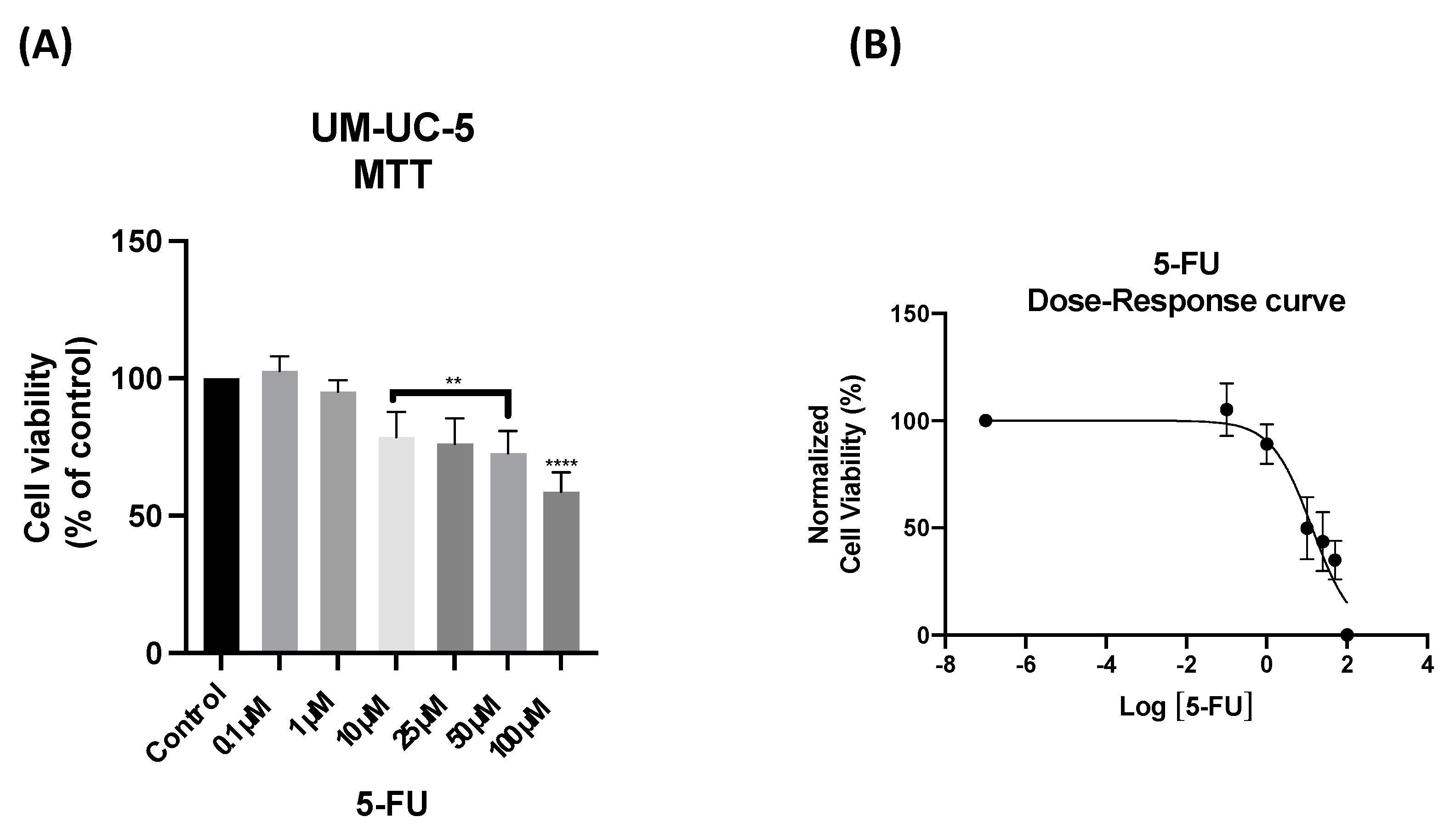
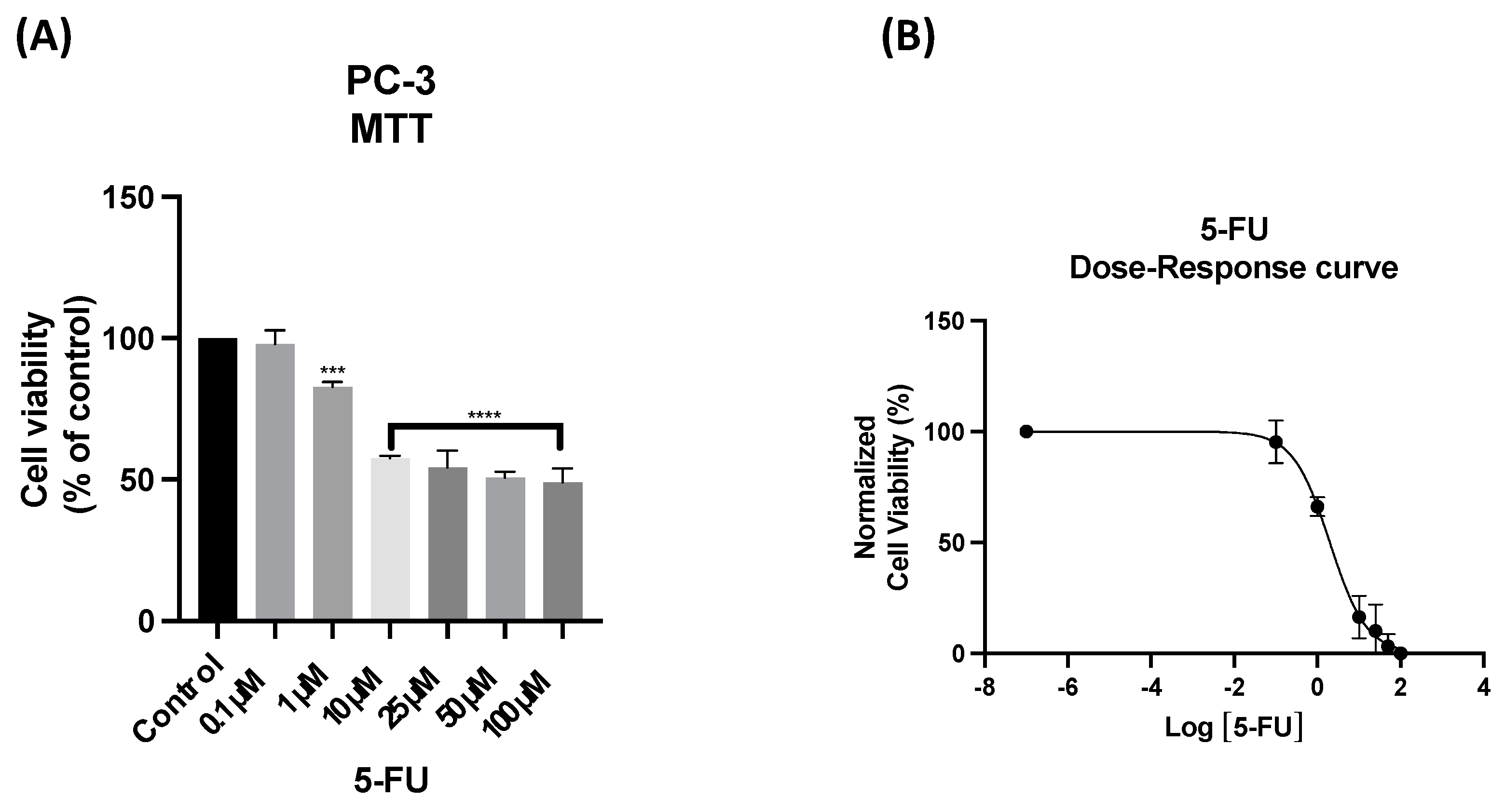
3.2. The Effect of 5-FU as a Single Agent on PC-3 and UM-UC-5 Cellular Viability
3.3. The Effect of Sildenafil as a Single Agent and in Combination with 5-FU
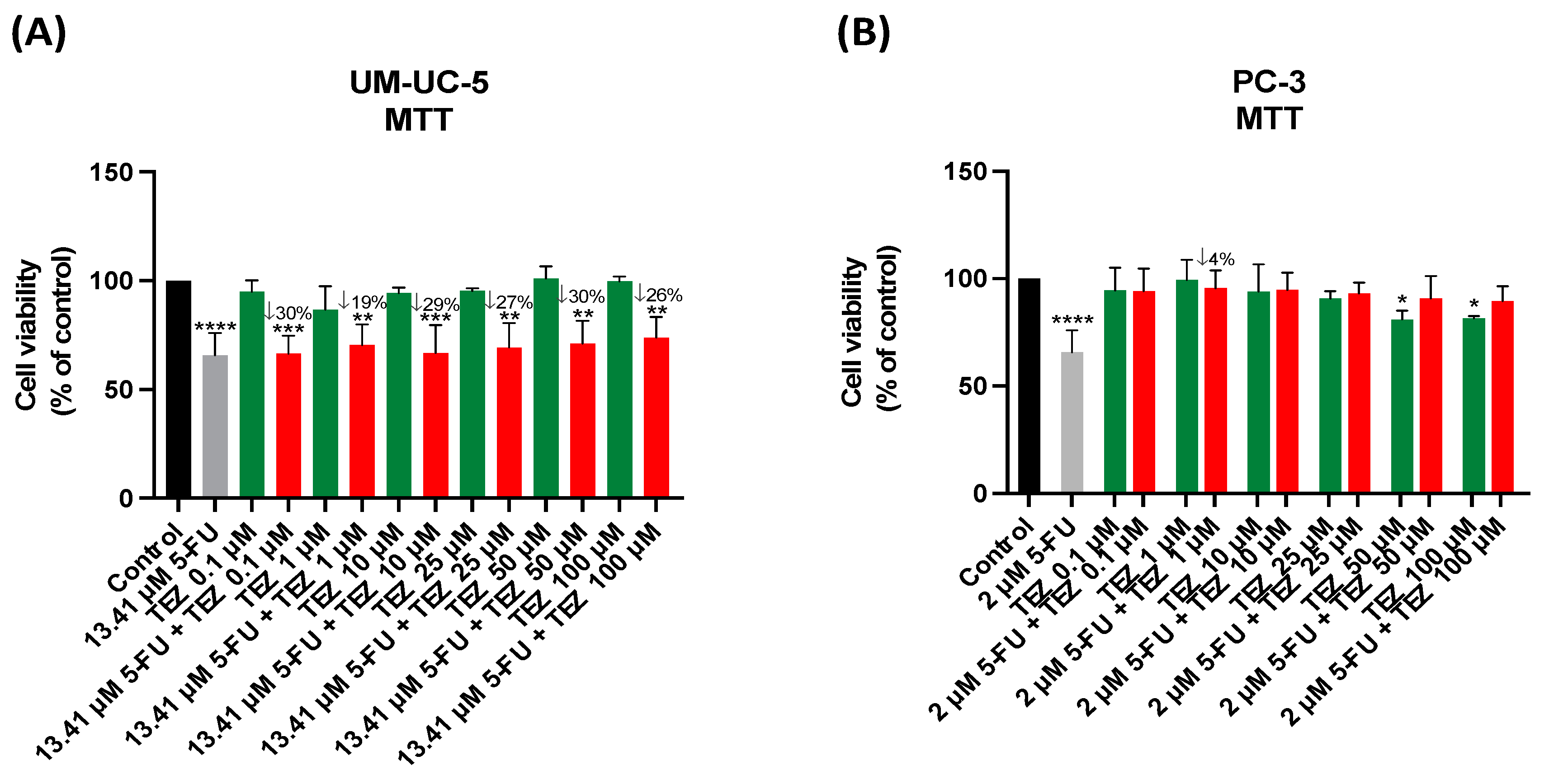
3.4. The Effect of Tezosentan as a Single Agent and in Combination with 5-FU
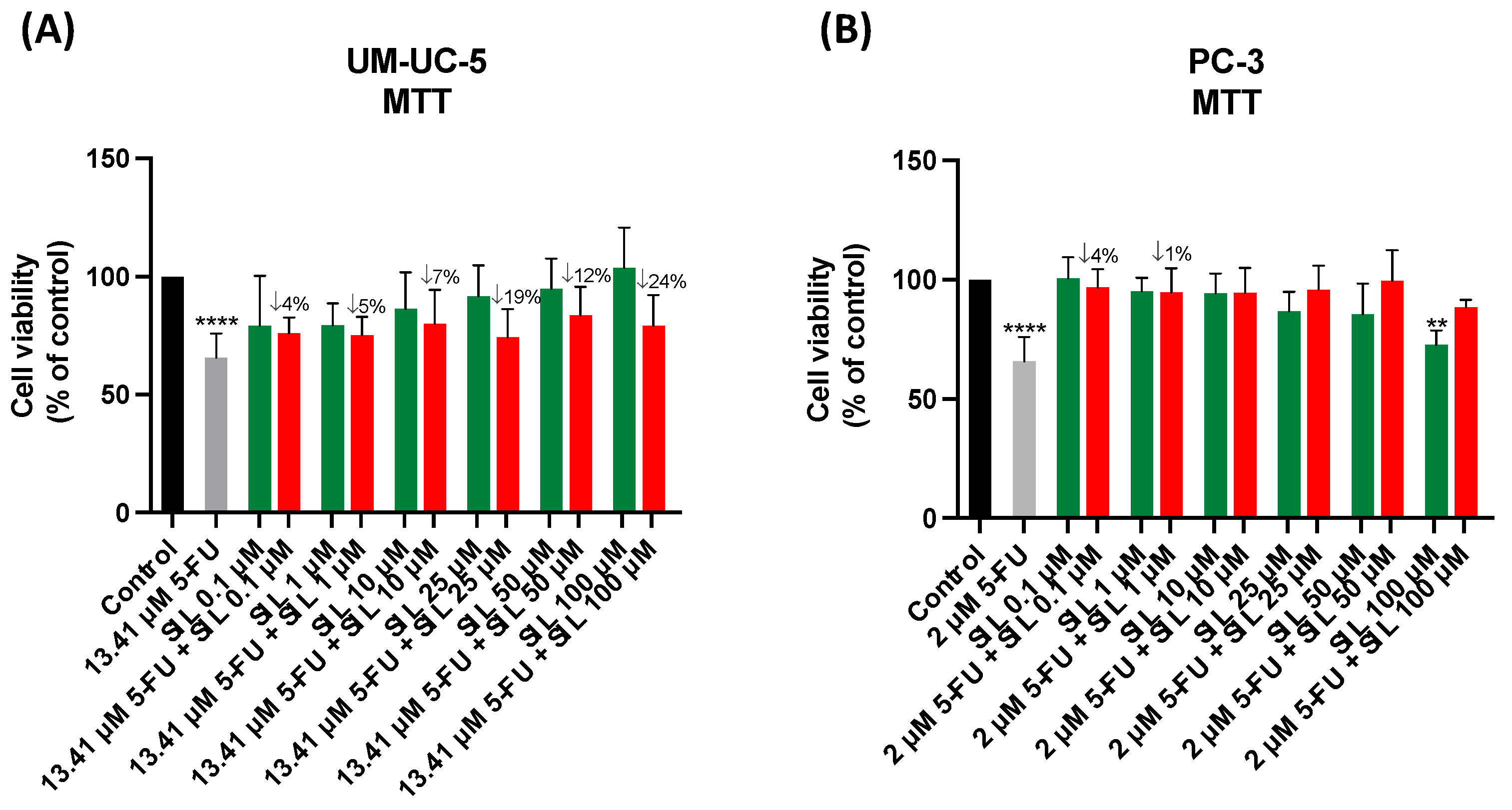
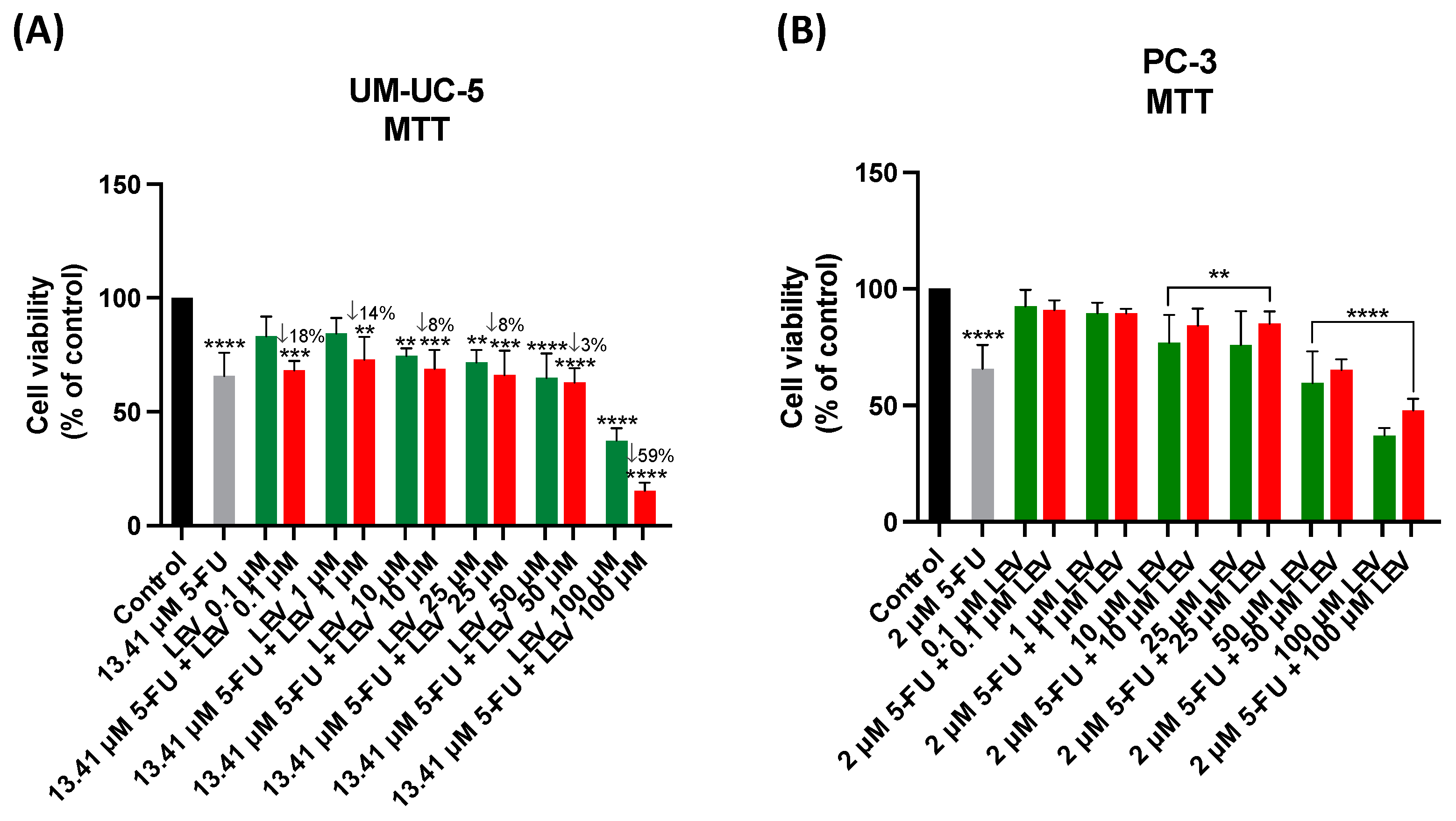
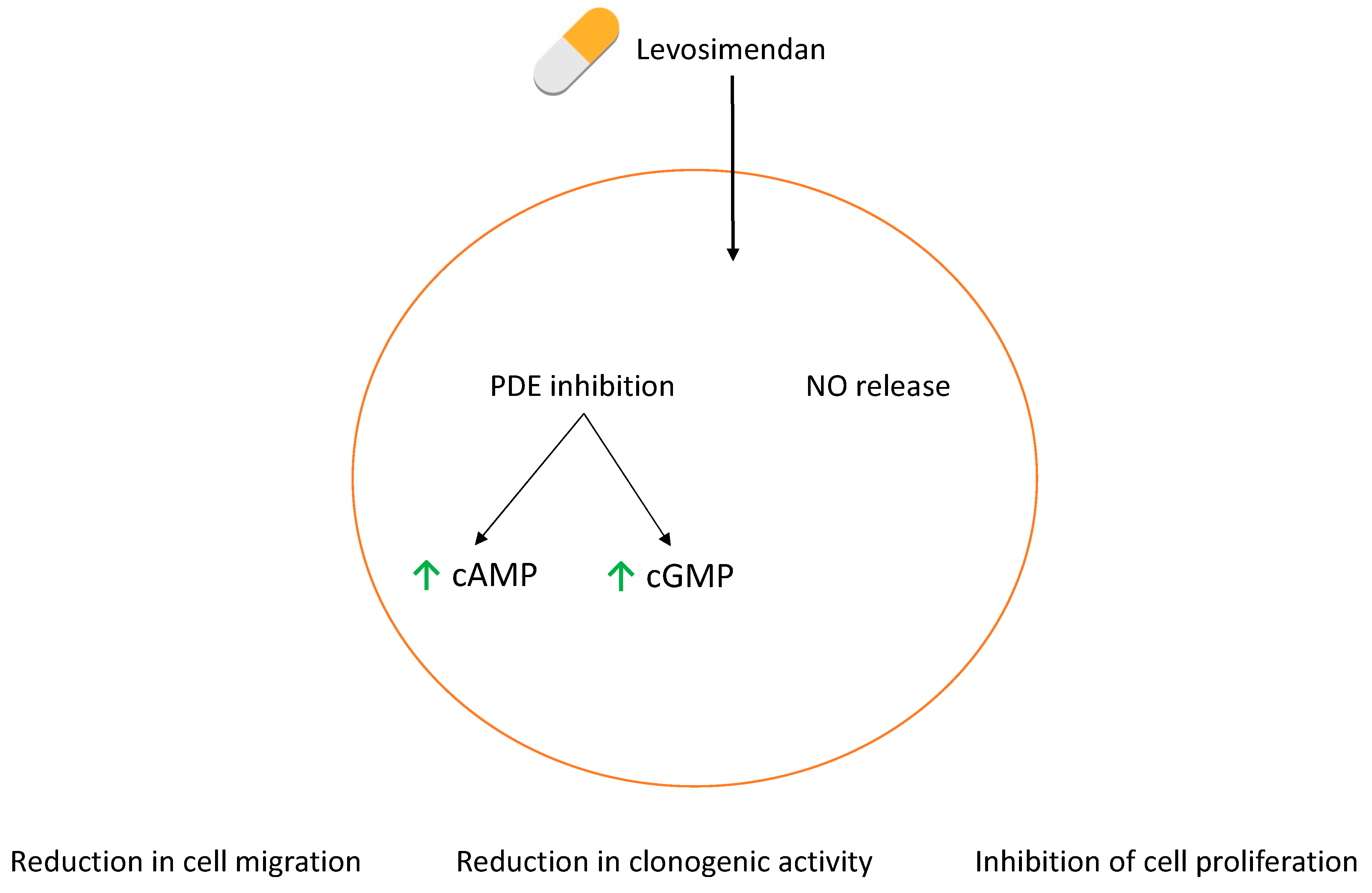
3.5. The Effect of Levosimendan as a Single Agent and in Combination with 5-FU
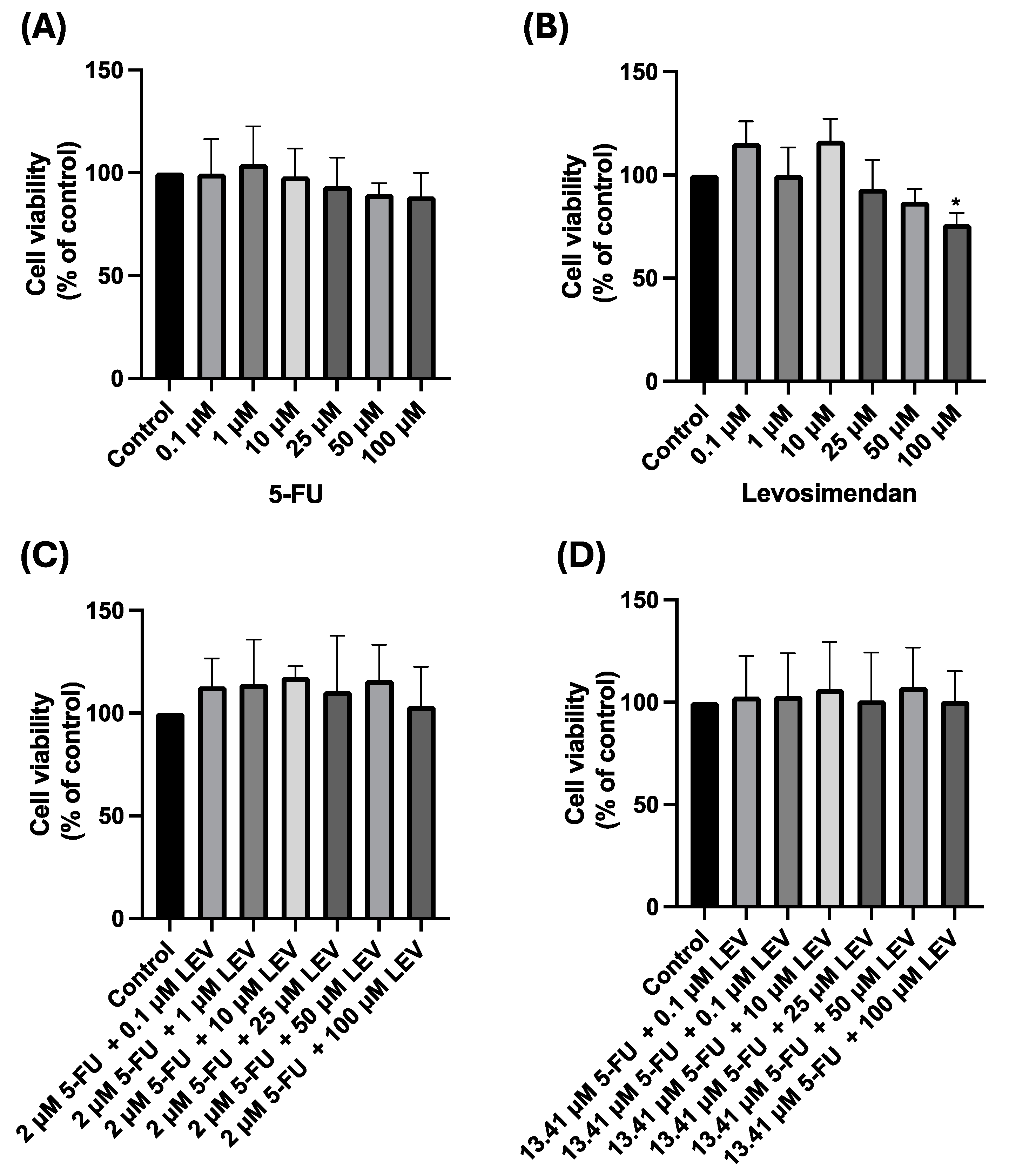
3.6. Safety Assessment of 5-FU, Levosimendan, and Their Combination in Non-Cancerous MRC-5 Cells
3.7. In Silico Validation of the Effect of the Combination of Levosimendan with 5-FU
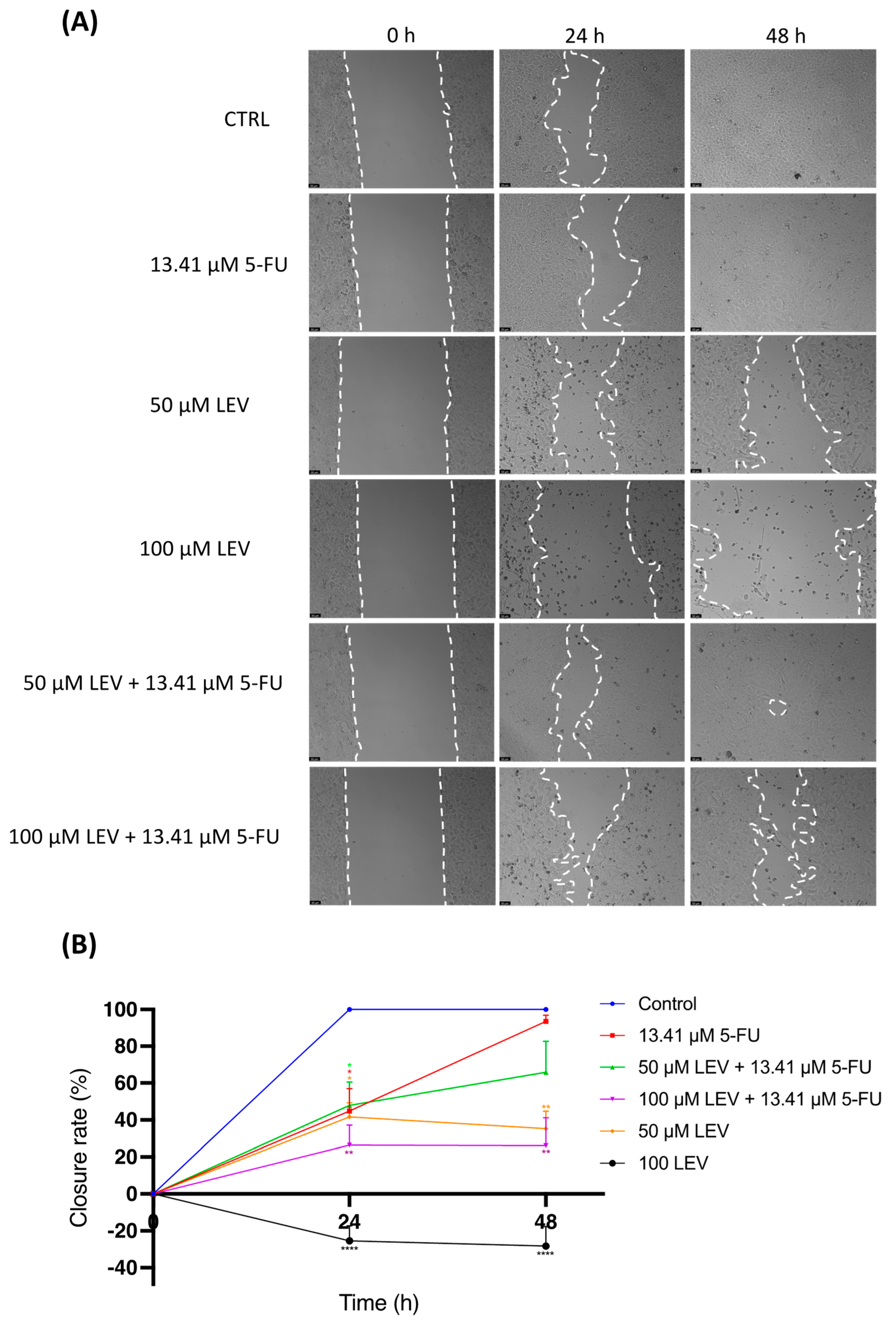
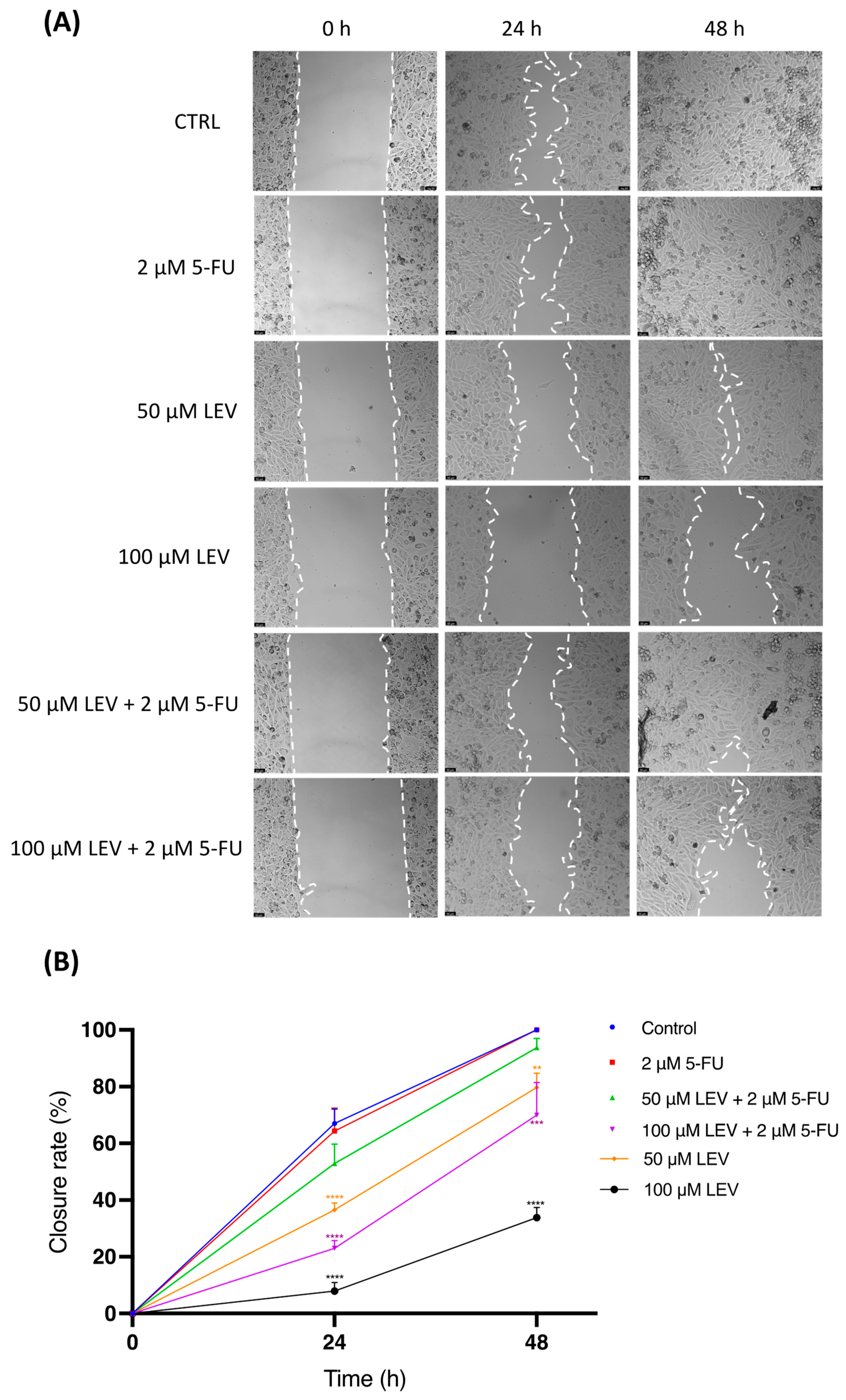
3.8. Levosimendan Reduces Migration of Bladder and Prostate Cancer Cells
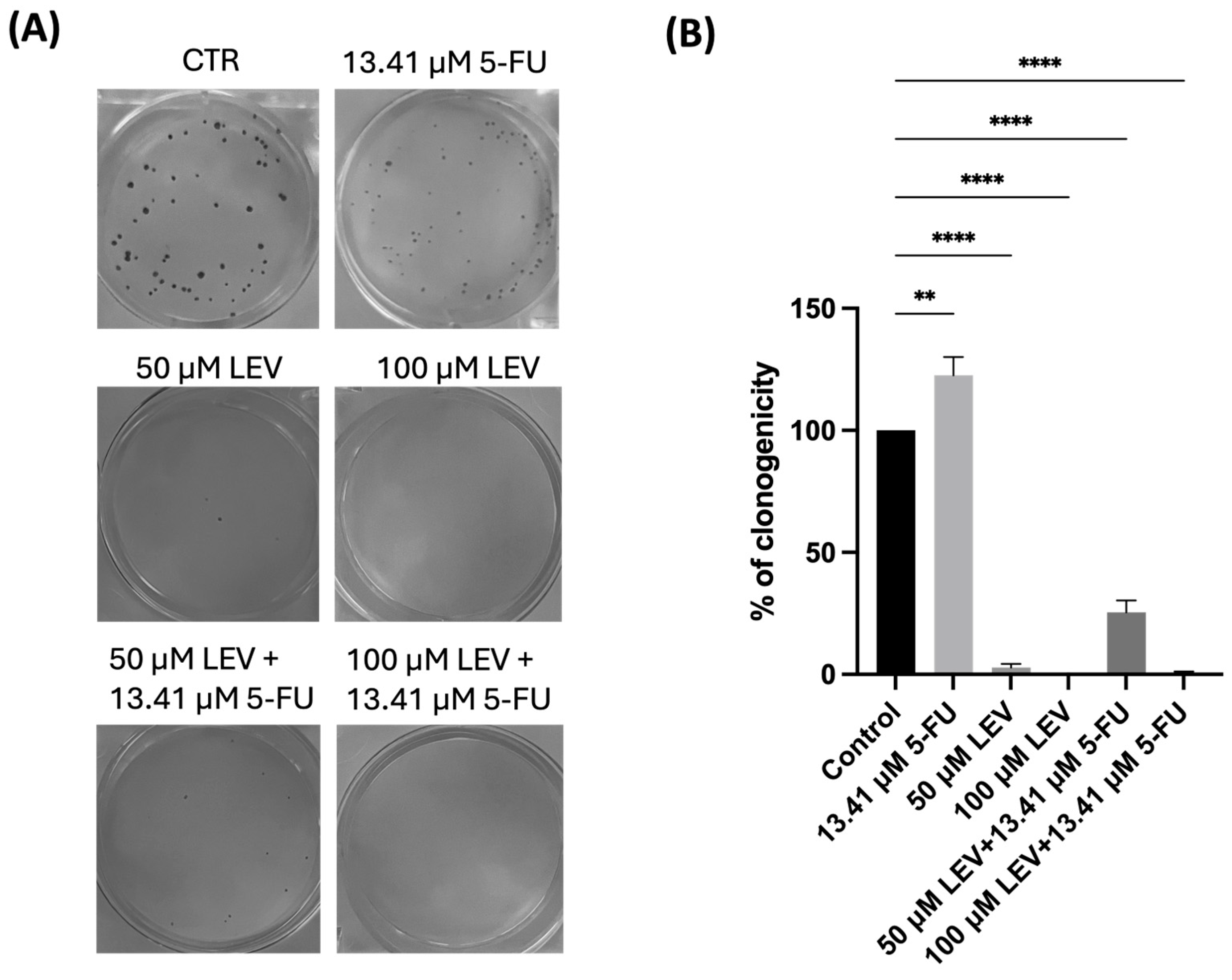
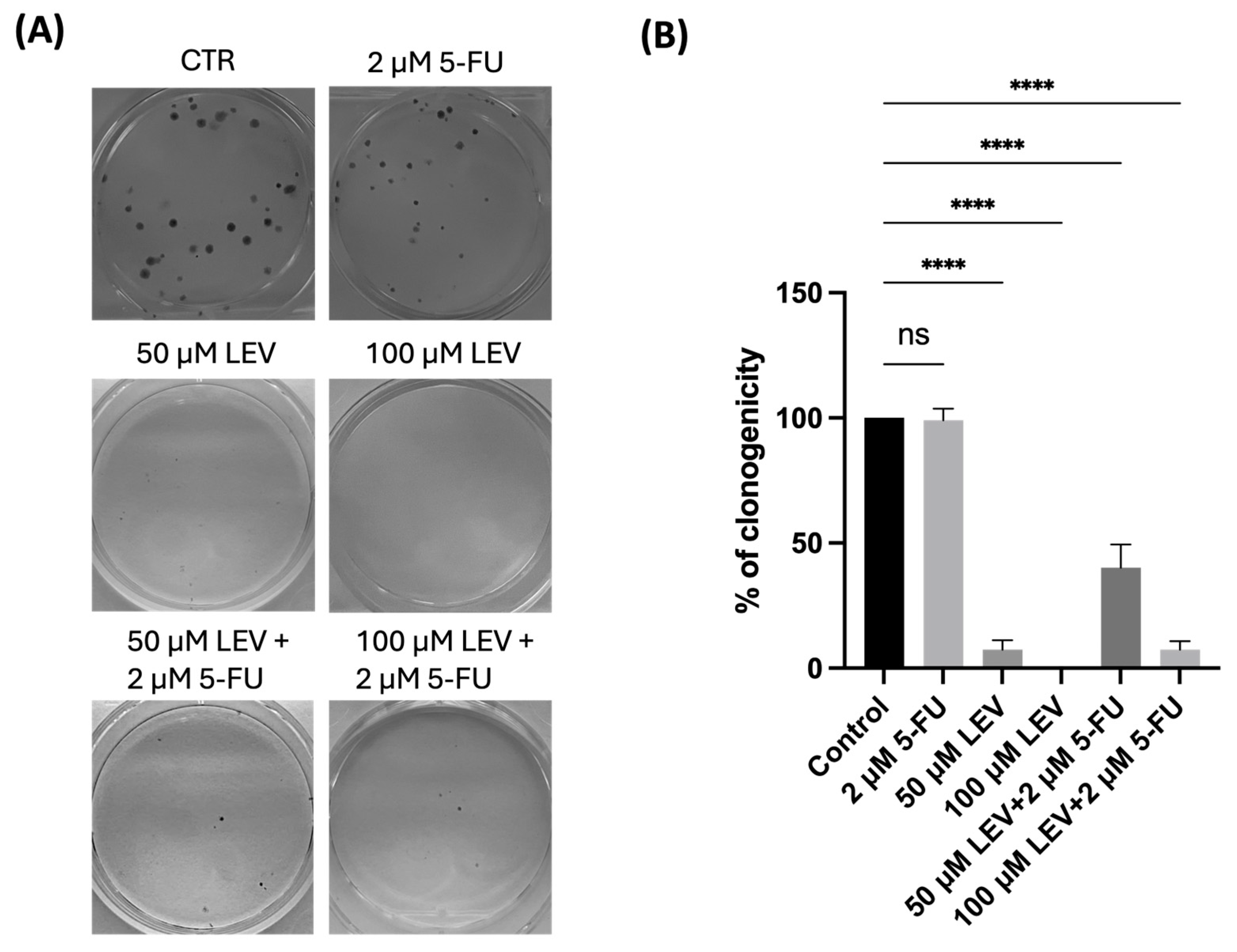
3.9. Levosimendan Reduces the Clonogenic Ability of Bladder and Prostate Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Fitch, M.I. Reducing the global burden of cancer. Asia Pac. J. Oncol. Nurs. 2022, 9, 100087. [Google Scholar] [CrossRef] [PubMed]
- Stangl, A.L.; Earnshaw, V.A.; Logie, C.H.; van Brakel, W.; Simbayi, L.C.; Barré, I.; Dovidio, J.F. The Health Stigma and Discrimination Framework: A global, crosscutting framework to inform research, intervention development, and policy on health-related stigmas. BMC Med. 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, X.; Qiu, S.; Gu, Y.; Zhang, Y. Nanomedicine for urologic cancers: Diagnosis and management. Semin. Cancer Biol. 2022, 86, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ellinger, J. Insights into Urologic Cancer. Cancers 2023, 15, 3108. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Andersson, K.E.; Arner, A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef]
- Shih, D.-H.; Shih, P.-L.; Wu, T.-W.; Lee, C.-X.; Shih, M.-H. Distinguishing Bladder Cancer from Cystitis Patients Using Deep Learning. Mathematics 2023, 11, 4118. [Google Scholar] [CrossRef]
- Belkahla, S.; Nahvi, I.; Biswas, S.; Nahvi, I.; Ben Amor, N. Advances and development of prostate cancer, treatment, and strategies: A systemic review. Front. Cell Dev. Biol. 2022, 10, 991330. [Google Scholar] [CrossRef]
- Gann, P.H. Risk factors for prostate cancer. Rev. Urol. 2002, 4 (Suppl. S5), S3–S10. [Google Scholar]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer—Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirzaei, S.; Barati, M.; Hejazi, E.S.; Kakavand, A.; Entezari, M.; Salimimoghadam, S.; Kalbasi, A.; Rashidi, M.; Taheriazam, A.; et al. Curcumin in the treatment of urological cancers: Therapeutic targets, challenges and prospects. Life Sci. 2022, 309, 120984. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Chawla, A.; Sharma, P.; Mir, P.A.; Potoo, F.H.; Reiner, Ž.; Reiner, I.; Ateşşahin, D.A.; Sharifi-Rad, J.; Mir, R.H.; et al. Repurposing approved non-oncology drugs for cancer therapy: A comprehensive review of mechanisms, efficacy, and clinical prospects. Eur. J. Med. Res. 2023, 28, 345. [Google Scholar] [CrossRef]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimers Dement. 2017, 3, 651–657. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Okuyama, R. Advancements in Drug Repurposing: Examples in Psychiatric Medications. Int. J. Mol. Sci. 2023, 24, 11000. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Vale, N. Repurposing of the Drug Tezosentan for Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5118–5131. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Vale, N. Understanding the Clinical Use of Levosimendan and Perspectives on its Future in Oncology. Biomolecules 2023, 13, 1296. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Ravi Kumar, B.V.V.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Sadeghi, H.; Adeli, I.; Mousavi, T.; Daniali, M.; Nikfar, S.; Abdollahi, M. Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Life 2021, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.I.; Wang, S.; Yang, D.; Pan, K.; Li, L.; Yuan, S. Preclinical pharmacodynamic evaluation of antibiotic nitroxoline for anticancer drug repurposing. Oncol. Lett. 2016, 11, 3265–3272. [Google Scholar] [CrossRef]
- Ueda, M.; Kumagai, K.; Ueki, K.; Inoki, C.; Orino, I.; Ueki, M. Growth inhibition and apoptotic cell death in uterine cervical carcinoma cells induced by 5-fluorouracil. Int. J. Cancer 1997, 71, 668–674. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Ziasarabi, P.; Sahebkar, A.; Ghasemi, F. Evaluation of the Effects of Nanomicellar Curcumin, Berberine, and Their Combination with 5-Fluorouracil on Breast Cancer Cells. Adv. Exp. Med. Biol. 2021, 1328, 21–35. [Google Scholar] [CrossRef]
- He, L.; Chen, H.; Qi, Q.; Wu, N.; Wang, Y.; Chen, M.; Feng, Q.; Dong, B.; Jin, R.; Jiang, L. Schisandrin B suppresses gastric cancer cell growth and enhances the efficacy of chemotherapy drug 5-FU in vitro and in vivo. Eur. J. Pharmacol. 2022, 920, 174823. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Vale, N. Repurposing Alone and in Combination of the Antiviral Saquinavir with 5-Fluorouracil in Prostate and Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12240. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, R.; Kusunoki, M.; Yanagi, H.; Noda, M.; Furuyama, J.I.; Yamamura, T.; Hashimoto-Tamaoki, T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: A novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001, 61, 1029–1037. [Google Scholar]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Bouklihacene, M.; Thorne, R.F. 5-Fluorouracil-induced apoptosis in colorectal cancer cells is caspase-9-dependent and mediated by activation of protein kinase C-δ. Oncol. Lett. 2014, 8, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Steger, F.; Hautmann, M.G.; Kölbl, O. 5-FU-induced cardiac toxicity—An underestimated problem in radiooncology? Radiat. Oncol. 2012, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Balachandran, C. Serpentine supravenous pigmentation. A rare vasculo-cutaneous effect induced by systemic 5-fluorouracil. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 714–715. [Google Scholar] [CrossRef] [PubMed]
- Boolell, M.; Allen, M.J.; Ballard, S.A.; Gepi-Attee, S.; Muirhead, G.J.; Naylor, A.M.; Osterloh, I.H.; Gingell, C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996, 8, 47–52. [Google Scholar] [PubMed]
- AboYoussef, A.M.; Khalaf, M.M.; Malak, M.N.; Hamzawy, M.A. Repurposing of sildenafil as antitumour; induction of cyclic guanosine monophosphate/protein kinase G pathway, caspase-dependent apoptosis and pivotal reduction of Nuclear factor kappa light chain enhancer of activated B cells in lung cancer. J. Pharm. Pharmacol. 2021, 73, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Sager, G.; Berg, T.; Nergaard, B.; Moe, B.T.; Ørbo, A. Increased gene expression of the ABCC5 transporter without distinct changes in the expression of PDE5 in human cervical cancer cells during growth. Anticancer Res. 2012, 32, 3055–3061. [Google Scholar]
- Zhu, B.; Strada, S.J. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr. Top. Med. Chem. 2007, 7, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsen, T.; Benson, L.; Hagerman, I.; Dahlström, U.; Edner, M.; Lund, L.H. Planned repetitive use of levosimendan for heart failure in cardiology and internal medicine in Sweden. Int. J. Cardiol. 2014, 175, 55–61. [Google Scholar] [CrossRef]
- Pathak, A.; Lebrin, M.; Vaccaro, A.; Senard, J.M.; Despas, F. Pharmacology of levosimendan: Inotropic, vasodilatory and cardioprotective effects. J. Clin. Phar. Ther. 2013, 38, 341–349. [Google Scholar] [CrossRef]
- Kamath, S.R.; Jaykumar, I.; Matha, S. Levosimendan. Indian Pediatr. 2009, 46, 593–596. [Google Scholar]
- Yokoshiki, H.; Katsube, Y.; Sunagawa, M.; Sperelakis, N. Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytes. Eur. J. Pharmacol. 1997, 333, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kaheinen, P.; Pollesello, P.; Levijoki, J.; Haikala, H. Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channels. J. Cardiovasc. Pharmacol. 2001, 37, 367–374. [Google Scholar] [CrossRef]
- Pataricza, J.; Höhn, J.; Petri, A.; Balogh, Á.; Papp, J.G. Comparison of the Vasorelaxing Effect of Cromakalim and the New Inodilator, Levosimendan, in Human Isolated Portal Vein. J. Pharm. Pharmacol. 2010, 52, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Pataricza, J.; Krassói, I.; Höhn, J.; Kun, A.; Papp, J.G. Functional role of potassium channels in the vasodilating mechanism of levosimendan in porcine isolated coronary artery. Cardiovasc. Drugs Ther. 2003, 17, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Nacitarhan, C.; Seyrek, M. Potassium channels in the vasodilating action of levosimendan on the human umbilical artery. J. Soc. Gynecol. Investig. 2006, 13, 312–315. [Google Scholar] [CrossRef]
- Erdei, N.; Papp, Z.; Pollesello, P.; Édes, I.; Bagi, Z. The levosimendan metabolite OR-1896 elicits vasodilation by activating the KATP and BKCa channels in rat isolated arterioles. Br. J. Pharmacol. 2006, 148, 696–702. [Google Scholar] [CrossRef]
- Gruhn, N.; Nielsen-Kudsk, J.E.; Theilgaard, S.; Bang, L.; Olesen, S.P.; Aldershvile, J. Coronary vasorelaxant effect of levosimendan, a new inodilator with calcium-sensitizing properties. J. Cardiovasc. Pharmacol. 1998, 31, 741–749. [Google Scholar] [CrossRef] [PubMed]
- De Witt, B.J.; Ibrahim, I.N.; Bayer, E.; Fields, A.M.; Richards, T.A.; Banister, R.E.; Kaye, A.D. An analysis of responses to levosimendan in the pulmonary vascular bed of the cat. Anesth. Analg. 2002, 94, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Grossini, E.; Molinari, C.; Caimmi, P.; Uberti, F.; Vacca, G. Levosimendan induces NO production through p38 MAPK, ERK and Akt in porcine coronary endothelial cells: Role for mitochondrial KATP channel. Br. J. Pharmacol. 2009, 156, 250–261. [Google Scholar] [CrossRef]
- Dingemanse, J.; Clozel, M.; van Giersbergen, P.L. Pharmacokinetics and pharmacodynamics of tezosentan, an intravenous dual endothelin receptor antagonist, following chronic infusion in healthy subjects. Br. J. Clin. Pharmacol. 2002, 53, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W. Tezosentan in the management of decompensated heart failure. Cardiol. Rev. 2005, 13, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Wu, B.; Quan, Y.; Liu, D.; Li, Y.; Zhang, X. Synergistic Activity of an Antimetabolite Drug and Tyrosine Kinase Inhibitors against Breast Cancer Cells. Chem. Pharm. Bull. 2017, 65, 768–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miskimins, W.K.; Ahn, H.J.; Kim, J.Y.; Ryu, S.; Jung, Y.S.; Choi, J.Y. Synergistic anti-cancer effect of phenformin and oxamate. PLoS ONE 2014, 9, e85576. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Chidley, C.; Sorger, P.K. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. Elife 2019, 8, e50036. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.-S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef]
- Ribeiro, E.; Costa, B.; Vasques-Nóvoa, F.; Vale, N. In Vitro Drug Repurposing: Focus on Vasodilators. Cells 2023, 12, 671. [Google Scholar] [CrossRef]
- Wang, J.; Re, J.; Wang, Z. Mode of action of sildenafil. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1999, 21, 493–496. [Google Scholar]
- Pashkovetsky, E.; Gupta, C.A.; Aronow, W.S. Use of levosimendan in acute and advanced heart failure: Short review on available real-world data. Ther. Clin. Risk Manag. 2019, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Conti, N.; Gatti, M.; Raschi, E.; Diemberger, I.; Potena, L. Evidence and Current Use of Levosimendan in the Treatment of Heart Failure: Filling the Gap. Drug Des. Devel. Ther. 2021, 15, 3391–3409. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. New Trends for Antimalarial Drugs: Synergism between Antineoplastics and Antimalarials on Breast Cancer Cells. Biomolecules 2020, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Cardoso, A.; Vale, N. Synergistic Growth Inhibition of HT-29 Colon and MCF-7 Breast Cancer Cells with Simultaneous and Sequential Combinations of Antineoplastics and CNS Drugs. Int. J. Mol. Sci. 2021, 22, 7408. [Google Scholar] [CrossRef]
- Correia, A.S.; Marques, L.; Cardoso, A.; Vale, N. Exploring the Role of Drug Repurposing in Bridging the Hypoxia-Depression Connection. Membranes 2023, 13, 800. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Marques, L.; Vale, N. The Involvement of Hypoxia in the Response of Neuroblastoma Cells to the Exposure of Atorvastatin. Curr. Issues Mol. Biol. 2023, 45, 3333–3346. [Google Scholar] [CrossRef]
- Marques, L.; Vale, N. Prediction of CYP-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Modeling: A Case Study of Salbutamol and Fluvoxamine. Pharmaceutics 2023, 15, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, G.; Ji, J.; Wu, J.; Sun, X.; Shen, J.; Jiang, H.; Wang, H. PDE5 inhibitor promotes melanin synthesis through the PKG pathway in B16 melanoma cells. J. Cell. Biochem. 2012, 113, 2738–2743. [Google Scholar] [CrossRef]
- Karami-Tehrani, F.; Moeinifard, M.; Aghaei, M.; Atri, M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch. Med. Res. 2012, 43, 470–475. [Google Scholar] [CrossRef]
- Hossain, K.R.; Clarke, R.J. General and specific interactions of the phospholipid bilayer with P-type ATPases. Biophys. Rev. 2019, 11, 353–364. [Google Scholar] [CrossRef]
- Manallack, D.T. The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar]
- Antila, S.; Kivikko, M.; Lehtonen, L.; Eha, J.; Heikkilä, A.; Pohjanjousi, P.; Pentikäinen, P.J. Pharmacokinetics of levosimendan and its circulating metabolites in patients with heart failure after an extended continuous infusion of levosimendan. Br. J. Clin. Pharmacol. 2004, 57, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Evrard, S.; Khatib, A.M. The Give-and-Take Interaction Between the Tumor Microenvironment and Immune Cells Regulating Tumor Progression and Repression. Front. Immunol. 2022, 13, 850856. [Google Scholar] [CrossRef] [PubMed]
- Kipka, H.; Schaflinger, R.; Tomasi, R.; Pogoda, K.; Mannell, H. The Effects of the Levosimendan Metabolites OR-1855 and OR-1896 on Endothelial Pro-Inflammatory Responses. Biomedicines 2023, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Leo, S.; Rimessi, A.; Wieckowski, M.R.; Fiorica, F.; Giorgi, C.; Pinton, P. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim. Biophys. Acta 2021, 1868, 119061. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Trimarco, B.; Iaccarino, G.; Santulli, G. New Insights in Cardiac Calcium Handling and Excitation-Contraction Coupling. Adv. Exp. Med. Biol. 2018, 1067, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, X.; He, R.; Meng, W.; Chen, W.; Wang, F.; Meng, X. Enhanced anti-breast cancer efficacy of co-delivery liposomes of docetaxel and curcumin. Front. Pharmacol. 2022, 13, 969611. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Lewis, T.A.; de Waal, L.; Wu, X.; Youngsaye, W.; Wengner, A.; Kopitz, C.; Lange, M.; Gradl, S.; Ellermann, M.; Lienau, P.; et al. Optimization of PDE3A Modulators for SLFN12-Dependent Cancer Cell Killing. ACS Med. Chem. Lett. 2019, 10, 1537–1542. [Google Scholar] [CrossRef]
- Hou, Y.; Wren, A.; Mylarapu, N.; Browning, K.; Islam, B.N.; Wang, R.; Vega, K.J.; Browning, D.D. Inhibition of Colon Cancer Cell Growth by Phosphodiesterase Inhibitors Is Independent of cGMP Signaling. J. Pharmacol. Exp. Ther. 2022, 381, 42–53. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Favot, L.; Keravis, T.; Holl, V.; Le Bec, A.; Lugnier, C. VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb. Haemost. 2003, 90, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Shimizu, K.; Narita, M.; Manganiello, V.C.; Tagawa, T. Characterization of phosphodiesterase 3 in human malignant melanoma cell line. Anticancer Res. 2002, 22, 3171–3174. [Google Scholar]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef]
- Huguenin, S.; Vacherot, F.; Kheuang, L.; Fleury-Feith, J.; Jaurand, M.C.; Bolla, M.; Riffaud, J.P.; Chopin, D.K. Antiproliferative effect of nitrosulindac (NCX 1102), a new nitric oxide-donating non-steroidal anti-inflammatory drug, on human bladder carcinoma cell lines. Mol. Cancer Ther. 2004, 3, 291–298. [Google Scholar] [CrossRef]
- Seabra, A.B.; Durán, N. Nitric oxide donors for prostate and bladder cancers: Current state and challenges. Eur. J. Pharmacol. 2018, 826, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Lowe, J.E.; Knowles, R.G.; Green, I.C.; Green, M.H. Factors affecting the DNA damaging activity of superoxide and nitric oxide. Mutat. Res. 1998, 402, 77–84. [Google Scholar] [CrossRef]
- Marshall, H.E.; Stamler, J.S. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 2001, 40, 1688–1693. [Google Scholar] [CrossRef]
- Plenchette, S.; Romagny, S.; Laurens, V.; Bettaieb, A. S-Nitrosylation in TNF superfamily signaling pathway: Implication in cancer. Redox Biol. 2015, 6, 507–515. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grünert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.P.; Roy, I.; Hauser, A.D.; Wilson, J.M.; Williams, C.L.; Dwinell, M.B. Cyclic AMP regulates the migration and invasion potential of human pancreatic cancer cells. Mol. Carcinog. 2015, 54, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.J.; Huang, X.Y. cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J. Biol. Chem. 2008, 283, 13799–13805. [Google Scholar] [CrossRef] [PubMed]
- Orstavik, O.; Ata, S.H.; Riise, J.; Dahl, C.P.; Andersen, G.; Levy, F.O.; Skomedal, T.; Osnes, J.B.; Qvigstad, E. Inhibition of phosphodiesterase-3 by levosimendan is sufficient to account for its inotropic effect in failing human heart. Br. J. Pharmacol. 2014, 171, 5169–5181. [Google Scholar] [CrossRef]
- Gordon, J.L.; Hinsen, K.J.; Reynolds, M.M.; Smith, T.A.; Tucker, H.O.; Brown, M.A. Anticancer potential of nitric oxide (NO) in neuroblastoma treatment. RSC Adv. 2021, 11, 9112–9120. [Google Scholar] [CrossRef]
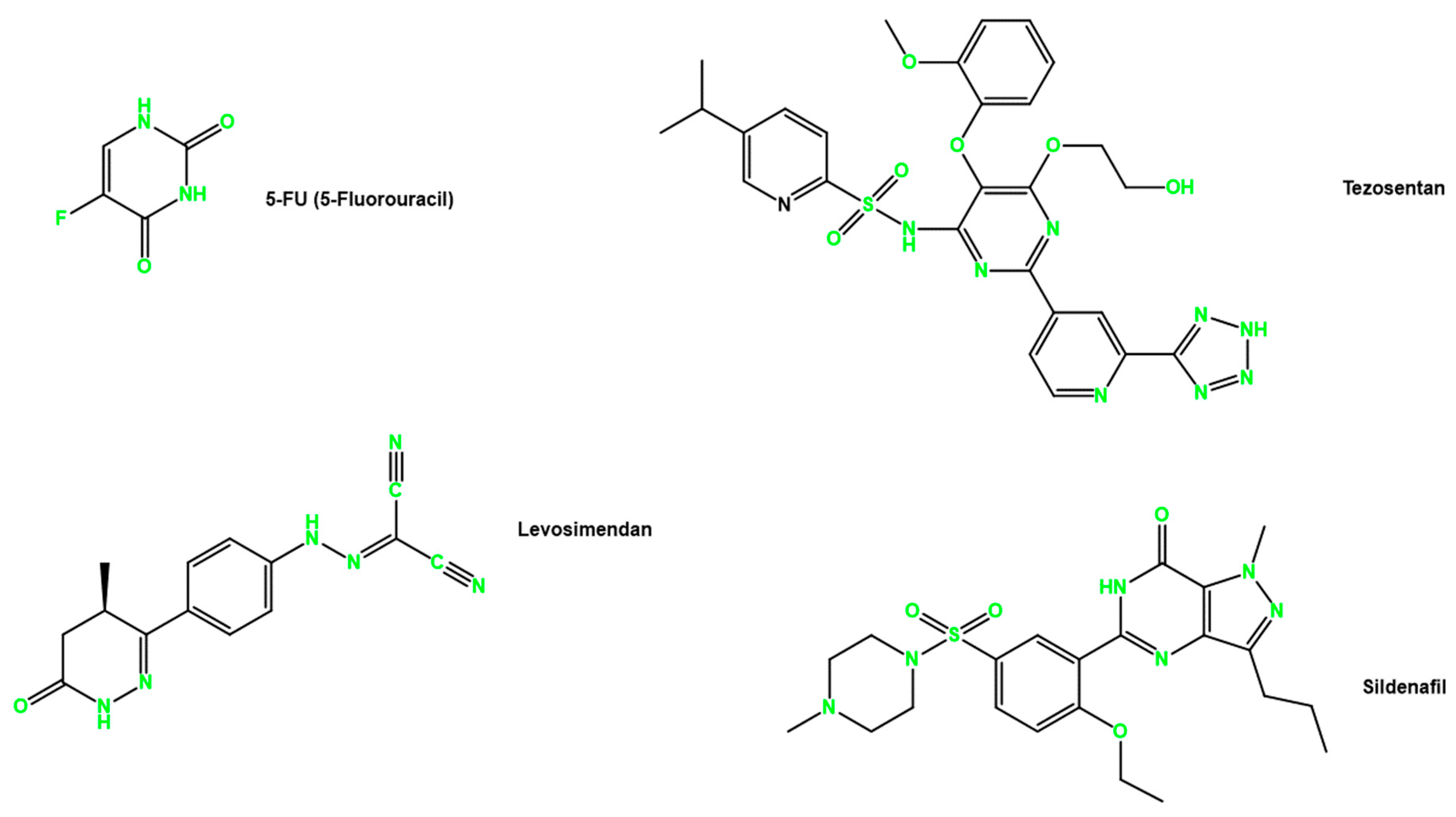
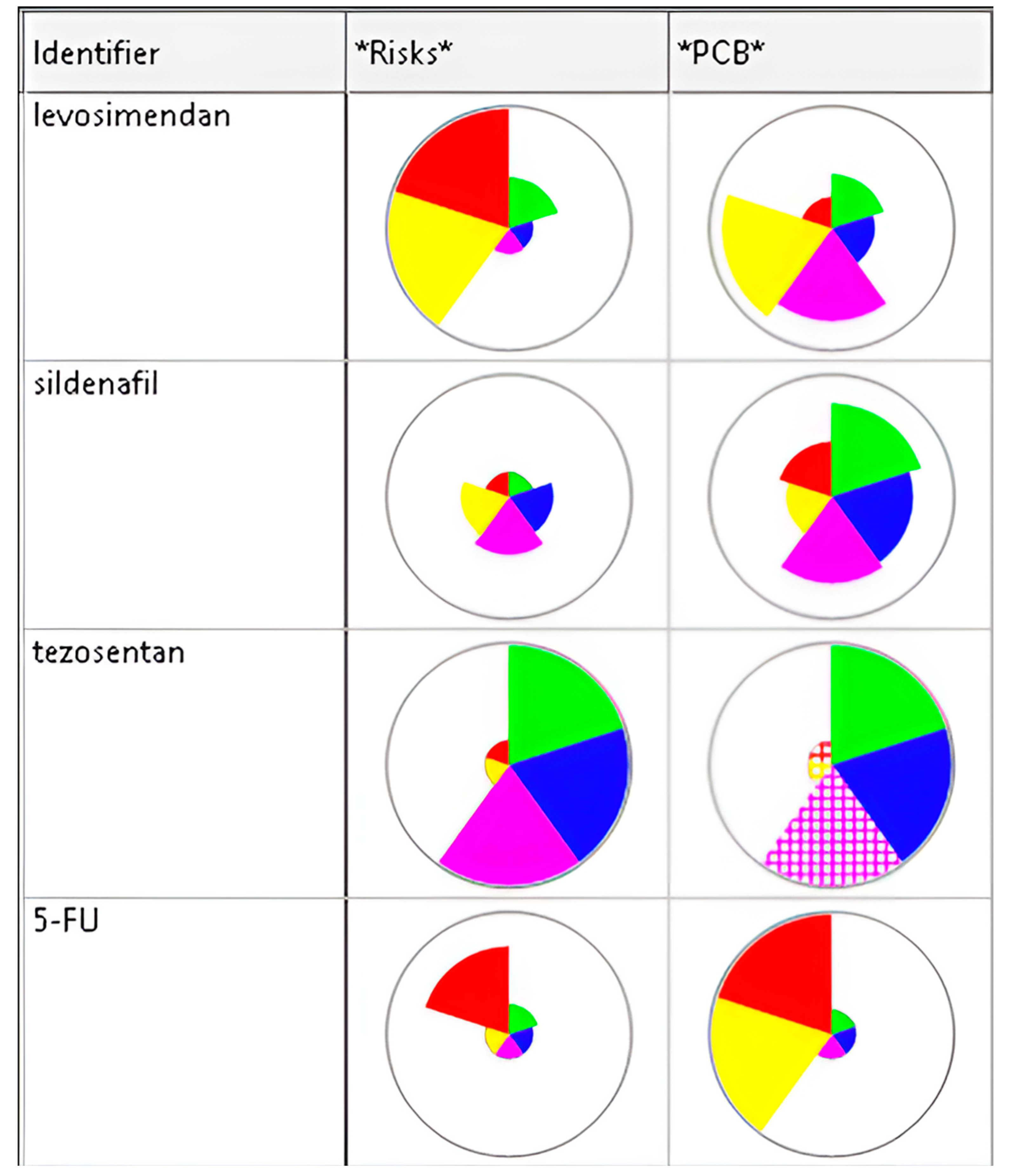
| Drug | MOA | Indication | References |
|---|---|---|---|
| Sildenafil | PDE5 inhibitor | Erectile dysfunction and pulmonary hypertension | [60] |
| Tezosentan | Endothelin receptor antagonist | Pulmonary arterial hypertension | [51] |
| Levosimendan | Calcium sensitizer | Acute and advanced heart failure and hypertension | [61,62] |
| Drug | Cell Line | IC50 (µM) |
|---|---|---|
| 5-FU | UM-UC-5 | 13.41 |
| PC-3 | 2.00 | |
| Sildenafil | UM-UC-5 | >100 |
| PC-3 | >100 | |
| Tezosentan | UM-UC-5 | >100 |
| PC-3 | 18.73 | |
| Levosimendan | UM-UC-5 | 12.14 |
| PC-3 | 21.74 |
| Properties | Predicated Value | Observed Value | Reference |
|---|---|---|---|
| Molecular weight (g/mol) | 280.291 | 280.28 | [52] |
| Ionization constant (pKa) | 7.23 | 6.3 | [60] |
| LogP | 2.352 | 2.16–2.69 | [63] |
| PSA (Å2) | 113.43 | 113 | [52] |
| Water solubility (mg/mL) | 0.121 | 0.04–0.0881 | [52,64] |
| 5-FU | Sildenafil | Tezosentan | Levosimendan | |
|---|---|---|---|---|
| Water Solubility (mg/mL) | 5.86 | 0.410 | 0.084 | 0.121 |
| Log P | −0.66 | 2.070 | 3.583 | 2.352 |
| PSA (Å2) | 58.2 | 113.42 | 200.11 | 113.43 |
| pKa | 7.18 | 8.74 | 6.10 | 10.41 |
| P-gp substrate (%) | Yes (82) | Yes (50) | Yes * | No * |
| P-gp inhibitor (%) | No (93) | No (56) | Yes (97) | No (61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, E.; Costa, B.; Marques, L.; Vasques-Nóvoa, F.; Vale, N. Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes. J. Clin. Med. 2024, 13, 4113. https://doi.org/10.3390/jcm13144113
Ribeiro E, Costa B, Marques L, Vasques-Nóvoa F, Vale N. Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes. Journal of Clinical Medicine. 2024; 13(14):4113. https://doi.org/10.3390/jcm13144113
Chicago/Turabian StyleRibeiro, Eduarda, Barbara Costa, Lara Marques, Francisco Vasques-Nóvoa, and Nuno Vale. 2024. "Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes" Journal of Clinical Medicine 13, no. 14: 4113. https://doi.org/10.3390/jcm13144113
APA StyleRibeiro, E., Costa, B., Marques, L., Vasques-Nóvoa, F., & Vale, N. (2024). Enhancing Urological Cancer Treatment: Leveraging Vasodilator Synergistic Potential with 5-FU for Improved Therapeutic Outcomes. Journal of Clinical Medicine, 13(14), 4113. https://doi.org/10.3390/jcm13144113








